Back to Journals » Clinical and Experimental Gastroenterology » Volume 16
Gastroparesis: Myths, Misconceptions, and Management
Authors Cangemi DJ, Lacy BE
Received 9 September 2022
Accepted for publication 29 March 2023
Published 6 June 2023 Volume 2023:16 Pages 65—78
DOI https://doi.org/10.2147/CEG.S362879
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Santosh Shenoy
David J Cangemi, Brian E Lacy
Division of Gastroenterology and Hepatology, Mayo Clinic, Jacksonville, FL, USA
Correspondence: David J Cangemi, Division of Gastroenterology, Mayo Clinic, 4500 San Pablo Road, Jacksonville, FL, USA, 32224, Email [email protected]
Abstract: Gastroparesis (GP), a historically vexing disorder characterized by symptoms of nausea, vomiting, abdominal pain, early satiety, and/or bloating, in the setting of an objective delay in gastric emptying, is often difficult to treat and carries a tremendous burden on the quality of patients’ lives, as well as the healthcare system in general. Though the etiology of GP has been fairly well defined, much work has been done recently to better understand the pathophysiology of GP, as well as to identify novel effective and safe treatment options. As our understanding of GP has evolved, many myths and misconceptions still abound in this rapidly changing field. The goal of this review is to identify myths and misconceptions regarding the etiology, pathophysiology, diagnosis, and treatment of GP, in the context of the latest research findings which have shaped our current understanding of GP. Recognition and dispelling of such myths and misconceptions is critical to moving the field forward and ultimately advancing the clinical management of what will hopefully become a better understood and more manageable disorder in the future.
Keywords: gastroparesis, functional dyspepsia, nausea, vomiting, abdominal pain
Introduction
Gastroparesis (GP) is a syndrome defined by characteristic symptoms (nausea, vomiting, early satiety, abdominal pain, and/or bloating) in the setting of an objective delay in gastric emptying and the absence of mechanical obstruction.1 As such, a diagnosis of GP requires objective measurement of gastric emptying, traditionally performed with a 4-hour scintigraphic study (GES) using a standardized test meal; however, assessment of gastric emptying can also now be performed with a stable isotope 13-carbon spirulina (13C) breath test.2,3 Historically, the epidemiology of GP has been difficult to estimate since many patients with GP may be labeled with alternative ICD-10 codes, such as nausea and vomiting; alternatively, patients who have perhaps not undergone formal assessment for GP may be inappropriately labeled with the code for GP based on symptoms alone. One frequently referenced study, utilizing data from 1996 to 2006 in Olmsted County, Minnesota, found the prevalence of GP to be 37.8 in women and 9.6 in men per 100,000 persons.4 More recently, a systematic review of 13 epidemiologic studies of GP, mostly involving US databases or registries, identified a prevalence range of 13.8–267.7 per 100,000 adults. When considering those with diabetes specifically, the estimated 10-year cumulative incidence of GP in type 1 diabetes was 5.2%, compared to 1% in patients with type 2 diabetes.5
The impact of GP on patients’ quality of life can be significant. A recently published survey study utilizing the 36-item Short Form (SF-36), a validated questionnaire to assess quality of life, identified that mean scores for mental health and social functioning in patients with GP were similar to scores for patients with irritable bowel syndrome (IBS) and depression.6 From an economic standpoint, a previous study estimated that GP accounted for a total of 3500 million dollars in hospital charges and 911,963 hospital days in 2004.7 A more recent study reported that the number of Emergency Department visits for patients with GP as a primary diagnosis in the US increased from 15,459 in 2006 to 36,820 in 2013.8 Given the tremendous physical, psychologic, and economic burden attributed to GP, it is easy to understand why this topic is of great interest to the field today. Much work has been done recently in an attempt to better understand GP, and to ultimately identify novel safe and effective treatment options. As a result, our understanding of GP is rapidly evolving. The purpose of this review is to identify myths and misconceptions based on our current understanding of GP and to discuss the current state of management for this historically vexing disorder.
Etiology
The etiology of GP is diverse but has been well defined. A common misconception is that GP is nearly always a result of diabetes mellitus with associated peripheral neuropathy, though this is false, as connective tissue disorders, surgery (ie, hiatal hernia repair, fundoplication surgery, lung transplantation), ischemia, and a variety of inflammatory or neurologic disorders are also well-established risk factors for the development of GP.9–11 Notably, prior data suggested that the majority of patients with GP could be categorized with idiopathic GP, meaning an underlying cause cannot be identified – though it has been theorized that many of these patients developed GP as a post-infectious phenomenon.9–12 However, updated epidemiologic data suggest that the majority of patients with GP (57.4%) have a diabetic etiology13 (Myth/Misconception #1). In a large, retrospective, cross-sectional analysis of over 71,000 patients with GP, postsurgical (15%), drug-induced (11.8%) and idiopathic (11.3%) classifications followed diabetes as the most prevalent etiologies. Thus, recent data reaffirm the importance of diabetes as a risk factor for GP (though not the sole risk factor) and suggest that idiopathic GP may now be less prevalent, perhaps due in part to our improved understanding of GP and recognition of this disorder over time.
Pathophysiology
Gastroparesis is defined by an objectively measured delay in gastric emptying, which is often associated with decreased amplitude or frequency of gastric antral contractions. A common misconception is that delayed gastric emptying occurs largely due to vagal nerve dysfunction (Myth/Misconception #2); however, delayed gastric emptying can also occur due to decreased gastric fundic tone, antroduodenal dyscoordination, gastric dysrhythmias, abnormal duodenal feedback, as well as pyloric dysfunction (Figure 1).9,10,14 Historically, the extent of gastric emptying delay has not been shown to reliably predict symptom severity (Myth/Misconception #3). However, this sentiment has recently been challenged by a large meta-analysis which identified that, when gastric emptying is optimally measured (ie, a scintigraphy study measuring the emptying of a solid meal for at least 3 hours), delayed gastric emptying does indeed correlate with GP symptoms.15 These data emphasize the critical importance of utilizing standardized means of gastric emptying assessment in the evaluation of GP.
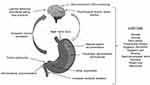 |
Figure 1 Pathophysiologic mechanisms involved in the generation of gastroparesis symptoms. Notes: Reproduced from Lacy BE, Tack J, Gyawali CP. AGA clinical practice update on management of medically refractory gastroparesis: expert review. Clin Gastroenterol Hepatol. 2022;20(3):491–500. Copyright 2022, with permission from Elsevier.39 |
In addition to the severity of gastric emptying delay, a recent study suggests that the distribution of an ingested meal within the stomach may predict different upper gastrointestinal symptoms. In a study of 100 patients with GP, those with immediate and 1-hour distal distribution of food contents on a standard GES, as measured by regional intragastric meal distribution analysis, more often had symptoms of early satiety, whereas those with more proximal delayed (4-hour) distribution of food had more severe nausea and vomiting.16 Further, recent data suggest that small bowel motility abnormalities may also contribute to symptoms in patients with GP. In a small study of patient with symptoms of GP, enteric dysmotility, as measured by small bowel manometry, was identified in nearly all patients (96%) with delayed gastric emptying (n = 25).17 On a microscopic level, delays in GE may occur due to a loss of interstitial cells of Cajal (the so-called gastric pacemaker cells), which have been identified in the gastric body, antrum, and pylorus in patients with GP, alterations in inhibitory and excitatory components of the enteric nervous system, as well as degeneration and fibrosis of gastric smooth muscle.18–22 Additionally, hyperglycemia and vagal nerve denervation are relevant factors in diabetic GP specifically.1,2
Though once thought to be entirely separate disorders, there has been growing sentiment among experts in the field that there is significant overlap between GP and functional dyspepsia (FD), a common disorder of gut–brain interaction (DGBI), defined by Rome IV criteria, and characterized by symptoms similar to GP, including abdominal pain, nausea, early satiety, and bloating23–26 (Myth/Misconception #4). Though symptoms of nausea and vomiting are traditionally more prominent in GP compared to FD, for many patients, symptoms of GP may frequently be indistinguishable from FD.9,10,27,28 A recent publication by the Gastroparesis Consortium (funded by the National Institutes of Health) described the results of a 48-week prospective study involving 944 patients, 720 of whom had GP, in which 42% of patients with GP were found to have normal gastric emptying at the conclusion of the study and 37% of patients with normal gastric emptying transitioned to delayed gastric emptying.29 Given the significant transition between diagnoses over time, the authors put forth the view that GP and FD should be considered as part of the same spectrum of gastric sensorimotor dysfunction. Though this landmark study was not without limitations (ie, all patients included were referred for tertiary evaluation, Rome III criteria was used, information was not provided on which patients underwent pyloric interventions over the course of the study), the overall premise of the study is in keeping with a growing movement within the field to recognize the significant overlap between GP and FD.23–26
Moreover, there is evidence that both visceral and central hypersensitivity are relevant from a pathophysiologic standpoint for some patients with GP. In a study of 58 patients with idiopathic GP, 29% had evidence of gastric hypersensitivity as measured by gastric barostat study, and gastric hypersensitivity was associated with symptoms of epigastric pain, early satiety, and weight loss.30 Additionally, a recent pilot study using functional MRI brain imaging in 10 GP patients with symptoms of nausea identified a significant reduction in functional network connectivity in the insula of GP patients, compared to controls, after 30 minutes of exposure to a visual cue meant to provoke motion sickness.31 Though the study was small, these results provide intriguing insight into the possibility that some patients with GP may develop symptoms due to changes in the central processing of peripheral stimuli.
Diagnosis
Updated clinical guidelines by the American College of Gastroenterology (ACG) reaffirm GES as the standard test for assessment of gastric emptying in patients with upper gastrointestinal symptoms, such as nausea, vomiting, early satiety, abdominal pain, and/or bloating.2 Notably, the guidelines specify the importance of utilizing a solid meal to measure gastric emptying – ideally for 4 hours. The stable isotope gastric emptying breath test using 13C and wireless motility capsule (WMC) testing are considered reasonable alternative means of measuring gastric emptying. However, while the WMC has been shown to have reasonable agreement with GES, it is important to recognize that the WMC measures emptying of a nondigestible object, as opposed to a solid meal, and therefore depends primarily on the migrating motor complex (MMC), which is responsible for clearing the stomach between meals.32 Additionally, cutaneous electrogastrography (ECG) can be used to measure gastric myoelectric activity and different patterns of gastric electrical signal amplitude and frequency have been described in patients with gastric outlet obstruction compared to patients with idiopathic GP, for example.33 However, importantly, cutaneous ECG does not measure gastric emptying and therefore its clinical utility remains unclear and it is not currently recommended as a diagnostic test for GP in clinical practice.2,3 Greater than 10% retention of a solid meal on GES has historically been utilized as the cutoff to distinguish delayed gastric emptying from normal gastric emptying. However, in light of the recent evidence suggesting that GP and FD be considered a part of the same spectrum of gastric sensorimotor dysfunction, the updated clinical guidelines suggest that this cutoff may need to be reconsidered as mild delays in gastric emptying are commonly found in patients with FD.34
Notably, these authors recently identified that GP is frequently misdiagnosed in the community. In a retrospective study involving 339 patients referred to our tertiary referral center for evaluation of GP, 80.5% of referred patients ultimately received alternative diagnoses after tertiary evaluation, with FD being the most common alternative diagnosis (44.5% of patients).35 Of note, only 130 of these patients (38.3%) had undergone a 4-hour GES, but only 23 patients (6.8%) were known to have ingested a standard test meal of radiolabeled eggs. These data emphasize the importance of performing a GES properly, and adhering to a well-established, standardized protocol, in order to make an accurate diagnosis of GP36 (Myth/Misconception #5). However, a recent questionnaire-based study assessing key GES protocol measures identified overall poor compliance with GES protocol guidelines among US medical institutions.37 Among the 121 responses (40.4% of which were academic or teaching medical centers), the average rate of protocol adherence to the 52 different measures assessed was only 64%. Moreover, only 58% of responding medical institutions performed scintigraphic imaging for the validated 4-hour length of time, and only 59% used the recommended test meal of egg whites, two slices of white toast, jelly, and water.
Finally, an important misconception as it relates to the diagnosis of GP is the relevance of retained food content seen on esophagogastroduodenoscopy (EGD). In the aforementioned study from our center, patients correctly diagnosed with GP after tertiary evaluation more often had retained food in the stomach on upper endoscopy [22.7% vs 8.8%, p = 0.004].35 However, current data suggests that the identification of retained food during endoscopy is not diagnostic for GP. In a recent retrospective study of nearly 3000 patients who underwent both EGD and GES, findings of retained gastric contents were associated with the positive predictive value of retained gastric contents for delayed gastric emptying was only 55%.38 Further, retained gastric contents were noted to be present in 3% of all upper endoscopy procedures assessed (85,116 EGDs performed in 55,537 patients), a small but not insignificant number. Moreover, other risk factors were identified as being associated with retained gastric contents aside from GP, such as diabetes, acid suppressant medications, some cardiovascular medications, and opioids. Indeed, the updated ACG clinical guidelines caution that retained gastric food should not be considered diagnostic for GP2(Myth/Misconception #6).
Treatment
Introduction
The treatment of GP should be individualized based on the patient’s predominant symptom and the severity of reported symptoms (Figure 2).39 For example, a patient with idiopathic GP with the predominant symptom of occasional nausea may simply require dietary modifications. In contrast, a patient with long-standing diabetic GP with daily nausea and vomiting and severe abdominal pain may require multimodal medical therapy as well as endoscopic intervention. The data presented below primarily focus on single-agent studies compared to placebo, as data from large, head-to-head comparison studies do not exist.
 |
Figure 2 Proposed management for the treatment of gastroparesis based on predominant symptom and overall symptom severity. Abbreviations: GES, gastric emptying study; CAM, complementary and alternative medicine; CBT, cognitive and behavioral therapy; G-POEM, gastric per oral endoscopic myotomy. Notes: Reproduced from Lacy BE, Tack J, Gyawali CP. AGA clinical practice update on management of medically refractory gastroparesis: expert review. Clin Gastroenterol Hepatol. 2022;20(3):491–500. Copyright 2022, with permission from Elsevier.39 |
Dietary Interventions
Patients with GP frequently report meal-related symptoms of early satiety, abdominal pain and/or discomfort, and nausea and vomiting. Intuitively, eating smaller, more frequent meals to improve meal-related symptoms makes sense, although data supporting this concept are quite limited. A small study of diabetic patients with GP showed that a diet with large particle size emptied more slowly than a diet with small particle size.40 A prospective, 20-week parallel group dietary intervention study in diabetic patients (n = 56; 36 with type I diabetes) found that a small particle diet substantially improved symptoms of early satiety, bloating, nausea and vomiting compared to a large particle diet.41 A retrospective analysis of 86 patients with GP found that a low-fiber, low-fat, small frequent meal diet combined with prokinetic therapy improved symptoms in 58% of patients.42 Although many patients with GP report that fatty foods worsen GI symptoms, no prospective study has evaluated a low fat versus a normal fat diet over a prolonged course of time. It is important to highlight that tight glycemic control is essential for patients with diabetic GP as elevated blood sugar levels impede gastric emptying. We recommend that for patients with mild GP symptoms with nausea predominance a small particle, reduced fat diet should be employed for a minimum of 4 weeks. If patients do not report symptom improvement, then the prior diet can be resumed. Of note, many patients with GP voluntarily restrict food intake in order to minimize symptoms, potentially leading to malnutrition, vitamin and nutrient deficiencies (eg, D, E, K, folate, calcium, iron) and even the development of avoidant-restrictive eating behaviors.43
The prevalence of eating disorders among patients with gastrointestinal disorders has perhaps been underrecognized to date, though this topic is gaining increased attention. For example, a recent study of 158 patients presenting for evaluation of GP/dyspepsia symptoms identified the presence of clinically significant eating disorders in nearly 55% of patients, with approximately 40% meeting cutoff for avoidant restrictive food intake disorder (ARFID).44 Thus, it is critically important that providers exercise caution in recommending dietary therapy for patients with GP, particularly those with a history of an eating disorder or who may be deemed to be at risk for an eating disorder based on psychologic assessment. Additionally, gastric emptying can be delayed in patients with eating disorders such as anorexia nervosa and bulimia nervosa, and can improve with weight gain.45 Therefore, it is important for clinicians to understand that delayed gastric emptying may occur as a result of weight loss associated with an eating disorder in the appropriate patient. Referral to Psychiatry/Psychology should be considered in patients with signs of an eating disorder, and referral to a dietitian should be considered when malnutrition is a concern based on laboratory testing and symptom severity. In patients with severe refractory symptoms which result in malnutrition, enteral therapy, such as via jejunostomy tube, may need to be considered.2,39
Medical Therapies for Nausea and Vomiting
Multiple agents are available to treat nausea and vomiting; however, most have never been tested in prospective studies involving GP patients (Table 1). The following section summarizes commonly used medications to treat symptoms of nausea and vomiting in patients with GP, beginning with the dopamine receptor antagonists metoclopramide and domperidone. Prokinetic agents which improve gastric emptying but without a documented mechanism to improve nausea and vomiting are discussed in a later section.
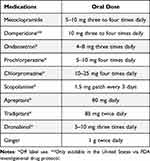 |
Table 1 Suggested Medications and Dosing for Treatment of Nausea and Vomiting in Gastroparesis |
Metoclopramide is a centrally acting dopamine type-2 (D2) receptor antagonist. It is widely available in the EU and is the only FDA-approved medication for the treatment of GP symptoms in the US. Metoclopramide exhibits antiemetic effects via inhibition of D2 and 5-HT3 receptors in the brain, as well as prokinetic effects due to its 5-HT4 receptor agonist activity.46 Metoclopramide can be administered through multiple routes (oral, subcutaneous, intravenous, nasal) depending upon the clinical situation and patient preferences. Clinical guidelines recommend starting at the lowest possible effective dose for each patient beginning with 5 mg t.i.d. 30 minutes before meals with a maximum dose of 40 mg/day.9 A recent review identified 8 separate trials of metoclopramide for the treatment of GP.47 Six studies involved a comparator (placebo in 4 cases; domperidone in one; erythromycin in one); 5 studies were double-blinded; 2 studies were open label. The sample size in these studies ranged from 1 to 45 patients; the majority of the patients were diabetic; most studies were 3–4 weeks in duration; metoclopramide dosing was generally 10 mg by mouth four times daily. Overall, GP symptom improvement was rated at approximately 30%. Metoclopramide’s use is often limited by side effects including mild sedation, agitation, hyperprolactinemia, and extrapyramidal effects. In the US, metoclopramide carries a black box warning restricting its use to ≥12 weeks for the risk of tardive dyskinesia, although this risk has been estimated to be small (<1%).48 More recently, a retrospective analysis of studies assessing the presence of tardive dyskinesia in patients treated with metoclopramide found the risk of tardive dyskinesia to be even smaller than previously thought (0.1% per 1000 patient years)49 (Myth/Misconception #7).
Domperidone, a dopamine type-2 (D2) receptor antagonist, is similar in action to metoclopramide, although it does not readily cross the blood–brain barrier.50 Domperidone is thus associated with fewer central side effects than metoclopramide. Providers should be aware of an association of domperidone with QT prolongation and ventricular tachycardia.51 Domperidone is widely available throughout most of the world and is sold over-the-counter in some countries. It is only available in the US through an FDA investigational drug application. The recommended starting dose is 10 mg three times daily with escalation to 20 mg four times daily including at bedtime.9 A recent analysis identified 11 separate trials of domperidone for the treatment of GP.47 Six were randomized, controlled trials; 4 were open label. Four were placebo-controlled, while two were comparator studies (one each for cisapride and metoclopramide). Sample size ranged from 3 to 208 patients. Most studies involved patients with diabetic GP and were 4 weeks in duration, although a retrospective study lasted just over one year. The typical dose used was 10 mg taken four times daily. Symptom improvement was reported by most patients; in the one comparator study involving metoclopramide, no difference was noted between the two groups. Adverse effects occurred in up to 16–17% of patients; elevated prolactin levels were identified in all patients receiving domperidone in one open-label study. In a separate, single-center cohort study (n = 115) not included in this meta-analysis, the majority (68%) of GP patients treated with domperidone reported improvement in symptom scores. This study also noted that 7% (8 of 115) of patients had cardiac-type side effects necessitating drug cessation.52
Serotonin type-3 (5-HT3) receptor antagonists and phenothiazines are commonly used (off-label) for the treatment of nausea and vomiting related to GP. 5-HT3 receptor antagonists inhibit vagal afferent nerves and receptors in the chemoreceptor trigger zone but do not affect gastric motility.53,54 All first-generation 5-HT3 antagonists are considered to have similar efficacy, and therefore selection can be determined by availability, price, and tolerability.55 Ondansetron is available in tablet form as well as an oral disintegrating tablet, which may ease administration in patients with symptoms of severe nausea; granisetron is available as a transdermal patch. In two studies, transdermal granisetron (3.1 mg/24 hr) was moderately effective in reducing symptoms of nausea and/or vomiting in patients with GP with symptoms refractory to conventional treatment.56,57 Phenothiazines, such as prochlorperazine and chlorpromazine, inhibit both D1 and D2 receptors in the brain leading to antiemetic effects. No studies have directly compared 5-HT3 receptor agonists to phenothiazines.9
Other medications are available to treat nausea and vomiting, but there is no, or limited, data to support their use in patients with GP. Scopolamine is a muscarinic cholinergic receptor antagonist that can be considered for the treatment of nausea in GP patients, although it is important to note that there have been no clinical studies which support its use for treatment of GP. Aprepitant is a NK-1 receptor antagonist that reduces nausea and vomiting through the inhibition of substance P in the area postrema within the brainstem. A randomized, placebo-controlled trial of 126 patients (most, but not all, with documented GP) treated with aprepitant (125 mg/day, n = 63) resulted in mixed results as it failed to meet the primary outcome of a reduction in nausea but did meet some secondary outcomes in terms of reducing symptom severity.58 Tradipitant, another NK-1 antagonist, yielded better results in a slightly larger study (n = 152) involving only patients with documented idiopathic or diabetic GP.59 During this 4-week study, 85 mg twice daily of tradipitant was more effective than placebo at reducing nausea severity and frequency (p = 0.0099). Synthetic cannabinoids, such as dronabinol and nabilone, are approved for the treatment of nausea and vomiting associated with chemotherapy but have not been studied specifically for the treatment of GP. It is important to recognize that cannabinoids may slow gastric emptying and thus could potentially worsen some GP symptoms.
Prokinetic Therapy
One common misconception is that all symptoms of GP will resolve if gastric emptying is improved or normalized (Myth/Misconception #8). Research efforts for many years thus focused on accelerating gastric emptying. A number of agents (reviewed in the following section) have been tested, although the benefits of prokinetic therapy are much less than hoped for. Erythromycin, a macrolide antibiotic that acts as a motilin receptor agonist, is commonly used off-label to treat GP symptoms. Activation of motilin receptors in the GI tract stimulates cholinergic activity in the gastric antrum and helps initiate the MMC, thus accelerating movement of material from the stomach to the small intestine.60,61 Hospitalized GP patients may benefit with a course of intravenous erythromycin at a dose of 3 mg/kg (over 45 minutes) every 8 hours.9 For outpatients, oral doses of 50–100 mg four times daily given 30–45 minutes prior to meals and at bedtime may improve symptoms.62 Unfortunately, most patients develop tachyphylaxis to erythromycin fairly rapidly thus limiting its effectiveness and many patients report a worsening of nausea. Ghrelin is an endogenous peptide that is also involved in stimulation of Phase III of the MMC.63 Relamorelin is a potent synthetic ghrelin agonist, which has been shown to improve gastric emptying and symptoms in diabetic GP patients.64,65 Improvement in vomiting frequency was noted in a small subset of patients. Overall lack of efficacy, however, prompted cessation of further research trials. In a placebo-controlled, cross-over study, 34 patients with GP (28 idiopathic) were treated with prucalopride, a 5-HT4 agonist for 4 weeks.66 Prucalopride (2 mg q.d.) led to a statistically significant improvement in symptoms of early satiety, nausea, vomiting and bloating, compared to placebo; as well, overall quality of life was improved. Large, multicenter studies are needed to confirm these interesting results.
Complementary and Alternative Therapy
Patients are increasingly turning to complementary and alternative medicines for relief of their GP symptoms. Ginger is commonly used to treat symptoms of nausea, although its exact antiemetic mechanism of action is unclear. Although not directly tested in patients with GP, a meta-analysis of five randomized trials demonstrated that a dose of 1 gram of ginger was significantly better in preventing post-operative nausea and vomiting (PONV) than placebo.67 STW 5 (Iberogast), a combination herbal product, has been shown to be superior to placebo in improving dyspeptic symptoms in a prospective, multicenter study involving over 300 patients with FD.68 STW 5 is thought to improve gastric antral motility and enhance relaxation of the proximal stomach but does not appear to negatively affect gastric emptying.69,70 Although STW 5 has not been studied for the treatment of GP, it was noted that patients with “dysmotility-like” FD had a greater decrease in symptom scores with STW 5, compared to those with “ulcer-like” and non-specific FD.70 Acupuncture improves some medical conditions, although its efficacy for treatment of GP remains unclear. A meta-analysis of 14 randomized controlled trials assessing acupuncture treatment in diabetic patients with dyspeptic symptoms demonstrated higher response rates with acupuncture, though the results were overall inconclusive given the low quality of trials included in the analysis.71 Similarly, a recent comprehensive meta-analysis of 32 studies involving 2601 participants using several different acupuncture techniques provided very low-certainty evidence of benefit in the setting of evidence of publication bias and a positive bias of small study effects.72 Based on this data, we would not recommend acupuncture for GP symptoms. Relaxation techniques, including guided imagery and hypnosis, have proved useful in treating symptoms of nausea and vomiting in patients undergoing chemotherapy, but these techniques have not been studied in GP patients.73
Medical Therapies for Abdominal Pain
Abdominal pain is a cardinal, but perhaps underrecognized, symptom of GP (Myth/Misconception #9). In a recent prospective study of 346 GP patients (212 idiopathic) whose symptoms were assessed by numerous questionnaires and who underwent various physiologic tests (such as GES and WMC), abdominal pain was found to be present in 90% of patients.74 Further, abdominal pain was found to be more common in women, and over 50% of patients reported daily pain. Moreover, over one-third of patients reported severe or very severe pain, and pain severity was associated with depression, anxiety, and elevated somatic symptom scores. Notably, the severity of abdominal pain was not found to be associated with the severity of delayed gastric emptying, suggesting that there are separate pathophysiologic processes involved in the generation of pain symptoms in patients with GP.
Tricyclic antidepressants (TCA), such as amitriptyline, desipramine, and nortriptyline, now commonly referred to as neuromodulators, have been extensively used in clinical practice to treat pain. In low doses, these medications may also reduce symptoms of nausea and vomiting. One placebo-controlled, randomized trial assessed the efficacy of a TCA for the treatment of GP. The NORIG trial evaluated nortriptyline, dose escalated at 3-week intervals up to 75 mg at 12 weeks, on overall symptoms of idiopathic GP in 130 patients.75 Overall, this study showed no difference between patients treated with nortriptyline compared to those who received placebo, though the strict primary outcome measurement of ≥50% reduction in overall GCSI scores on two consecutive assessments compared to the baseline is worth noting. Despite this being a negative study, low-dose neuromodulator therapy using a tertiary amine TCA (eg, amitriptyline or imipramine) may provide some pain relief, based on studies demonstrating positive results for the treatment of FD and other DGBIs.76
Mirtazapine, an antidepressant with both noradrenergic, serotonergic activity and antihistaminic activity, has been shown to be effective in treating refractory nausea and vomiting in multiple case reports. A recent trial in patients with FD indicated that it was effective in reducing dyspeptic symptoms and resulted in a significant increase in body weight.77 In a prospective, 4-week trial, open-label trial, patients with GP (n = 30; 26 idiopathic) reported improvement in symptoms of nausea, vomiting, retching and reduced appetite.78 Although data from large, placebo-controlled trials are lacking, mirtazapine may be considered a reasonable option for treating some patients with GP, especially those with symptoms of refractory nausea and vomiting with weight loss.
Pregabalin binds to a subunit of the voltage gated calcium channels within the central nervous system and modulates calcium influx, inhibiting release of excitatory neurotransmitter and exerting anti-nociceptive and anticonvulsant effects. It is structurally related to gabapentin but has no activity at GABA or benzodiazepine receptors, though both pregabalin and gabapentin are frequently used to treat neuropathic pain. A pooled analysis from seven randomized clinical trials involving a total of 1510 patients with painful diabetic neuropathy identified a statistically significant reduction in mean pain scores with pregabalin at treatment doses of 150 mg, 300 mg and 600 mg daily.79 Though pregabalin and gabapentin have not been studied for the treatment of GP symptoms specifically, a recent study of randomized control trial involving 72 FD patients found that treatment with pregabalin 75 mg daily for 4 weeks resulted in significant overall improvement in dyspeptic symptoms, particularly abdominal pain, compared to placebo.80
Behavioral Therapies
Behavioral therapies such as cognitive behavioral therapy (CBT) and hypnotherapy (now termed “brain-gut behavior therapies (BGBTs)”) have gained increased recognition and acceptance as treatments for various gastrointestinal disorders, particularly DGBIs.81 Cognitive behavioral therapy, in particular, is one of the most widely used forms of behavioral therapy for gastrointestinal disorders characterized by pain (ie, FD and IBS) and recurrent vomiting and/or regurgitation (ie, rumination syndrome).81–83 Cognitive behavioral therapy is also used to treat psychologic distress, such as anxiety, which is important to highlight as patients with GP have been shown to have elevated scores for both anxiety and depression on the validated Hospital Anxiety and Depression Scale (HADS).84 Though there have been no published studies to date which have assessed CBT for the treatment of GP, it stands to reason that CBT may be effective for treating symptoms of GP, as CBT has been shown to improve symptoms of FD, and considering the sentiment that GP and FD exist along a spectrum of gastric sensorimotor dysfunction. In a study of 158 patients with FD, a 10-week course of coping-focused CBT yielded significant improvement of dyspeptic symptoms and quality of life compared to medical therapy alone; further, these improvements were shown to be maintained at 6 months.85 Cognitive behavioral therapy, administered over 4 months, was also found to result in a significantly greater reduction in dyspeptic symptoms, as well as improvement in psychologic parameters, compared to no medical care, when assessed at 1-year follow-up in a study of 100 patients with FD.86 Similarly, gut-directed hypnotherapy has yet to be studied for the treatment of GP but has been shown to improve symptoms and quality of life in patients with FD87,88 As such, BGBTs would seem to represent a treatment domain, which is ripe for investigation to understand the potential benefit for patients with GP.
Interventional Therapy
It is thought that some patients with GP may have delayed gastric emptying due to abnormalities in gastric pyloric tone and pressure, so-called “pylorospasm”, as well as dyscoordination between pyloric relaxation and antral contractions.11,89 Thus, the pylorus has become a fitting target for interventional therapies with a goal to reduce pylorospasm. Endoscopic injection of botulinum toxin A (BoTox) was previously viewed as a promising intervention after multiple open-label studies demonstrated that pyloric injection of botulinum toxin resulted in improvement in gastric emptying and patients’ symptoms.90,91 However, two subsequent randomized control trials showed that injection of botulinum toxin was not superior to placebo in improving gastric emptying or symptoms.92,93 As such, the role of intrapyloric injection of botulinum toxin as a valid treatment for GP has become controversial, and updated ACG guidelines currently do not recommend intrapyloric botulinum toxin injection for the treatment of GP (Myth/Misconception #10). That said, a recent prospective study of 35 patients with refractory GP demonstrated that intrapyloric botulinum toxin injection significantly improved symptoms in patients with altered pyloric distensibility, as measured by endoluminal functional lumen imaging probe (EndoFLIP).94 Therefore, the utilization of EndoFLIP to assess pyloric distensibility could possibly reinvigorate interest in intrapyloric injection for the treatment of GP.
Updated ACG guidelines acknowledge the potential role for EndoFLIP to assess pyloric function in some patients with GP, as well as to predict treatment response to gastric peroral endoscopic myotomy (G-POEM), an intervention which has gained considerable intrigue in recent years. Several open-label studies have previously demonstrated that G-POEM improves symptoms and gastric emptying in GP patients.2 Further, more recent studies have shown favorable long-term outcome data for treatment of GP with G-POEM, up to 4 years.95–97
While sham-controlled trials were previously lacking, one recent sham-controlled study demonstrated that G-POEM resulted in significant improvement in symptoms in GP patients over sham therapy. In a study of 41 GP patients (17 with diabetic GP, 13 with postsurgical GP, and 11 with idiopathic GP), in which 21 were randomized to G-POEM and 20 underwent a sham procedure (EGD lasting at least 40 minutes, without pyloric intervention), significantly more patients undergoing G-POEM experienced treatment success, defined as at least a 50% improvement in GCSI score at 6-months follow-up, compared to the patients who underwent the sham procedure (77% vs 22%, p = 0.005).98 Notably, the study was terminated early after interim analysis by the Data and Safety Monitoring Board revealed a significant difference in response among the two groups. Also, of note, patients included in the study had an abnormal gastric emptying scintigraphy, defined by >60% gastric retention at 2 hours and/or >10% retention at 4 hours, as well as severe symptoms (assessed by GCSI score) which persisted for at least 6 months. While this work certainly adds to the excitement of G-POEM for the treatment of GP, additional sham-controlled studies are needed to help determine which patients may be best suited for treatment with G-POEM, as well as to better define the role of EndoFLIP in predicting treatment success with pyloric interventions.
Finally, the role of gastric electrical stimulation (GES) for the treatment of GP continues to be debated. Though approved for the treatment of refractory nausea and vomiting symptoms due to GP by the FDA in 2000, GES’s precise mechanism of action remains unclear, as it has not been shown to effectively improve gastric emptying, and prospective randomized studies have yielded mixed results.99–103 That said, multiple individual studies to date have demonstrated significant improvement in symptoms of nausea and vomiting with GES treatment in patients with diabetic or idiopathic GP; abdominal pain has not been shown to improve. Therefore, GES is currently thought of as a reasonable treatment option in GP patients, not taking opioids, with nausea/vomiting predominant symptoms refractory to standard therapy.2,39
Conclusions
Gastroparesis remains a troubling disorder, which has a tremendous impact on patients’ quality of life and the healthcare system in general. Recent epidemiologic data suggest that GP is more prevalent than previously thought. Though historically characterized by an objective delay in gastric emptying, the pathophysiology of GP is complex and recent data has shed light on other pathophysiologic mechanisms beyond delayed gastric emptying, such as visceral and central hypersensitivity, which provide further evidence to dispel the misconception that GP is solely a disorder of delayed gastric emptying. Further, the recent movement among experts in the field to classify GP along the same spectrum of gastric sensorimotor dysfunction as FD has the potential to fundamentally transform how GP is viewed as a disorder, with tremendous implications for future research. Regarding treatment, the evidence in support of G-POEM for treatment of GP continues to mount, though additional large, sham-controlled trials are still needed to help identify the specific GP patient population which would be best suited for pyloric intervention, as well as to better define the potential role for EndoFLIP in this evaluation. Finally, increased attention to the complexity of GP from a pathophysiologic standpoint brings hope of novel treatments beyond traditional antiemetics and prokinetics whose overall efficacy remains modest at best for the GP population at large. As it stands, metoclopramide remains the only FDA-approved treatment for GP. Moving forward, gut-brain directed therapies, such as behavioral therapies and neuromodulators, seem worthy of further investigation as treatment options for GP given emerging evidence linking GP with FD.
Disclosure
BEL is consultant for Ironwood, Urovant, Salix, Sanofi. The authors report no other conflicts of interest in this work.
References
1. Camilleri M, Sanders KM. Gastroparesis. Gastroenterology. 2022;162(1):68–87 e1. doi:10.1053/j.gastro.2021.10.028
2. Camilleri M, Kuo B, Nguyen L, et al. ACG clinical guideline: gastroparesis. Am J Gastroenterol. 2022;117(8):1197–1220. doi:10.14309/ajg.0000000000001874
3. Lacy BE, Crowell MD, Cangemi DJ, et al. Diagnostic evaluation of gastric motor and sensory disorders. Am J Gastroenterol. 2021;116(12):2345–2356. doi:10.14309/ajg.0000000000001562
4. Jung HK, Choung RS, Locke GR, et al. The incidence, prevalence and outcomes of patients with gastroparesis in Olmsted County, Minnesota from 1996 to 2006. Gastroenterology. 2009;136:1225–1233. doi:10.1053/j.gastro.2008.12.047
5. Dilmaghani S, Zheng T, Camilleri M. Epidemiology and healthcare utilization in patients with gastroparesis: a systematic review. Clin Gastroenterol Hepatol. 2022. doi:10.1016/j.cgh.2022.07.011
6. Lacy BE, Crowell MD, Mathis C, et al. Gastroparesis: quality of life and health care utilization. J Clin Gastroenterol. 2018;52:20–24. doi:10.1097/MCG.0000000000000728
7. Wang YR, Fisher RS, Parkman HP. Gastroparesis-related hospitalizations in the United States: trends, characteristics, and outcomes, 1995–2004. Am J Gastroenterol. 2008;103:313–322. doi:10.1111/j.1572-0241.2007.01658.x
8. Hirsch W, Nee J, Ballou S, et al. Emergency department burden of gastroparesis in the United States, 2006 to 2013. J Clin Gastroenterol. 2019;53:109–113. doi:10.1097/MCG.0000000000000972
9. Camilleri M, Parkman HP, Shafi MA, et al. Clinical guideline: management of gastroparesis. Am J Gastroenterol. 2013;108:18–37. doi:10.1038/ajg.2012.373
10. Parkman HP, Hasler WL, Fisher RS, et al.; American Gastroenterological Association. American Gastroenterological Association medical position statement: diagnosis and treatment of gastroparesis. Gastroenterology. 127;2004:1589–1591. doi:10.1053/j.gastro.2004.09.054
11. Moshiree B, Potter M, Talley NJ. Epidemiology and pathophysiology of gastroparesis. Gastrointest Endosc Clin N Am. 2019;29(1):1–14. doi:10.1016/j.giec.2018.08.010
12. Hasler WL, May KP, Wilson L, et al. Post-infectious gastroparesis: differences in baseline clinical characteristics and evolution of symptoms and disease severity after 48 weeks versus patients without infectious prodromes. Gastroenterology. 2011;140:S707. doi:10.1016/S0016-5085(11)62936-3
13. Ye Y, Yin Y, Huh SY, et al. Epidemiology, etiology, and treatment of gastroparesis: real-world evidence from a large US national claims database. Gastroenterology. 2022;162(1):109–121 e5. doi:10.1053/j.gastro.2021.09.064
14. Camilleri M, Chedid V, Ford AC, et al. Gastroparesis. Nat Rev Dis Primers. 2018;4(1):41. doi:10.1038/s41572-018-0038-z
15. Vijayvargiya P, Jameie-Oskooei S, Camilleri M, et al. Association between delayed gastric emptying and upper gastrointestinal symptoms: a systematic review and meta-analysis. Gut. 2019;68(5):804–813. doi:10.1136/gutjnl-2018-316405
16. Silver PJ, Dadparvar S, Maurer AH, et al. Proximal and distal intragastric meal distribution during gastric emptying scintigraphy: relationships to symptoms of gastroparesis. Neurogastroenterol Motil. 2022;2022:e14436.
17. Cogliandro RF, Rizzoli G, Bellacosa L, et al. Is gastroparesis a gastric disease? Neurogastroenterol Motil. 2019;31(5):e13562. doi:10.1111/nmo.13562
18. Harberson J, Thomas RM, Harbison SP, et al. Gastric neuromuscular pathology in gastroparesis: analysis of full-thickness antral biopsies. Dig Dis Sci. 2010;55(2):359–370. doi:10.1007/s10620-009-1071-2
19. Bernard CE, Gibbons SJ, Mann IS, et al. Association of low numbers of CD206-positive cells with loss of ICC in the gastric body of patients with diabetic gastroparesis. Neurogastroenterol Motil. 2014;26(9):1275–1284. doi:10.1111/nmo.12389
20. Grover M, Farrugia G, Lurken MS, et al. Cellular changes in diabetic and idiopathic gastroparesis. Gastroenterology. 2011;140(5):1575–85 e8. doi:10.1053/j.gastro.2011.01.046
21. Grover M, Farrugia G, Stanghellini V. Gastroparesis: a turning point in understanding and treatment. Gut. 2019;68(12):2238–2250. doi:10.1136/gutjnl-2019-318712
22. Bashashati M, Moraveji S, Torabi A, et al. Pathological findings of the antral and pyloric smooth muscle in patients with gastroparesis-like syndrome compared to gastroparesis: similarities and differences. Dig Dis Sci. 2017;62(10):2828–2833. doi:10.1007/s10620-017-4629-4
23. Lacy BE. Functional dyspepsia and gastroparesis: one disease or two? Am J Gastroenterol. 2012;107(11):1615–1620. doi:10.1038/ajg.2012.104
24. Kim BJ, Kuo B. Gastroparesis and functional dyspepsia: a blurring distinction of pathophysiology and treatment. J Neurogastroenterol Motil. 2019;25(1):27–35. doi:10.5056/jnm18162
25. Cangemi DJ, Lacy BE. Gastroparesis and functional dyspepsia: different diseases or different ends of the spectrum? Curr Opin Gastroenterol. 2020;36(6):509–517. doi:10.1097/MOG.0000000000000677
26. Stanghellini V, Tack J. Gastroparesis: separate entity or just a part of dyspepsia? Gut. 2014;63(12):1972–1978. doi:10.1136/gutjnl-2013-306084
27. Stanghellini V, Chan FK, Hasler WL, et al. Gastroduodenal Disorders. Gastroenterology. 2016;150(6):1380–1392. doi:10.1053/j.gastro.2016.02.011
28. Lacy BE, Everhart K, Crowell MD. Functional dyspepsia: clinical symptoms, psychological findings, and GCSI Scores. Dig Dis Sci. 2019;64(5):1281–1287. doi:10.1007/s10620-018-5347-2
29. Pasricha PJ, Grover M, Yates KP, et al. Functional dyspepsia and gastroparesis in tertiary care are interchangeable syndromes with common clinical and pathologic features. Gastroenterology. 2021;160(6):2006–2017. doi:10.1053/j.gastro.2021.01.230
30. Karamanolis G, Caenepeel P, Arts J, et al. Determinants of symptom pattern in idiopathic severely delayed gastric emptying: gastric emptying rate or proximal stomach dysfunction? Gut. 2007;56(1):29–36. doi:10.1136/gut.2005.089508
31. Snodgrass P, Sandoval H, Calhoun VD, et al. Central nervous system mechanisms of nausea in gastroparesis: an fMRI-based case-control study. Dig Dis Sci. 2020;65(2):551–556. doi:10.1007/s10620-019-05766-5
32. Lee AA, Rao S, Nguyen LA, et al. Validation of diagnostic and performance characteristics of the wireless motility capsule in patients with suspected gastroparesis. Clin Gastroenterol Hepatol. 2019;17(9):1770–1779. doi:10.1016/j.cgh.2018.11.063
33. Brzana RJ, Koch KL, Bingaman S. Gastric myoelectrical activity in patients with gastric outlet obstruction and idiopathic gastroparesis. Am J Gastroenterol. 1998;93(10):1803–1809. doi:10.1111/j.1572-0241.1998.00524.x
34. Park S, Acosta A, Camilleri M, et al. Gastric motor dysfunction in patients with functional gastroduodenal symptoms. Am J Gastroenterol. 2017;112(11):1689–1699. doi:10.1038/ajg.2017.264
35. Cangemi DJ, Stephens L, Lacy BE. Misdiagnosis of gastroparesis is common: a retrospective review of patients referred to a tertiary gastroenterology practice. Clin Gastroenterol Hepatol. 2023;2023:S1542–3565.
36. Tougas G, Eaker EY, Abell TL, et al. Assessment of gastric emptying using a low fat meal: establishment of international control values. Am J Gastroenterol. 2000;95:1456–1462. doi:10.1111/j.1572-0241.2000.02076.x
37. Wise J, Vazquez-Roque M, McKinney CJ, et al. Gastric emptying scans: poor adherence to national guidelines. Dig Dis Sci. 2021;66(9):2897–2906. doi:10.1007/s10620-020-06314-2
38. Bi D, Choi C, League J, et al. Food residue during esophagogastroduodenoscopy is commonly encountered and is not pathognomonic of delayed gastric emptying. Dig Dis Sci. 2021;66(11):3951–3959. doi:10.1007/s10620-020-06718-0
39. Lacy BE, Tack J, Gyawali CP. AGA clinical practice update on management of medically refractory gastroparesis: expert review. Clin Gastroenterol Hepatol. 2022;20(3):491–500. doi:10.1016/j.cgh.2021.10.038
40. Olausson EA, Alpsten M, Larsson A, et al. Small particle size of a solid meal increases gastric emptying and late postprandial glycaemic response in diabetic subjects with gastroparesis. Diabetes Res Clin Pract. 2008;80(2):231–237. doi:10.1016/j.diabres.2007.12.006
41. Olausson EA, Storsrud S, Grundin H, et al. A small particle size diet reduces upper gastrointestinal symptoms in patients with diabetic gastroparesis: a randomized controlled trial. Am J Gastroenterol. 2014;109(3):375–385. doi:10.1038/ajg.2013.453
42. Strijbos D, Keszthelyi D, Smets FGM, et al. Therapeutic strategies in gastroparesis: results of stepwise approach with diet and prokinetics, gastric rest, and PEG-J: a retrospective analysis. Neurogastroenterol Motil. 2019;31:e13588. doi:10.1111/nmo.13588
43. Parkman HP, Yates KP, Hasler WL, et al. Dietary intake and nutritional deficiencies in patients with diabetic or idiopathic gastroparesis. Gastroenterology. 2011;141(2):486–498. doi:10.1053/j.gastro.2011.04.045
44. Burton murray H, Jehangir A, Silvernale CJ, et al. Avoidant/restrictive food intake disorder symptoms are frequent in patients presenting for symptoms of gastroparesis. Neurogastroenterol Motil. 2020;32(12):e13931. doi:10.1111/nmo.13931
45. Benini L, Todesco T, Dalle Grave R, et al. Gastric emptying in patients with restricting and binge/purging subtypes of anorexia nervosa. Am J Gastroenterol. 2004;99(8):1448–1454. doi:10.1111/j.1572-0241.2004.30246.x
46. Perkel MS, Moore C, Hersh T, Davidson ED. Metoclopramide therapy in patients with delayed gastric emptying. Dig Dis Sci. 1979;24(9):662–666. doi:10.1007/BF01314461
47. Acosta A, Camilleri M. Prokinetics in gastroparesis. Gastroenterol Clin N Am. 2015;44(1):97–111. doi:10.1016/j.gtc.2014.11.008
48. Rao AS, Camilleri M. metoclopramide and tardive dyskinesia. Aliment Pharmacol Therapeut. 2010;31(1):11–19. doi:10.1111/j.1365-2036.2009.04189.x
49. Al-Saffar A, Lennernäs H, Hellström PM. Gastroparesis, metoclopramide, and tardive dyskinesia: risk revisited. Neurogastroenterol Motil. 2019;31(11):e13617. doi:10.1111/nmo.13617
50. Barone JA. Domperidone: a peripherally acting dopamine2-receptor antagonist. Ann Pharmacother. 1999;33(4):429–440. doi:10.1345/aph.18003
51. Drolet B, Rousseau G, Daleau P, Cardinal R, Turgeon J. Domperidone should not be considered a no-risk alternative to cisapride in the treatment of gastrointestinal motility disorders. Circulation. 2000;102(16):1883–1885. doi:10.1161/01.CIR.102.16.1883
52. Schey R, Saadi M, Midani D, Roberts AC, Parupalli R, Parkman HP. Domperidone to treat symptoms of gastroparesis: benefits and side effects from a large single-center cohort. Dig Dis Sci. 2016;61(12):3545–3551. doi:10.1007/s10620-016-4272-5
53. Hasler WL. Serotonin and the GI tract. Curr Gastroenterol Rep. 2009;11(5):383–391. doi:10.1007/s11894-009-0058-7
54. Youssef AS, Parkman HP, Nagar S. Drug‐drug interactions in pharmacologic management of gastroparesis. Neurogastroenterol Motil. 2015;27(11):1528–1541. doi:10.1111/nmo.12614
55. Navari R, Gandara D, Hesketh P, et al. Comparative clinical trial of granisetron and ondansetron in the prophylaxis of cisplatin-induced emesis. The Granisetron study group. J Clin Onc. 1995;13(5):1242–1248. doi:10.1200/JCO.1995.13.5.1242
56. Simmons K, Parkman HP. Granisetron transdermal system improves refractory nausea and vomiting in gastroparesis. Dig Dis Sci. 2014;59(6):1231–1234. doi:10.1007/s10620-014-3097-3
57. Midani D, Parkman HP. Granisetron transdermal system for treatment of symptoms of gastroparesis: a prescription registry study. J Neurogastroenterol Motil. 2016;22(4):650–655. doi:10.5056/jnm15203
58. Pasricha PJ, Yates KP, Sarosiek I, et al. Aprepitant has mixed effects on nausea and reduces other symptoms in patients with gastroparesis and related disorders. Gastroenterology. 2018;154(1):65–76. doi:10.1053/j.gastro.2017.08.033
59. Carlin JL, Lieberman R, Dahal A, et al. Efficacy and safety of tradipitant in patients with diabetic and idiopathic gastroparesis in a randomized, placebo-controlled trial. Gastroenterology. 2021;160(1):76–87. doi:10.1053/j.gastro.2020.07.029
60. Broad J, Mukherjee S, Samadi M, Martin J, Dukes G, Sanger G. Regional- and agonist-dependent facilitation of human neurogastrointestinal functions by motilin receptor agonists. Br J Pharmacol. 2012;167(4):763–774. doi:10.1111/j.1476-5381.2012.02009.x
61. Sanger GJ, Furness JB. Ghrelin and motilin receptors as drug targets for gastrointestinal disorders. Nat Rev Gastroenterol Hepatol. 2016;13(1):38–48. doi:10.1038/nrgastro.2015.163
62. Lacy BE, Weiser K. Gastric motility, gastroparesis, and gastric stimulation. Surg Clin. 2005;85(5):967–987. doi:10.1016/j.suc.2005.05.005
63. Tack J, Depoortere I, Bisschops R, et al. Influence of ghrelin on interdigestive gastrointestinal motility in humans. Gut. 2006;55(3):327–333. doi:10.1136/gut.2004.060426
64. Lembo A, Camilleri M, McCallum R, et al. Relamorelin reduces vomiting frequency and severity and accelerates gastric emptying in adults with diabetic gastroparesis. Gastroenterology. 2016;151(1):87–96. doi:10.1053/j.gastro.2016.03.038
65. Camilleri M, McCallum RW, Tack J, et al. Efficacy and safety of relamorelin in diabetics with symptoms of gastroparesis: a randomized, placebo-controlled study. Gastroenterology. 2017;153(5):1240–1250. doi:10.1053/j.gastro.2017.07.035
66. Carbone F, Ven den Houte K, Clevers E, et al. Prucalopride in gastroparesis: a randomized, placebo-controlled crossover study. Am J Gastroenterol. 2019;114(8):1265–1274. doi:10.14309/ajg.0000000000000304
67. Chaiyakunapruk N, Kitikannakorn N, Nathisuwan S, Leeprakobboon K, Leelasettagool C. The efficacy of ginger for the prevention of postoperative nausea and vomiting: a meta-analysis. Am J Obstet Gyn. 2006;194(1):95–99. doi:10.1016/j.ajog.2005.06.046
68. von Arnim U, Peitz U, Vinson B, Gundermann KJ, Malfertheiner P. STW 5, a phytopharmacon for patients with functional dyspepsia: results of a multicenter, placebo-controlled double-blind study. Am J Gastroenterol. 2007;102(6):1268–1275. doi:10.1111/j.1572-0241.2006.01183.x
69. Pilichiewicz AN, Horowitz M, Russo A, et al. Effects of Iberogast® on proximal gastric volume, antropyloroduodenal motility and gastric emptying in healthy men. Am J Gastroenterol. 2007;102(6):1276–1283. doi:10.1111/j.1572-0241.2007.01142.x
70. Braden B, Caspary W, Börner N, Vinson B, Schneider AR. Clinical effects of STW 5 (Iberogast®) are not based on acceleration of gastric emptying in patients with functional dyspepsia and gastroparesis. Neurogastroenterol Motil. 2009;21(6):632–638. doi:10.1111/j.1365-2982.2008.01249.x
71. Yang M, Li X, Liu S, et al. Meta-analysis of acupuncture for relieving non-organic dyspeptic symptoms suggestive of diabetic gastroparesis. BMC Compl Altern Med. 2013;13(1):311. doi:10.1186/1472-6882-13-311
72. Kim KH, Lee MS, Choi TY, Kim TH. Acupuncture for symptomatic gastroparesis. Cochrane Database Sys Rev. 2018;12(12):CD0096676.
73. Rhodes VA, McDaniel RW. Nausea, vomiting, and retching: complex problems in palliative care. A Cancer J Clin. 2001;51(4):232–248. doi:10.3322/canjclin.51.4.232
74. Parkman HP, Wilson LA, Hasler WL, et al. Abdominal pain in patients with gastroparesis: associations with gastroparesis symptoms, etiology of gastroparesis, gastric emptying, somatization, and quality of life. Dig Dis Sci. 2019;64(8):2242–2255. doi:10.1007/s10620-019-05522-9
75. Parkman HP, Van Natta ML, Abell TL, et al. Effect of nortriptyline on symptoms of idiopathic gastroparesis: the NORIG randomized clinical trial. JAMA. 2013;310(24):2640–2649. doi:10.1001/jama.2013.282833
76. Drossman DA, Tack J, Ford AC, Szigethy E, Tornblom H, Van Oudenhove L. Neuromodulators for functional gastrointestinal disorders (disorders of gut-brain interaction): a Rome foundation working team report. Gastroenterology. 2018;154:1140–71 e1. doi:10.1053/j.gastro.2017.11.279
77. Tack J, Ly HG, Carbone F, et al. Efficacy of mirtazapine in patients with functional dyspepsia and weight loss. Clin Gastroenterol Hepatol. 2016;14(3):385–392.e4. doi:10.1016/j.cgh.2015.09.043
78. Malamood M, Roberts A, Kataria R, Parkman HP, Schey R. Mirtazapine for symptom control in refractory gastroparesis. Drug Des Devel Ther. 2017;30:11:1035–1041.
79. Freeman R, Durso-DeCruz E, Emir B. Efficacy, safety, and tolerability of pregabalin treatment for painful diabetic peripheral neuropathy: findings from seven randomized, controlled trials across a range of doses. Diabetes Care. 2008;31(7):1448–1454. doi:10.2337/dc07-2105
80. Kotikula I, Thinrungroj N, Pinyopornpanish K, et al. Randomised clinical trial: the effects of pregabalin vs placebo on functional dyspepsia. Aliment Pharmacol Ther. 2021;54(8):1026–1032. doi:10.1111/apt.16588
81. Keefer L, Ballou SK, Drossman DA, et al. A Rome working team report on brain-gut behavior therapies for disorders of gut-brain interaction. Gastroenterology. 2022;162(1):300–315. doi:10.1053/j.gastro.2021.09.015
82. Thomas JJ, Murray HB. Cognitive-behavioral treatment of adult rumination behavior in the setting of disordered eating: a single case experimental design. Int J Eat Disord. 2016;49(10):967–972. doi:10.1002/eat.22566
83. Murray HB, Zhang F, Call CC, et al. Comprehensive cognitive-behavioral interventions augment diaphragmatic breathing for rumination syndrome: a proof-of-concept trial. Dig Dis Sci. 2021;66(10):3461–3469. doi:10.1007/s10620-020-06685-6
84. Bielefeldt K, Raza N, Zickmund SL. Different faces of gastroparesis. World J Gastroenterol. 2009;15(48):6052–6060. doi:10.3748/wjg.15.6052
85. Orive M, Barrio I, Orive VM, et al. A randomized controlled trial of a 10 week group psychotherapeutic treatment added to standard medical treatment in patients with functional dyspepsia. J Psychosom Res. 2015;78(6):563–568. doi:10.1016/j.jpsychores.2015.03.003
86. Haug TT, Wilhelmsen I, Svebak S, et al. Psychotherapy in functional dyspepsia. J Psychosom Res. 1994;38(7):735–744. doi:10.1016/0022-3999(94)90026-4
87. Calvert EL, Houghton LA, Cooper P, et al. Long-term improvement in functional dyspepsia using hypnotherapy. Gastroenterology. 2002;123(6):1778–1785. doi:10.1053/gast.2002.37071
88. Kinsinger SW, Joyce C, Venu M, et al. Pilot study of a self-administered hypnosis intervention for functional dyspepsia. Dig Dis Sci. 2022;67(7):3017–3025. doi:10.1007/s10620-021-07183-z
89. Mearin F, Camilleri M, Malagelada JR. Pyloric dysfunction in diabetics with recurrent nausea and vomiting. Gastroenterology. 1986;90(6):1919–1925. doi:10.1016/0016-5085(86)90262-3
90. Bromer MQ, Friedeberg F, Miller LS, et al. Endoscopic pyloric injection of botulinum toxin A for the treatment of refractory gastroparesis. Gastrointest Endosc. 2005;61:833–839. doi:10.1016/S0016-5107(05)00328-7
91. Arts J, van Gool S, Caenepeel P, et al. Influence of intrapyloric botulinum toxin injection on gastric emptying and meal-related symptoms in gastroparesis patients. Aliment Pharmacol Ther. 2006;24:661–667. doi:10.1111/j.1365-2036.2006.03019.x
92. Arts J, Holvoet L, Caenepeel P, et al. Clinical trial: a randomized-controlled crossover study of intrapyloric injection of botulinum toxin in gastroparesis. Aliment Pharmacol Ther. 2007;26:1251–1258. doi:10.1111/j.1365-2036.2007.03467.x
93. Friedenberg FK, Palit A, Parkman HP, Hanlon A, Nelson DB. Botulinum toxin A for the treatment of delayed gastric emptying. Am J Gastroenterol. 2008;2008:103416–103423.
94. Desprez C, Melchior C, Wuestenberghs F, et al. Pyloric distensibility measurement predicts symptomatic response to intrapyloric botulinum toxin injection. Gastrointest Endosc. 2019;90:754–60.e1. doi:10.1016/j.gie.2019.04.228
95. Abdelfatah MM, Noll A, Kapil N, et al. Long-term outcome of gastric per-oral endoscopic pyloromyotomy in treatment of gastroparesis. Clin Gastroenterol Hepatol. 2021;19:816–824. doi:10.1016/j.cgh.2020.05.039
96. Vosoughi K, Ichkhanian Y, Benias P, et al. Gastric per-oral endoscopic myotomy (G-POEM) for refractory gastroparesis: results from an international prospective trial. Gut. 2021;71(1):25–33. doi:10.1136/gutjnl-2020-322756
97. Hernández Mondragón OV, Contreras LFG, Velasco GB, et al. Gastric peroral endoscopic myotomy outcomes after 4 years of follow-up in a large cohort of patients with refractory gastroparesis (with video). Gastrointest Endosc. 2022;96(3):487–499. doi:10.1016/j.gie.2022.03.025
98. Martinek J, Hustak R, Mares J, et al. Endoscopic pyloromyotomy for the treatment of severe and refractory gastroparesis: a pilot, randomized, sham-controlled trial. Gut. 2022;71:2170–2178. doi:10.1136/gutjnl-2022-326904
99. Abell T, McCallum R, Hocking M, et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 2003;125(2):421–428. doi:10.1016/S0016-5085(03)00878-3
100. McCallum RW, Snape W, Brody F, et al. Gastric electrical stimulation with Enterra therapy improves symptoms from diabetic gastroparesis in a prospective study. Clin Gastroenterol Hepatol. 2010;8(11):947–54;quiz e116. doi:10.1016/j.cgh.2010.05.020
101. McCallum RW, Sarosiek I, Parkman HP, et al. Gastric electrical stimulation with Enterra therapy improves symptoms of idiopathic gastroparesis. Neurogastroenterol Motil. 2013;25(10):815–e636. doi:10.1111/nmo.12185
102. Levinthal DJ, Bielefeldt K. Systematic review and meta-analysis: gastric electrical stimulation for gastroparesis. Auton Neurosci. 2021;202:45–55. doi:10.1016/j.autneu.2016.03.004
103. Ducrotte P, Coffin B, Bonaz B, et al. Gastric electrical stimulation reduces refractory vomiting in a randomized crossover trial. Gastroenterology. 2020;158(3):506–514 e2. doi:10.1053/j.gastro.2019.10.018
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
