Back to Journals » Cancer Management and Research » Volume 12
Gastric Signet Ring Cell Carcinoma: Current Management and Future Challenges
Authors Li Y , Zhu Z, Ma F, Xue L , Tian Y
Received 17 June 2020
Accepted for publication 15 August 2020
Published 3 September 2020 Volume 2020:12 Pages 7973—7981
DOI https://doi.org/10.2147/CMAR.S268032
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chien-Feng Li
Yang Li,1 Zhikai Zhu,2 Fuhai Ma,1 Liyan Xue,3 Yantao Tian1
1Department of Pancreatic and Gastric Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, People’s Republic of China; 2School of Public Health, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, People’s Republic of China; 3Department of Pathology, National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, People’s Republic of China
Correspondence: Yantao Tian
Department of Pancreatic and Gastric Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, 17 South Panjiayuan Lane, Suite 806, Surgical Building, Chaoyang District, Beijing 100021, People’s Republic of China
Tel +86 1087787120
Email [email protected]
Abstract: Recent advances in the epidemiology, pathology, molecular mechanisms, and combined modality therapy (CMT) fields have shown that gastric signet ring cell carcinoma (GSRC) should be considered a distinct cancerous entity. Clinical management of this cancer is challenging, with chemoradioresistance and poor outcomes in advanced stages. Pathological and molecular sets of GSRC demonstrate different features of poor cohesion and differentiation according to the WHO, Japanese Gastric Cancer Association, and Laurén classifications. These features also result in poor response to adjuvant and neoadjuvant chemoradiotherapy. Certain studies of GSRC showed the disputed effectiveness of hyperthermic intraperitoneal chemotherapy and immunotherapy. Our aim was to discuss how an improved understanding of these therapeutic benefits may provide better treatment selection for patients, and therefore improve survival. The challenges in the new understanding of GSRC in routine practice and pathology, and the current limitations of treatment will also be discussed.
Keywords: gastric cancer, signet ring cell, pathology, combined modality therapy
Introduction
Gastric cancer is one of the most common causes of cancer-related death and imposes a significant burden on global health care. There are over 1,000,000 new cases of gastric cancer annually, and it is the third leading cause of cancer-related mortality worldwide, resulting in approximately 783,000 deaths in 2018.1 There has been a continuous decrease in gastric cancer incidence in the past decades in most parts of the world.2 These downward trends can be attributed to the unexpected success of prevention, such as improvements in the treatment of H. pylori infection.3 However, gastric signet ring cell carcinoma (GSRC), a distinct type of gastric cancer, is persistently increasing in Asia, Europe and the United States, and accounted for 35–45% of new adenocarcinoma cases.4,5 Despite important advances in the understanding of its epidemiology, pathology, molecular mechanisms, therapeutic options, and strategies, the diagnosis and treatment burden of GSRC remains high.
GSRC faces many clinical challenges. The endoscopic and pathological tests are impractical for early-stage screening purposes. GSRC is usually diagnosed at a more advanced stage with metastases in lymph nodes, distant organs, or both, in which case incomplete resections were more frequent.6 Many patients have recurrent disease or complications after resection with curative intent.7 Thus, the combined modality therapy (CMT) should be considered viable. Currently, treatment largely depends on conventional chemotherapy, which is less effective in the majority of GSRC patients.6 Perioperative adjuvant or neoadjuvant chemoradiotherapy can be used, but many patients continue to develop drug resistance and metastatic disease.8–10 These treatments are associated with limited benefits in overall survival, and median survival in the majority of clinical trials is 12 to 20 months.9,11-13
Although several prognostic and predictive factors have been investigated, including patient and tumor characteristics, none of these have provided strategies based on the unique biomarkers in GSRC. Due to inadequate predictive biomarkers, some patients were detrimentally overtreated with chemotherapy. Exploration of biomarkers that would allow treatment to be tailored to specific patient molecular characteristics is of great importance. Initial trials of CMT in GSRC have produced conflicting results.8,14,15 In addition to these clinical challenges, it has been increasingly recognized that the poor prognosis of GSRC is closely related to unique biological behaviors.5,16,17
This review assesses how a better understanding of the CMT and genetic features of GSRC might improve patient outcomes. First, recent progress in the field of pathological and molecular classification of GSRC is discussed as a framework for further analysis. This is followed by addressing our current understanding of CMT in GSRC. Finally, how advances in CMT might influence the outcome of GSRC and a further review of areas of investigation will be presented.
Pathological and Molecular Classification of GSRC
Pathological Classification
Most gastric cancers are adenocarcinomas but are highly heterogeneous in terms of growth, cell differentiation, histogenesis, and molecular pathogenesis. Although the understanding of the etiology and pathogenesis of gastric cancer has been developed, there is less molecular pathology in clinical use, especially concerning the molecular markers that are relevant to the diagnosis and treatment.18,19
The most commonly used classifications are these published by the Japanese Gastric Cancer Association (JGCA),20 WHO,19 Nakamura21 and Laurén.22 (Figure 1) There are five subtypes of gastric cancer are divided as following: tubular, papillary, poorly cohesive, mucinous, and mixed adenocarcinomas. Poorly cohesive carcinomas (PCC) are composed predominantly or exclusively of signet ring cells. These cells were classified by WHO in 2019 as having an optically clear, globoid droplet or cytoplasmic mucin center, with an eccentrically placed nucleus.19 A signet ring cell type also corresponds to diffuse, undifferentiated, and poorly differentiated adenocarcinoma types in the Laurén classification (1965), Nakamura’s classification (1968),23 and JGCA (2017), respectively.19,21,22,24 A tumor including single signet ring cell or more distinct histological components is considered to be indicative of mixed adenocarcinoma, where phenotypic divergence is attributed to somatic mutation in the gene encoding CDH1.5
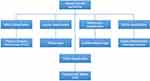 |
Figure 1 The pathological classifications of GSRC. |
There are also some disputations for signet ring cell carcinoma (SRCC). First, it is difficult to distinguish the type between GSRC and poorly cohesive (PC) carcinoma types, mainly because GSRC can transform into a PC type in the invasive layer, while losing its morphology during the transformation.24 Second, differentiation or adhesiveness should be taken more seriously. Japan and WHO defined gastric adenocarcinoma differently, with little glandular differentiation, so it was reclassified as PCC based on a focus on the mutual adhesiveness of cancer cells.19,24 Third, the specific proportion of GSRC in gastric cancer is controversial. GSRC is diagnosed in more than 50% of poorly cohesive cells having signet ring cell morphology, according to the 2010 WHO classification. However, Mariette, on behalf of European Chapter of International Gastric Cancer Association, proposed that only WHO PC carcinomas containing more than 90% PCCs with classical GSRC morphology should be classified as GSRC carcinomas. This classification reflects the possibility that the proportion of signet ring cells may represent the differentiation grade in PC and GSRC carcinomas.5 However, the definition has not yet reached global consensus. Finally, the discrepancy in endoscopic biopsies and resected specimens is still uncertain, which may lead to the adoption of different treatment strategies.
Molecular Classification
Recent advances in molecular studies have not only shed light on the carcinogenesis of gastric cancer, but have also offered novel approaches for prevention, diagnosis and therapeutic intervention.25 Accumulation of genetic and molecular abnormalities occurs during gastric carcinogenesis, including activation of oncogenes, overexpression of growth receptors, inactivation of tumor suppression genes, DNA repair genes and cell adhesion molecules, loss of heterogeneity and point mutations of tumor suppressor genes, and silencing of tumor suppressors.7,26 GSRC carcinogenesis is also a multistep and multifactorial process. The revelation and understanding of molecular issues and pathways have contributed to the application of molecular mechanisms in the prevention, early diagnosis, tumor classification and therapeutic intervention.
The Cancer Genome Atlas (TCGA) Network published an analysis of primary gastric cancers, classifying the following subgroups: Epstein Barr virus (EBV) associated, microsatellite instability (MSI) associated, chromosomal instability associated and genomically stable.26 Correlation with histological characteristics revealed enrichment of the diffuse subtype in the genomically stable group (73%). Shu et al reported that high CLDN18-ARHGAP26/6 fusion in GSRC leads to genetic differences with other subtypes of diffuse gastric cancer, onset at a young age, higher female/male ratio, advanced tumor stage, worse survival outcomes, and chemoresistance.16 Moreover, additional insights into the clinical and genomic features of SRCC for diagnosis, treatment strategy and evaluation of prognosis were presented in this study. In addition to sporadic gastric cancer, approximately 1–3% of gastric cancers arise from the inherited type, such as hereditary diffuse gastric carcinoma (HDGC), with a germline mutation in CDH1.27 The incidences are different for low-risk and high-risk areas. The potentially molecular mechanism is that the inactivation of E-cadherin is probably a key initiating event in HDGC tumorigenesis. And absence of a normal E-cadherin protein may lead to the disruption of the cell-cell adhesion complex. The different expression of E-cadherin protein in races may lead to different incidences of HDGC. The histologic phenotype of HDGC in the early stages includes patchy intramucosal signet ring cells in the lamina propria. Its unique feature is its association with pagetoid spread along the preserved basement membrane, which has the same biological behavior as SRCC.25 The molecular classification should be further investigated to determine prognosis and customize treatment.
Biomarkers
To our knowledge, the studies on specifically expressed biomarkers for GSRC are very limited. Chen et al reported that hsa-miR-665 and hsa-miR-95 were downregulated in GSRC but upregulated in intestinal-type gastric carcinoma, which may be related to the metastasis and chemoresistance of GSRC.28 Yan et al found that expression of miR-935 is lower both in GSRC cell lines and tissue samples than in non-GSRC, and enhanced expression of miR-935 in GSRC cell lines inhibit cell proliferation, migration, and invasion.29 Aihara et al reported that matrilysin played a key role in tumor progression and metastasis patients and preoperative evaluation of the matrilysin expression might be useful as to confirm submucosal invasion and lymph node metastasis for the early GSRC.30 Also, Saito et al found that high miR-99a-5p expression resulted in inhibition of proliferation in GSRC, and emerge as a biomarker for early GSRC and lymph node metastases.31 The significance of biomarkers was observed in the laboratory but not in clinics. Up to date, there is still a lack of mature biomarkers for GSRC in diagnoses and prognoses. Research at the molecular level have significant implications for an individual approach to treatment of GSRC. We may speculate that GSRC patients will undergo a treatment that is individual and different from current clinical guidelines in the future.
Management of Early and Locally Advanced GSRC
Endoscopic Treatment
Endoscopic submucosal dissection (ESD) is an optional treatment for early-stage gastric cancer with strict indications. There are two principles for the indications of endoscopic resection: suitability for en bloc resection and low possibility of lymph node metastasis.32 Absolute indication is defined as a tumor in which the possibility of harboring lymph node metastasis is less than 1%. The expanded indication of ESD is an undifferentiated-type adenocarcinoma without ulcerative findings in which the depth of invasion is clinically diagnosed as T1a and the diameter is ≤2 cm. The GSRC can reach the criterion of expanded indication for ESD. However, the key factor is the rate of lymph node metastasis. Pokala et al reported that the incidence of metastatic nodes for 244 T1a GSRC patients with a significantly associated tumor size of <1 cm and <2 cm was 2.7% and 5.4%, respectively.33 Lee et al reported that the incidence of lymph node metastasis was 1.9% with intramucosal cancer and proposed that early-stage GSRC can be treated through endoscopic resection if the diameter is smaller than 25 mm and intramucosal, excluding lymphatic vascular metastasis.34,35 Wang et al reported that the overall rate of lymph node metastasis in early-stage GSRC was 10.3% and no lymph node metastasis was observed in tumors <20mm without lymphatic vascular metastasis.36 Kim et al demonstrated that a large tumor size was the only significant factor related to incomplete resection in the early stage of GSRC.37 Additionally, ESD could be considered as a feasible local treatment for the early stage of GSRC if a complete resection is achieved. Generally speaking, whether endoscopic resection is appropriate for GSRC is still uncertain, and more confirmatory evidence is needed from randomized controlled trials.
Surgical Treatment
Adequate surgical resection is the main therapeutic option for GSRC. A standard gastrectomy is the principal surgical procedure performed with curative intent involving resection of at least two-thirds of the stomach with a D2 lymph node dissection.32,38 In principle, a D2 lymphadenectomy is indicated for cN+ or ≥ cT2 tumors.
A sufficient resection margin should be ensured when a standard gastrectomy is performed for curative intent. Piessen et al reported that the R0 resection rate of GSRC was 56.0%, significantly lower than the non-GSRC rate of 74% (P=0.019), with a higher peritoneal recurrence (52.2%) than the non-GSRC (21.4%, P=0.011).39 In this study, Piessen et al reported a distance of at least 5 cm between the proximal resection margin and the carcinoma. Moehler et al considered the proximal resection margin of 5–8 cm to be a safe distance in diffuse gastric carcinomas, with a very low probability of tumor detection in the resection margin.40 A proximal margin of at least 5 cm is recommended for those with an infiltrative cancer, according to the Japanese gastric cancer treatment guidelines 5th edition.32 There is no agreed-upon recommendation for a proximal resection margin distance for GSRC. As a result, an adopted principle could follow the guidelines of the normal adenocarcinoma type.
Adjuvant and Neoadjuvant Treatments
Adjuvant and neoadjuvant therapies are generally accepted to improve survival in patients who have adequate R0 resection of locally advanced cancer by eradicating microscopic disease locoregionally and at a distance from the primary tumor.7 In addition, such therapies are delivered with the intention of reducing recurrence by controlling residual tumor cells following a curative resection.32 Preoperative chemotherapy is viewed as a popular strategy in Europe, whereas postoperative chemoradiotherapy and postoperative chemotherapy are more common in the US and Asia, respectively.7 Various preoperative or postoperative regimens have been tested in numerous clinical trials. However, the specific regimens for GSRC remain uncertain.41,42 Several studies and retrospective analyses have shown that GSRC cannot benefit from chemotherapy or radiotherapy due to chemoresistance.6,8,10
Chemotherapy
GSRC has the characteristic of chemoresistance which has been confirmed by several studies. However, the effectiveness of chemotherapy for GSRC remains controversial.
Postoperative Chemotherapy
The Japanese ACTS-GC trial offered treatment benefits of postoperative chemotherapy.43 However, the results for GSRC were uncertain. A study with 899 GSRC patients conducted by Voron showed that postoperative chemotherapy did not significantly affect survival (HR=0.873, 95% CI: 0.708, 1.007). A signet ring cell is an independent adverse prognostic factor (HR=1.182) in multivariate analysis.6 Similarly, a study by Wei et al demonstrated that postoperative chemotherapy did not provide a dramatic survival benefit in 1303 GSRC patients (HR=0.935, 95% CI: 0.674, 1.296).44 Additionally, postoperative chemotherapy did not show a positive impact on survival in the 144 GSRC patients from Li’s study (HR=0.654, 95% CI: 0.271, 1.581).45 It appears that only Shi et al suggested that a selected group of stage IV GSRC patients benefited from postoperative chemotherapy (HR = 0.61, 95% CI: 0.51‐0.73).46 Most studies showed a rare survival benefit in GSRC patients, although positive trends could be obtained with postoperative chemotherapy. Thus, further improvements could result from discovery of effective anti-cancer target drugs.
Preoperative Chemotherapy
Although the MAGIC trial demonstrated the strengths of chemotherapy, preoperative chemotherapy has been controversial in GSRC.47 Messager et al reported that preoperative chemotherapy was an independent predictor of poor survival (HR = 1.4, 95% CI: 1.1, 1.9), with a shorter survival time compared to surgery alone (12.8 vs 14.0 months, P = 0.043) in 3010 GSRC patients (NCT01249859), as shown in Table 1.9 Piessen et al also pointed out that preoperative chemotherapy was a negative factor for survival (HR=1.25, 95% CI: 0.97, 1.59) among 499 patients.12 Similar results were obtained in the preoperative chemotherapy studies by Voron et al and Li et al.6,45 However, Heger claimed that there were positive and protective effects of preoperative chemotherapy on GSRC patients (HR=0.572, 95% CI: 0.353, 0.929).14 Preoperative chemotherapy as an optional treatment for advanced GSRC is disputed for several reasons such as chemoresistance and cancer progression during the preoperative regimen. Overall, more evidence is needed to prove its effectiveness.
 |
Table 1 Summary of Trials of the GSRC from ClinicalTrials.Gov |
Radiotherapy
The ARTIST trial in South Korea was conducted to assess the efficacy of postoperative chemotherapy with capecitabine and cisplatin, with or without radiation.48 The results did not significantly extend overall survival but improved disease-free survival of patients with lymph node metastasis using chemoradiotherapy compared to chemotherapy alone. The INT 0116 trial was the first mainly randomized trial to demonstrate a survival benefit for gastric cancer patients with adjuvant chemoradiotherapy.49,50 However, the effectiveness for patients with GSRC remains disputed. Shi et al claimed that postoperative radiotherapy did not improve the survival (HR=1.08, 95% CI: 0.76, 1.53).46 Nonetheless, from the perspective of Wei, postoperative radiotherapy provided more possibilities for survival (HR=0.788, 95% CI: 0.644, 0.009) of patients with locally advanced GSRC.44 Most previous studies did not offer compelling evidence on this issue. Therefore, more randomized controlled trials are required in further studies.
Management of Metastatic GSRC
Systemic Chemotherapy
The outlook for patients with metastatic gastric cancer is very poor in various studies. Patients with good performance status scores should be offered optional chemotherapy for palliation and to improve survival.7 Advanced GSRC is commonly considered to have a poor prognosis and inferior chemosensitivity compared to other types of gastric cancer. Lemoine et al reported that advanced GSRC appeared to benefit less from chemotherapy with a median overall survival of 5.6 months compared to 9.4 months for non-GSRC patients.8 However, triplet chemotherapy with docetaxel-5FU-oxaliplatin (TEFOX) has yielded favorable results among GSRC patients, and appears to be an effective as first-line treatment for advanced GSRC.41 Effective biological targets need to be explored to improve the survival of GSRC.
Hyperthermic Intraperitoneal Chemotherapy (HIPEC)
Several studies have confirmed that GSRC has the unique characteristic of peritoneal metastasis, which usually results in a poor prognosis.51–53 Li et al found a significantly higher peritoneal metastasis rate in GSRC, which was believed to be a risk factor for survival (HR=2.834, 95% CI: 2.32, 3.46).54 Some studies have suggested that peritoneal metastasis and recurrence occurs more frequently in GSRC.39,55
Peritoneal metastasis has always been considered to indicate an advanced stage of gastric cancer. Since the development of cytoreductive surgery (CRS) and HIPEC techniques, some patients with limited peritoneal involvement have been recategorized as having locoregional disease, which is now believed to be potentially treatable with a surgical strategy. This regionally focused approach is based on the synergistic effect between complete macroscopic removal of the tumor and all-involved peritoneal surfaces, along with the residual gold-standard treatment of systemic chemotherapy.
Königsrainer et al showed that the prognosis appears to be inferior irrespective of complete CRS and HIPEC. Complete cytoreduction could not be achieved in a considerable percentage of patients.52 Königsrainer proposed that CRS and HIPEC should be restricted to highly selective GSRC patients with peritoneal metastases. Yan et al proposed that the effective rate of HIPEC was worse in GSRC than in poorly or moderately differentiated adenocarcinoma at 44.4%, 69.2%, and 65.2%, respectively.53 Additionally, Shi et al demonstrated that GSRC did not benefit from intraperitoneal chemotherapy.51 According to the information retrieved, GSRC patients with peritoneal metastasis cannot benefits from HIPEC. However, based on the trials with an inferior quality of evidence, randomized controlled trials of HIPEC should be encouraged. To our knowledge, a randomized multicenter Phase III clinical study called GASTRICHIP is underway (Table 1). This trial is designed to evaluate the effects of a D2 resection and HIPEC in locally advanced gastric carcinoma and was launched in 2013 by Glehen from France (NCT01882933).56
Immunotherapy
In 1981, Yasue et al carried out a controlled study of maintenance chemoimmunotherapy, compared with immunotherapy alone, following palliative gastrectomy and induction chemoimmunotherapy for advanced gastric cancer (GC). These results suggest that Ok-432 (NSC-B116209) had better effects than chemoimmunotherapy, including MFC (mitomycin C, 5-fluorouracil, and cytosine arabinoside), particularly among patients with undifferentiated GC and SRCC.57 However, there are no dramatic advances in immunotherapy for GSRC.
With the development of immunology, immune checkpoint inhibitors targeting programmed death 1 (PD-1) and PD-1 ligand 1 (PD-L1), which are related to the efficiency of immunotherapy, have been recommended as an option for cancer treatment.7 Mismatch repair (MRR) proteins are the key elements in the DNA repair pathway, and deficient MMR (dMMR) is associated with immunotherapy. MSI occurs frequently in dMMR tumors. The prevalence of dMMR in SRCC may be related to the frequency of MSI in SRCC, but studies have yielded inconsistent results varying from 0% to 33%. Hirotsu et al reported that GSRC exhibits high MSI at low frequencies.58 A group of specifically selected GSRC patients with MSI may benefit from immunotherapy. Overall, more studies are required to identify the key mutations of therapeutic strategies for GSRC patients.
Updated Clinical Trials
The current clinical trials of GSRC are introduced in the following section and in Table 1.
PRODIGE-19-FFCD1103-ADCI002 is a prospective multicenter controlled randomized phase II/III trial comparing the current standard of care for perioperative chemotherapy with a strategy of primary surgery followed by adjuvant chemotherapy in patients with a stage IB-III GSRC.59 This trial was launched by Guillaume et al in France in 2013 and remains in the recruiting status (NCT01717924).
A randomized, multicenter, controlled study of XELOX vs XELOX combined with apatinib as a postoperative chemotherapy for a locally advanced GSRC with a D2 dissection was launched in 2017.60 This study is being conducted by Liang et al in China and has not yet been recruited (NCT03355612).
Glehen et al launched a phase III trial that compared overall 5-year survival rates in patients with advanced GSRC and/or positive peritoneal cytology, treated with a curative gastrectomy combined with adjuvant HIPEC, or curative gastrectomy alone (NCT01882933). This trial is underway and in the recruiting stage.56
Conclusion
Significant progress has been made in understanding the pathogenesis and the molecular biology of GSRC and in optimizing the available treatment options and modalities. However, improving outcomes for patients with GSRC remains a significant challenge. GSRC has several features, such as chemoresistance and peritoneal metastasis, which suggest poor response to anti-cancer drug-based therapies. This article has reviewed how improving the understanding of the pathological and molecular subgroups may facilitate the selection of patients that may benefit from CMT, including surgery, chemoradiation, immunotherapy, and HIPEC. Due to the absence of specific and effective molecular targets, challenges remain in the treatment strategy of GSRC. Thus, further studies should focus on the pathogenesis and molecular biology of GSRC.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Thrift AP, El-Serag HB. Burden of gastric cancer. Clin Gastroenterol Hepatol. 2020;18(3):534–542. doi:10.1016/j.cgh.2019.07.045
2. Arnold M, Abnet CC, Neale RE, et al. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology. 2020;159(1):335–349.e15. doi:10.1053/j.gastro.2020.02.068
3. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
4. Pernot S, Voron T, Perkins G, Lagorce-Pages C, Berger A, Taieb J. Signet-ring cell carcinoma of the stomach: impact on prognosis and specific therapeutic challenge. World J Gastroenterol. 2015;21(40):11428–11438. doi:10.3748/wjg.v21.i40.11428
5. Mariette C, Carneiro F, Grabsch HI, van der Post RS, Allum W, de Manzoni G. Consensus on the pathological definition and classification of poorly cohesive gastric carcinoma. Gastric Cancer. 2019;22(1):1–9. doi:10.1007/s10120-018-0868-0
6. Voron T, Messager M, Duhamel A, et al. Is signet-ring cell carcinoma a specific entity among gastric cancers? Gastric Cancer. 2016;19(4):1027–1040. doi:10.1007/s10120-015-0564-2
7. Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388(10060):2654–2664. doi:10.1016/S0140-6736(16)30354-3
8. Lemoine N, Adenis A, Bouche O, et al. Signet ring cells and efficacy of first-line chemotherapy in advanced gastric or oesogastric junction adenocarcinoma. Anticancer Res. 2016;36(10):5543–5549. doi:10.21873/anticanres.11138
9. Messager M, Lefevre JH, Pichot-Delahaye V, Souadka A, Piessen G, Mariette C. The impact of perioperative chemotherapy on survival in patients with gastric signet ring cell adenocarcinoma: a multicenter comparative study. Ann Surg. 2011;254(5):684–693. doi:10.1097/SLA.0b013e3182352647
10. Charalampakis N, Nogueras González GM, Elimova E, et al. The proportion of signet ring cell component in patients with localized gastric adenocarcinoma correlates with the degree of response to pre-operative chemoradiation. Oncology. 2016;90(5):239–247. doi:10.1159/000443506
11. Bekkar S, Gronnier C, Messager M, Robb WB, Piessen G, Mariette C. The impact of preoperative radiochemotherapy on survival in advanced esophagogastric junction signet ring cell adenocarcinoma. Ann Thorac Surg. 2014;97(1):303–310. doi:10.1016/j.athoracsur.2013.09.010
12. Piessen G, Messager M, Lefevre JH, et al. Signet ring cell adenocarcinomas: different clinical-pathological characteristics of oesophageal and gastric locations. Eur J Surg Oncol. 2014;40(12):1746–1755. doi:10.1016/j.ejso.2014.04.019
13. Wan Z, Huang Z, Chen L. Survival predictors associated with signet ring cell carcinoma of the esophagus (SRCCE): a population-based retrospective cohort study. PLoS One. 2017;12(7):e0181845. doi:10.1371/journal.pone.0181845
14. Heger U, Sisic L, Nienhüser H, et al. Neoadjuvant therapy improves outcomes in locally advanced signet-ring-cell containing esophagogastric adenocarcinomas. Ann Surg Oncol. 2018;25(8):2418–2427. doi:10.1245/s10434-018-6541-3
15. Stessin AM, Sison C, Schwartz A, Ng J, Chao CKS, Li B. Does adjuvant radiotherapy benefit patients with diffuse-type gastric cancer? Results from the surveillance, epidemiology, and end results database. Cancer. 2014;120(22):3562–3568. doi:10.1002/cncr.28913
16. Shu Y, Zhang W, Hou Q, et al. Prognostic significance of frequent CLDN18-ARHGAP26/6 fusion in gastric signet-ring cell cancer. Nat Commun. 2018;9(1):2447. doi:10.1038/s41467-018-04907-0
17. Ge S, Xia X, Ding C, et al. A proteomic landscape of diffuse-type gastric cancer. Nat Commun. 2018;9(1):1012. doi:10.1038/s41467-018-03121-2
18. Bosman FT, Carneiro F, Hruban RH. WHO Classification of Tumours of the Digestive System.
19. Nagtegaal ID, Odze RD, Klimstra D, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76(2):182–188. doi:10.1111/his.13975
20. Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14(2):101–112. doi:10.1007/s10120-011-0041-5
21. Nakamura K, Sugano H, Takagi K. Carcinoma of the stomach in incipient phase: its histogenesis and histological appearances. Gan. 1968;59(3):251–258.
22. Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi:10.1111/apm.1965.64.1.31
23. Sugano H, Nakamura K, Kato Y. Pathological studies of human gastric cancer. Acta Pathol Jpn. 1982;32(Suppl 2):329–347.
24. Arai T. Where does signet-ring cell carcinoma come from and where does it go? Gastric Cancer. 2019;22(4):651–652. doi:10.1007/s10120-019-00960-w
25. Hu B, El Hajj N, Sittler S, Lammert N, Barnes R, Meloni-Ehrig A. Gastric cancer: classification, histology and application of molecular pathology. J Gastrointest Oncol. 2012;3(3):251–261. doi:10.3978/j.issn.2078-6891.2012.021
26. Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513(7517):202–209. doi:10.1038/nature13480
27. van der Post RS, Gullo I, Oliveira C, et al. Histopathological, molecular, and genetic profile of hereditary diffuse gastric cancer: current knowledge and challenges for the future. Adv Exp Med Biol. 2016;908:371–391.
28. Chen J, Sun D, Chu H, et al. Screening of differential microRNA expression in gastric signet ring cell carcinoma and gastric adenocarcinoma and target gene prediction. Oncol Rep. 2015;33(6):2963–2971. doi:10.3892/or.2015.3935
29. Yan C, Yu J, Kang W, Liu Y, Ma Z, Zhou L. miR-935 suppresses gastric signet ring cell carcinoma tumorigenesis by targeting Notch1 expression. Biochem Biophys Res Commun. 2016;470(1):68–74. doi:10.1016/j.bbrc.2015.12.116
30. Aihara R, Mochiki E, Kamiyama Y, Ohno T, Asao T, Kuwano H. Matrilsin expression is a useful marker of submucosal invasion and lymph node metastasis in early stage signet ring cell carcinoma of the stomach. J Surg Oncol. 2006;93(6):491–497. doi:10.1002/jso.20439
31. Saito R, Maruyama S, Kawaguchi Y, et al. miR-99a-5p as possible diagnostic and prognostic marker in patients with gastric cancer. J Surg Res. 2020;250:193–199. doi:10.1016/j.jss.2020.01.004
32. Japanese Gastric Cancer Association jgca@ koto. kpu-m. ac. jp. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2020;1–21.
33. Pokala SK, Zhang C, Chen Z, et al. Incidence, survival, and predictors of lymph node involvement in early-stage gastric signet ring cell carcinoma in the US. J Gastrointest Surg. 2018;22(4):569–577. doi:10.1007/s11605-017-3500-4
34. Lee SH, Jee SR, Kim JH, Seol SY. Intramucosal gastric cancer: the rate of lymph node metastasis in signet ring cell carcinoma is as low as that in well-differentiated adenocarcinoma. Eur J Gastroenterol Hepatol. 2015;27(2):170–174. doi:10.1097/MEG.0000000000000258
35. Park JM, Kim SW, Nam KW, et al. Is it reasonable to treat early gastric cancer with signet ring cell histology by endoscopic resection? Analysis of factors related to lymph-node metastasis. Eur J Gastroenterol Hepatol. 2009;21(10):1132–1135. doi:10.1097/MEG.0b013e32832a21d8
36. Wang Z, Zhang X, Hu J, et al. Predictive factors for lymph node metastasis in early gastric cancer with signet ring cell histology and their impact on the surgical strategy: analysis of single institutional experience. J Surg Res. 2014;191(1):130–133. doi:10.1016/j.jss.2014.03.065
37. Kim MN, Kim HK, Shim CN, et al. Tumour size is related to the curability of signet ring cell early gastric cancer with endoscopic submucosal dissection: a retrospective single centre study. Dig Liver Dis. 2014;46(10):898–902. doi:10.1016/j.dld.2014.05.019
38. Ajani JA, D’Amico TA, Almhanna K, et al. Gastric cancer, version 3. 2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14(10):1286–1312. doi:10.6004/jnccn.2016.0137
39. Piessen G, Messager M, Leteurtre E, Jean-Pierre T, Mariette C. Signet ring cell histology is an independent predictor of poor prognosis in gastric adenocarcinoma regardless of tumoral clinical presentation. Ann Surg. 2009;250(6):878–887. doi:10.1097/SLA.0b013e3181b21c7b
40. Moehler M, Baltin CTH, Ebert M, et al. International comparison of the German evidence-based S3-guidelines on the diagnosis and multimodal treatment of early and locally advanced gastric cancer, including adenocarcinoma of the lower esophagus. Gastric Cancer. 2015;18(3):550–563. doi:10.1007/s10120-014-0403-x
41. Pernot S, Dubreuil O, Aparicio T, et al. Efficacy of a docetaxel-5FU-oxaliplatin regimen (TEFOX) in first-line treatment of advanced gastric signet ring cell carcinoma: an AGEO multicentre study. Br J Cancer. 2018;119(4):424–428. doi:10.1038/s41416-018-0133-7
42. Petrillo A, Pompella L, Tirino G, et al. Perioperative treatment in resectable gastric cancer: current perspectives and future directions. Cancers. 2019;11(3):399. doi:10.3390/cancers11030399
43. Sasako M, Sakuramoto S, Katai H, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29(33):4387–4393. doi:10.1200/JCO.2011.36.5908
44. Wei F, Lyu H, Wang S, Chu Y, Chen F. Postoperative radiotherapy improves survival in gastric signet-ring cell carcinoma: a SEER database analysis. J Gastric Cancer. 2019;19(4):393–407. doi:10.5230/jgc.2019.19.e36
45. Li Y, Ma F-H, Xue L-Y, Tian Y-T. Neoadjuvant chemotherapy upfront surgery for gastric signet ring cell carcinoma: a retrospective, propensity score-matched study. World J Gastroenterol. 2020;26(8):818–827. doi:10.3748/wjg.v26.i8.818
46. Shi T, Song X, Liu Q, et al. Survival benefit of palliative gastrectomy followed by chemotherapy in stage IV gastric signet ring cell carcinoma patients: a large population-based study. Cancer Med. 2019;8(13):6010–6020. doi:10.1002/cam4.2521
47. Smyth EC, Wotherspoon A, Peckitt C, et al. Mismatch repair deficiency, microsatellite instability, and survival: an exploratory analysis of the medical research council adjuvant gastric infusional chemotherapy (MAGIC) trial. JAMA Oncol. 2017;3(9):1197–1203. doi:10.1001/jamaoncol.2016.6762
48. Lee J, Lim DH, Kim S, et al. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol. 2012;30(3):268–273. doi:10.1200/JCO.2011.39.1953
49. Smalley SR, Benedetti JK, Haller DG, et al. Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol. 2012;30(19):2327–2333. doi:10.1200/JCO.2011.36.7136
50. Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345(10):725–730. doi:10.1056/NEJMoa010187
51. Shi C, Yang B, Chen Q, Yang J, Fan N. Retrospective analysis of adjuvant intraperitoneal chemotherapy effect prognosis of resectable gastric cancer. Oncology. 2011;80(5–6):289–295. doi:10.1159/000329075
52. Königsrainer I, Horvath P, Struller F, Königsrainer A, Beckert S. Initial clinical experience with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in signet-ring cell gastric cancer with peritoneal metastases. J Gastric Cancer. 2014;14(2):117–122. doi:10.5230/jgc.2014.14.2.117
53. Yan K, Wu K, Yan L, Liang L, Yuan Y. Efficacy of postoperative intraperitoneal hyperthermic perfusion chemotherapy with oxaliplatin + 5-Fluorouracil in the treatment of gastric cancer patients with peritoneal carcinomatosis. J BUON. 2019;24(4):1587–1594.
54. Li C, Kim S, Lai JF, et al. Advanced gastric carcinoma with signet ring cell histology. Oncology. 2007;72(1–2):64–68. doi:10.1159/000111096
55. Zhang M, Zhu G, Zhang H, Gao H, Xue Y. Clinicopathologic features of gastric carcinoma with signet ring cell histology. J Gastrointest Surg. 2010;14(4):601–606. doi:10.1007/s11605-009-1127-9
56. Glehen O, Passot G, Villeneuve L, et al. GASTRICHIP: D2 resection and hyperthermic intraperitoneal chemotherapy in locally advanced gastric carcinoma: a randomized and multicenter phase III study. BMC Cancer. 2014;14:183. doi:10.1186/1471-2407-14-183
57. Yasue M, Murakami M, Nakazato H, Suchi T, Ota K. A controlled study of maintenance chemoimmunotherapy vs immunotherapy alone immediately following palliative gastrectomy and induction chemoimmunotherapy for advanced gastric cancer. Tokai cooperative study group for adjuvant chemoimmunotherapy of stomach cancer. Cancer Chemother Pharmacol. 1981;7(1):5–10.
58. Hirotsu Y, Mochizuki H, Amemiya K, et al. Deficiency of mismatch repair genes is less frequently observed in signet ring cell compared with non-signet ring cell gastric cancer. Med Oncol. 2019;36(3):23. doi:10.1007/s12032-019-1246-4
59. Piessen G, Messager M, Le Malicot K, et al. Phase II/III multicentre randomised controlled trial evaluating a strategy of primary surgery and adjuvant chemotherapy versus peri-operative chemotherapy for resectable gastric signet ring cell adenocarcinomas - PRODIGE 19 - FFCD1103 - ADCI002. BMC Cancer. 2013;13:281. doi:10.1186/1471-2407-13-281
60. Liang H. XELOX plus apatinib vs XELOX as post-operative chemotherapy in locally advanced gastric signet ring carcinoma. Published 2017. Available from: https://clinicaltrials.gov/ct2/show/NCT03355612?term=NCT03355612. NLM identifier: NCT03355612.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
