Back to Journals » Neuropsychiatric Disease and Treatment » Volume 18
Gender Difference in Cognitive Function Among Stable Schizophrenia: A Network Perspective
Authors Chen M , Zhang L, Jiang Q
Received 15 October 2022
Accepted for publication 6 December 2022
Published 22 December 2022 Volume 2022:18 Pages 2991—3000
DOI https://doi.org/10.2147/NDT.S393586
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Mengyi Chen, Lei Zhang, Qi Jiang
Department of Geriatric, Shanghai Pudong New Area Mental Health Center, Tongji University School of Medicine, Shanghai, People’s Republic of China
Correspondence: Mengyi Chen, Shanghai Pudong New Area Mental Health Center, Tongji University School of Medicine, Shanghai, 200124, People’s Republic of China, Tel/Fax +8602168306699, Email [email protected]
Objective: To investigate the gender differences and influencing factors of cognitive function in stable schizophrenic patients, and to explore the cognitive characteristics of male and female patients.
Methods: A total of 298 patients with chronic schizophrenia were divided into two groups according to gender. The differences of demographic and clinical characteristics between the two groups were firstly analyzed. Then the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) was used to measure their cognitive function, and the correlation between cognitive function and demographic characteristics and clinical characteristics was analyzed. Finally, the gender-based cognitive characteristics were explored through network analysis.
Results: There was no significant difference in the RBANS total score and sub-item score between the male schizophrenia and female schizophrenia patients. Correlation analysis showed that RBANS total score was inversely proportional with age, duration and Positive and Negative Syndrome Scale (PANSS) score in male schizophrenia, while being directly proportional with age at onset and inversely proportional with PANSS score in female schizophrenia. Network analysis showed that language was the core of cognitive function for male schizophrenia, and the delayed memory was the core of cognitive function for female schizophrenia.
Conclusion: There was no significant gender difference in cognitive function score among patients with stable schizophrenia. The core cognitive functions of male and female schizophrenia are language and delayed memory, respectively.
Keywords: schizophrenia, cognition, gender difference, network analysis
Introduction
Cognitive dysfunction is an important feature of schizophrenia,1 existing in almost all schizophrenia patients.2 The cognitive dysfunction is not only reflected in the acute phase of schizophrenia,3 but also obvious in the stable phase.4 Previous studies have shown that cognitive dysfunction in schizophrenia patients involves many fields, such as memory, attention, learning, executive functioning and cognitive processing speed.5 Moreover, the actual functional results of patients are negatively correlated with the degree of cognitive dysfunction,6 which makes it a difficult problem for schizophrenic patients to return to society, even for patients in the stable phase.7
There are differences in the cognitive dysfunction between male and female schizophrenia patients.8 Some studies consider male cognitive dysfunction is more serious,9 but also some studies hold the opposite view,10 or believe that there is no significant difference.11 With the development of magnetic resonance imaging (MRI), people have gradually realized the differences between male and female in brain regional structure and neural development,12 and believe that schizophrenia also has gender differences in neural pathway damage.13,14 Based on this view, some researchers hold that even if there is no significant difference in the total score of cognitive scale between male and female schizophrenia patients, they may still have subtle differences in specific domains of cognition, which may be reflected in the different subscale scores or the correlation of changes in scale items. The core cognitive function may become the target of psychopathology research and precision treatment in the future.
However, although the traditional correlation analysis can reflect the relationship between variables, it cannot observe the importance of a single variable in the whole network and the mutual influence of variables. In recent years, researchers have introduced network analysis into the psychopathology of mental disorders.15 Using this method, researchers can build a partial correlation network among cognitive function items, and also highlight the relationship and interplay among the variables.16 If a variable is closely related to most of the other variables in the network, its change would affect the whole cognitive function network.17 Up to now, there are no studies using network analysis to explore gender-based cognition differences in patients with schizophrenia. Therefore, this paper aims to investigate the gender differences and influencing factors of cognitive function in stable schizophrenia patients, and to explore the cognitive characteristics of male and female patients by network analysis. It is hoped to provide more valuable intervention targets for the rehabilitation of social function in chronic schizophrenia patients by exploring the core cognitive function.
Methods
Subjects
This is a case-control study, the total 298 subjects were recruited from Shanghai Pudong New Area Mental Health Center, Tongji University School of Medicine, Shanghai, China, from January 1, 2021 to May 1, 2022, aged 20 to 65 years. The diagnostic criteria are ICD-10 (International Classification of Diseases, 10th Revision),18 and we used Chinese version of MINI (mini-international neuropsychiatric interview)19 by 2 independent psychiatrists in the actual recruitment work to assess the patients. Inclusion criteria were: (1) meet the diagnostic criteria for schizophrenia; (2) age 18 years or older; (3) at stable phase; (4) ability to complete the cognition experiment. Exclusion criteria were: (1) any history of traumatic brain injuries; (2) substance abuse; (3) comorbid neurological disorders that may affect cognitive function.
This study was conducted in accordance with the Declaration of Helsinki. The study was approved by the Institutional Review Board (IRB) of the Shanghai Pudong New Area Mental Health Center, Tongji University School of Medicine, Shanghai, China (ID number: PDJWLL2020012). All the subjects signed the written informed consent for participating after the study process been fully explained.
Demographic and Clinical Variables
The demographic data of all the subjects was recorded, which were age, marital status, education, duration, age at onset, hospitalization times. The clinical aspects data associated with schizophrenia symptoms and medicines also was collected: the use of first generation antipsychotics (FGA), second generation antipsychotics (SGA), anticholinergic, mood stabilizer, benzodiazepine. Clinical symptoms were assessed using Positive and Negative Syndrome Scale (PANSS).20 The Cronbach’s α of the scale was 0.8707, which had good reliability and validity.21 All variables were grouped by gender.
Cognition Experiment
Cognition Experiment used Repeatable Battery for the Assessment of Neuropsychological Status (RBANS).22 RBANS had 12 sub-assessments: vocabulary learning, story retelling, graphics copy, picture naming, line orientation, semantic fluency, digit span, coding, vocabulary memory, vocabulary recognition, stories memory and graphics memory, and estimate one’s cognitive function from 5 dimensions: Immediate Memory, Visuospatial/Constructional, Language, Attention and Delayed Memory. The original score of each test item were converted into scale score by conversion norm. We used Chinese version with a Cronbach’s α >0.7, which had good reliability and validity.23
Statistical Analysis
The comparison between male and female groups about demographic and clinical variables were performed by SPSS 23.0 version. In terms of statistical methods, we used chi-square test for categorical variables, t-test and Mann–Whitney U-test for continuous variable. Spearman correlation analysis for correlation analysis. All tests were 2-tailed, significance was set at p < 0.05.
The GraphPad Prism 9.0 version was used as auxiliary means for graphing, which contributed to visualization of the results.
Network Analysis
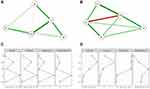
The R3.5.2 version24 was used to conducted network analysis. We used R package “qgraph” and “bootnet”.25 In the network model, the node represents a single cognitive item in the study, and the edge connecting the node can be interpreted as the conditional dependence between two items after controlling the value of other cognitive items.26 In this network, each variable (5 dimensions of RBANS) is indicated as a node and the associations between the two dimensions are represented as an edge connecting the nodes. While the green edges indicate the positive associations, red edges indicate the negative associations. In addition, the thickness of the edges shows their strength of associations. Individual nodes with a stronger connection with others are located in the center of the network, while with a lesser connection nodes are located in the periphery.
Centrality indices are calculated to examine which nodes are most connected to the others. Most often used metrics are closeness, betweenness, strength and expected influence (EI). Strength is identified as the most stable metric,27 and reflects the weight of every connection that a node has with other nodes with a view to all connections of other nodes. EI is used next to strength as the metric for it in view of possible negative edges.28 Betweenness inversely indicates the number of shortest paths between all pairs of nodes that include a given node.29 Closeness inversely indicates the mean shortest path from all other nodes to a given node.30
Results
Demographic and Clinical Characteristics
A total of 298 (157 male, 141 female) schizophrenia patients were enrolled. Table 1 summarizes the demographic and psychiatric clinical information of all the patients and separately for the male and female group. Male schizophrenia patients were more likely to take anticholinergics, and there were no significant differences in any other item between the male group and the female group.
 |
Table 1 Demographic and Clinical Variables of Male and Female Schizophrenia |
Cognition
Table 2 and Figure 1 show that there was no significant difference in the RBANS total score and each sub-item between the male schizophrenia and female schizophrenia, which suggested the cognitive impairment of schizophrenia has no gender difference. Spearman correlation analysis showed that RBANS total score were inversely proportional with age (P<0.001), duration (P=0.002) and PANSS (P<0.001) in male group (Figure 2A and B), as well as inversely proportional with PANSS (P<0.001) and directly proportional with age at onset (P=0.008) in female group (Figure 2C and D).
 |
Table 2 Comparison of RBANS Score Between Male and Female Schizophrenia |
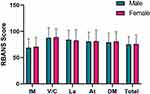 |
Figure 1 Comparison of RBANS score between male and female schizophrenia. Abbreviations: IM, Immediate Memory; V/C, Visuospatial/Constructional; La, Language; At, Attention; DM, Delayed Memory. |
Network Analysis
In male group (Figure 3A), generated network showed that all nodes were generally highly interconnected. Strong links were found between Language and Delayed Memory, Delayed Memory and Visuospatial/Constructional, Language and Immediate Memory, Language and Attention. However, we observed that Delayed Memory was inversely correlated to Immediate Memory and Attention, also the Visuospatial/Constructional was inversely correlated to Language, even though moderately.
In the female group (Figure 3B), the interconnection of each node was also obvious. Strong links were found between Delayed Memory and Visuospatial/Constructional, Attention and Delayed Memory, Visuospatial/Constructional and Attention, Delayed Memory and Language, Immediate Memory and Attention, among these the connection of Attention and Delayed Memory was severely negative, others were positive.
In terms of centrality analysis, in male group, Language demonstrated the highest centrality, which showed the highest indices in strength, closeness, betweenness and EI (Figure 3C). In female group, Delayed Memory demonstrated the highest centrality, which showed the highest indices in strength, closeness and betweenness, also had relatively high EI indices (Figure 3D).
Discussion
In clinical characteristics, the use of anticholinergic drugs was higher in the male patient group than in the female group, which may be due to the higher proportion of male patients who used more than one generation of antipsychotics. This is in line with the findings of a previous multicenter cross-sectional study conducted in East Asia.31 Several studies have shown that in schizophrenia, the use of anticholinergic drugs creates an additional cognitive burden,32,33 even though its effect on somatic side effects is significant.34 Multiple antipsychotic drug combinations may also be associated with a higher risk of metabolic syndrome, readmission, lower medication adherence, and lower quality of life.35,36 The awkward reality is that patients who have been on two or more antipsychotics for a long time often have experienced monotherapy, which is not enough to alleviate schizophrenia symptoms. In addition, compared with female patients, male patients have a higher risk of violent crimes in community and higher probability of impulsive behavior during hospitalization.37,38 Therefore, in the actual clinical work, we have to make a trade-off between safety and function maintenance.
There is no significant difference in the cognitive function between male and female schizophrenia, which is in line with the result of Cadenhead et al11 but different from others.9,10 Some studies pointed out that male schizophrenia had a relative advantage in spatial cognition while female schizophrenia had a relative advantage in verbal cognition, but there was no significant difference in their total cognition level.39 Combined with previous fMRI results, it has been shown that even for normal males and females, there were significant differences in brain structure and neural networks,40 so we considered that the decline of single cognitive function might be compensated by other cognitive functions.41 In this study, the proportion of anticholinergic drugs was different between the male group and female group, and anticholinergic drugs have been proven to cause a decline of language function.42 Therefore, in the future clinical assessment of cognitive function in schizophrenia, we should consider it comprehensively, based on more dimensions.
Correlation analysis showed that the RBANS total score was negatively correlated with age, duration of disease and PANSS score in the male group, as well as being negatively correlated with PANSS score and positively correlated with age of onset in the female group. Similar results have been confirmed in other studies.43,44 In this study, the relationship between cognition and age, cognition and duration of disease appeared to be only observed in the male group, and the relationship between cognition and age at onset was only observed in the female group. We consider that a woman’s menstrual cycle is a factor.45 On the one hand, since most of the subjects are between 20 and 50 years old, the cognitive function of women in this age could be affected by the menstrual cycle, and the representation of individual average cognition level by single test is weaker than that of men. On the other hand, estrogen and oxytocin have been shown to improve cognition in schizophrenia.46,47
In the network analysis of cognitive function in male patients, we found that Delayed Memory was negatively correlated with Immediate Memory and Attention, which seems to cause people’s confusion. In the general concept, Delayed Memory and Immediate Memory belong to the same broad category of Memory,48 and it seems that Delayed Memory and Immediate Memory should be positively correlated. However, since our study focused on schizophrenia patients, their cognitive function was impaired, integrity of their cognitive network was reduced,49 alertness was decreased,50 and they had to redistribute attention.51 As a result, the negative correlation between Delayed Memory and Immediate Memory was possible. Similarly, we found that Visuospatial/Constructional was negatively correlated to Language. Previous studies have shown that spatial and verbal cognition are separately managed by different brain regions,52 although they often communicate with each other to participate in specific tasks together.53 However, in this study, they showed a weak negative correlation, which further suggests that brain connectivity is impaired and cognitive function is dissociated in schizophrenia.54,55 In the centrality analysis of male patients, language has the highest centrality, which reflects its high importance for the cognition system in male schizophrenia. In actual clinical work, we can also carry out targeted intervention training on patients’ language to promote the whole cognitive function.
In the network analysis of cognitive function in female patients, the severe negative connection of Attention and Delayed Memory was observed. However, Visuospatial/Constructional showed a positive correlation with Attention and Delayed Memory at the same time. This result further supported our inference in male patients and showed the paradoxical expectation of schizophrenia,56 which is the reason that schizophrenia is called “Split-Mind Disorder” or “Attunement Disorder” in some countries.57 Network analysis visualized this paradoxical expectation, so that we can have an in-depth understanding of the cognitive function in schizophrenia. In the centrality analysis of female patients, Delayed Memory shows the highest centrality, which indicates that we can carry out intervention training about Delayed Memory, so as to promote the whole cognitive function and social function rehabilitation.
The limitations of this article were obvious. Firstly, this was a cross-sectional study, and we cannot define the processes by which schizophrenia alters cognitive function from this study. Secondly, the subjects were from hospitalized schizophrenia patients. They received regular rehabilitation treatment, which slowed down the speed of cognitive decline. However, schizophrenia patients in the community may not had such conditions. As a result, there might be admission bias. Thirdly, the sample size of the study was limited, which affected the persuasiveness of results. Fourthly, we used RBANS as a cognition measurement tool, but RBANS did not include executive function, which meant that our cognition measurement was not comprehensive. However, this study could still inspire future research. On the one hand, considering the completion degree of cognitive measurement, this study selected patients in the stable phase, who had no positive symptoms or weakly positive symptoms but obviously negative symptoms. Future studies might consider adding patients with positive symptoms and using network analysis to explore the relationship between symptoms and cognition. On the other hand, from this study, we know the core cognitive function of male and female schizophrenia patients, so that targeted cognition training can be carried out to maximize the recovery of patients’ social functions.
Conclusion
There was no significant gender difference in cognitive score function among patients with stable schizophrenia. The core cognitive functions of male and female schizophrenia are language and delayed memory, respectively.
Abbreviations
MRI, Magnetic Resonance Imaging; ICD-10, International Classification of Diseases, 10th Revision; MINI, mini-international neuropsychiatric interview; IRB, Institutional Review Board; FGA, first generation antipsychotics; SGA, second generation antipsychotics; PANSS, Positive and Negative Syndrome Scale; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status; EI, Expected influence; SD, Standard Deviation; IM, Immediate Memory; V/C, Visuospatial/Constructional; La, Language; At, Attention; DM, Delayed Memory; fMRI, Functional Magnetic Resonance Imaging.
Ethics Approval and Informed Consent
The study was approved by the Ethics Committee of Shanghai Pudong New Area Mental Health Center (reference number: PDJWLL2020012).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by the Special Fund for Livelihood Research (Medical and Health Care) of the Science and Technology Development Fund of Pudong New Area 2020 (PKJ2020-Y33).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mueser KT, McGurk SR. Schizophrenia. Lancet. 2004;363(9426):2063–2072. doi:10.1016/S0140-6736(04)16458-1
2. Fioravanti M, Carlone O, Vitale B, et al. A meta-analysis of cognitive deficits in adults with a diagnosis of schizophrenia. Neuropsychol Rev. 2005;15(2):73–95. doi:10.1007/s11065-005-6254-9
3. Mesholam-Gately RI, Giuliano AJ, Goff KP, et al. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23(3):315–336. doi:10.1037/a0014708
4. Wu C, Dagg P, Molgat C. A pilot study to measure cognitive impairment in patients with severeschizophrenia with the Montreal Cognitive Assessment (MoCA). Schizophr Res. 2014;158(1–3):151–155. doi:10.1016/j.schres.2014.07.006
5. Cognitive function in schizophrenia. Deficits, functional consequences, and future treatment. Psychiatr Clin N Am. 2003;26(1):25–40.
6. Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–330.
7. Lehman AF, Goldberg R, Dixon LB, et al. employment outcomes for persons with severe mental illnesses.ARCH. Gen Psychiat. 2002;59(2):165–172. doi:10.1001/archpsyc.59.2.165
8. Ferrer-Quintero M, Green MF, Horan WP, et al. The effect of sex on social cognition and functioning in schizophrenia. NPJ Schizophr. 2021;7(1):57. doi:10.1038/s41537-021-00188-7
9. Fiszdon JM, Silverstein SM, Buchwald J, Hull JW, Smith TE. Verbal memory in schizophrenia: sex differences over repeated assessments. Schizophr Res. 2003;61(2–3):235–243. doi:10.1016/S0920-9964(02)00285-2
10. Lewine RR, Walker EF, Shurett R, Caudle J, Haden C. Sex differences in neuropsychological functioning among schizophrenic patients. Am J Psychiatry. 1996;153(9):1178–1184. doi:10.1176/ajp.153.9.1178
11. Cadenhead KS, Geyer MA, Butler RW, et al. Information processing deficits of schizophrenia patients: relationship to clinical ratings, gender and medication status. Schizophr Res. 1997;28(1):51–62. doi:10.1016/S0920-9964(97)00085-6
12. Kovalev VA, Kruggel F, von Cramon DY. Gender and age effects in structural brain asymmetry as measured by MRI texture analysis. Neuroimage. 2003;19(3):895–905. doi:10.1016/S1053-8119(03)00140-X
13. Bryant NL, Buchanan RW, Vladar K, et al. Gender differences in temporal lobe structures of patients with schizophrenia: a volumetric MRI study. Am J Psychiat. 1999;156(4):603–609. doi:10.1176/ajp.156.4.603
14. Turetsky BI, Colbath EA, Gur RE. P300 subcomponent abnormalities in schizophrenia: i. Physiological evidence for gender and subtype specific differences in regional pathology. Biol Psychiat. 1998;43(2):84–96. doi:10.1016/S0006-3223(97)00258-8
15. Borsboom D. A network theory of mental disorders. World Psychiatry. 2017;16(1):5–13. doi:10.1002/wps.20375
16. Bringmann LF, Lemmens LH, Huibers MJ, et al. Revealing the dynamic network structure of the Beck Depression Inventory-II. Psychol Med. 2015;45(4):747–757. doi:10.1017/S0033291714001809
17. Borsboom D, Cramer AO. Network analysis: an integrative approach to the structure of psychopathology. Annu Rev Clin Psychol. 2013;9:91–121. doi:10.1146/annurev-clinpsy-050212-185608
18. ICD-10. International statistical classification of diseases and related health problems 10th revision. Commun Dis Rep CDR Wkly. 1992;2(28):125.
19. van Vliet IM, de Beurs E. The MINI-international neuropsychiatric interview. A brief structured diagnostic psychiatric interview for DSM-IV en ICD-10 psychiatric disorders. Tijdschr Psychiatr. 2007;49(6):393–397.
20. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia [J]. Schizophr Bull. 1987;13(2):261–276. doi:10.1093/schbul/13.2.261
21. SiT M, Yang JZ, Shu L, et al. Reliability and validity of Positive and Negative Symptom Scale (PANSS) . Chin J Ment Health. 2004;1:45–47.
22. Randolph C, Tierney MC, Mohr E, et al. Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J CLIN EXP NEUROPSYC. 1998;20(3):310–319. doi:10.1076/jcen.20.3.310.823
23. Zhang BH, Tan YL, Zhang WF, et al. Reliability and validity analysis of $. Chin Ment Health. 2008;22(12):865–869.
24. R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R foundation for statistical computing; 2018. Available from http://www.R-project.org/.
25. Epskamp S, Cramer AO, Waldorp LJ, Schmittmann VD, Borsboom D. Qgraph: network visualizations of relationships in psychometric data. J Stat Softw. 2012;48:1–18. doi:10.18637/jss.v048.i04
26. Epskamp S, Borsboom D, Fried EI. Estimating psychological networks and their accuracy: a tutorial paper. Behav Res Methods. 2018;50(1):195–212. doi:10.3758/s13428-017-0862-1
27. Barrat A, Barthelemy M, Pastor-Satorras R, Vespignani A. The architecture of complex weighted networks.
28. Robinaugh DJ, Millner AJ, McNally RJ. Identifying highly influential nodes in the complicated grief network. J Abnorm Psychol. 2016;125(6):747. doi:10.1037/abn0000181
29. Opsahl T, Agneessens F, Skvoretz J. Node centrality in weighted networks generalizing degree and shortest paths. Soc Networks. 2010;32:245–251. doi:10.1016/j.socnet.2010.03.006
30. Boccaletti S, Latora V, Moreno Y, Chavez M, Hwang DU. Complex networks: structure and dynamics. Phys Rep. 2006;424:175–308. doi:10.1016/j.physrep.2005.10.009
31. Sim K, Su A, Ungvari GS, et al. Depot antipsychotic use in schizophrenia: an East Asian perspective. Hum Psychopharm Clin. 2004;19(2):103–109. doi:10.1002/hup.571
32. Jochi YB, Thomas ML, Braff DL, et al. Anticholinergic medication burden-associated cognitive impairment in schizophrenia. Am J Psychiat. 2021;178(9):838–847. doi:10.1176/appi.ajp.2020.20081212
33. Vinogradov S, Fisher M, Warm H, et al. The cognitive cost of anticholinergic burden: decreased response to cognitive training in schizophrenia. Am J Psychiat. 2009;166(9):1055–1062. doi:10.1176/appi.ajp.2009.09010017
34. Ogino S, Miyamoto S, Miyake N, et al. Benefits and limits of anticholinergic use in schizophrenia: focusing on its effect on cognitive function. Psychiat Clin Neuros. 2014;68(1):37–49. doi:10.1111/pcn.12088
35. Civan Kahve A, Kaya H, Gui Cakil A, et al. Multiple antipsychotics use in patients with schizophrenia: why do we use it, what are the results from patient follow-ups? Asian J Psychiatr. 2020;52:102063. doi:10.1016/j.ajp.2020.102063
36. Wehmeier PM, Kluge M, Schacht A, et al. Quality of life and subjective well-being during treatment with antipsychotics in out-patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(3):703–712. doi:10.1016/j.pnpbp.2007.01.004
37. Modestin J, Ammann R. Mental disorder and criminality: male schizophrenia. Schizophrenia Bull. 1996;22(1):69–82. doi:10.1093/schbul/22.1.69
38. Zhu X, Li W, Wang X. Characteristics of aggressive behavior among male inpatients with schizophrenia. Shanghai Arch Psychiatry. 2016;28(5):280–288. doi:10.11919/j.issn.1002-0829.216052
39. Halari R, Mehrotra R, Sharma T, Ng V, Kumari V. Cognitive impairment but preservation of sexual dimorphism in cognitive abilities in chronic schizophrenia. Psychiatry Res. 2006;141(2):129–139. doi:10.1016/j.psychres.2005.07.021
40. Gron G, Wunderlich AP, Spitzer M, et al. Brain activation during human navigation: gender-different neural networks as substrate of performance. Nat Neurosci. 2000;3(4):404–408. doi:10.1038/73980
41. Gonzaleaz-Burgos L, Hernande-Cabrera JA, Westman E, et al. Cognitive compensatory mechanisms in normal aging: a study on verbal fluency and the contribution of other cognitive functions. Aging. 2019;11(12):4090–4106. doi:10.18632/aging.102040
42. Brébion G, Bressan RA, Amador X. et al. Medications and verbal memory impairment in schizophrenia: the role of anticholinergic drugs. Psychol Med. 2004;34(2):369–374. doi:10.1017/s0033291703008900
43. Chen M, Jiang Q, Zhang L. CACNA1C gene rs1006737 polymorphism affects cognitive performance in Chinese han schizophrenia. Neuropsychiatr Dis Treat. 2022;18:1697–1704. doi:10.2147/NDT.S373492
44. Han M, Huang X-F, Chen DC, et al. Gender differences in cognitive function of patients with chronic schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39(2):358–363. doi:10.1016/j.pnpbp.2012.07.010
45. Gurvich C, Gavrilidis E, Worsley R, et al. Menstrual cycle irregularity and menopause status influence cognition in women with schizophrenia. Psychoneuroendocrino. 2018;96:173–178. doi:10.1016/j.psyneuen.2018.06.022
46. Rubin LH, Carter CS, Drogos LL, et al. Effects of sex, menstrual cycle phase, and endogen- ous hormones on cognition in schizophrenia. Schizophr Res. 2015;166(1–3):269–275. doi:10.1016/j.schres.2015.04.039
47. Chafari E, Fararouie M, Shirazi HG, et al. Combination of estrogen and antipsychotics in the treatment of women with chronic schizophrenia: a double-blind, randomized, placebo-controlled clinical trial. Clin Schizophr Relat Psychoses. 2013;6(4):172–176. doi:10.3371/CSRP.GHFA.01062013
48. Voineskos AN, Felsky D, Kovacevic N, et al. Neural correlates of immediate and delayed word recognition memory: an MEG study. Brain Res. 2008;1240:132–142. doi:10.1016/j.brainres.2008.08.061
49. Voineskos AN, Felsky D, Kovacevic N. Oligodendrocyte genes, white matter tract integrity, and cognition in schizophrenia. Cereb Cortex. 2013;23(9):2044–2057. doi:10.1093/cercor/bhs188
50. Nestor PG, Kubicki M, Spencer KM, et al. Attentional networks and cingulum bundle in chronic schizophrenia. Schizophr Res. 2007;90(1–3):308–315. doi:10.1016/j.schres.2006.10.005
51. Potts GF, O’Donnell BF, Hirayasu Y, et al. Disruption of neural systems of visual attention in schizophrenia. Arch Gen Psychiat. 2002;59(5):418–424. doi:10.1001/archpsyc.59.5.418
52. Wang X, Men W, Gao J, et al. Two forms of knowledge representations in the human brain. Neuron. 2020;107(2):383–393.e5. doi:10.1016/j.neuron.2020.04.010
53. Bonner MF, Epstein RA. Object representations in the human brain reflect the co-occurrence statistics of vision and language. Nat Commun. 2021;12(1):4081. doi:10.1038/s41467-021-24368-2
54. Birkett P, Sigmundsson T, Sharma T, et al. Reaction time and sustained attention in schizophrenia and its genetic predisposition. Schizophr Res. 2007;95(1–3):76–85. doi:10.1016/j.schres.2007.05.030
55. Li A, Zalesky A, Howes O, et al. A neuroimaging biomarker for striatal dysfunction in schizophrenia. Nat Med. 2020;26(4):558–565. doi:10.1038/s41591-020-0793-8
56. Billeke P, Armijo A, Castillo D, et al. Paradoxical expectation: oscillatory brain activity reveals social interaction impairment in schizophrenia. Biol Psychiat. 2015;78(6):421–431. doi:10.1016/j.biopsych.2015.02.012
57. Cho JW, Jang EY, Woo HJ, et al. Effects of renaming schizophrenia in Korea: from “Split-Mind Disorder” to “Attunement Disorder”. Psychiat Invest. 2018;15(7):656–662. doi:10.30773/pi.2018.02.18.2
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.