Back to Journals » OncoTargets and Therapy » Volume 13
γ-Catenin Overexpression in AML Patients May Promote Tumor Cell Survival via Activation of the Wnt/β-Catenin Axis
Authors Qian J, Huang X, Zhang Y, Ye X, Qian W
Received 13 September 2019
Accepted for publication 3 February 2020
Published 12 February 2020 Volume 2020:13 Pages 1265—1276
DOI https://doi.org/10.2147/OTT.S230873
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Jianmin Xu
Jiejin Qian, 1,* Xianbo Huang, 1,* Yinyin Zhang, 1, 2 Xiujin Ye, 1 Wenbin Qian 1, 2
1Department of Hematology, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou 310003, People’s Republic of China; 2Malignant Lymphoma Diagnosis and Therapy Center, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou 310003, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wenbin Qian; Xiujin Ye
Institute of Hematology, The First Affiliated Hospital, College of Medicine, Zhejiang University, 79# Qingchun Road, Hangzhou 310003, People’s Republic of China
Tel +86-571-87235983
; +86-571-87235875
Fax +86-571-87235560
Email [email protected]; [email protected]
Background: Canonical Wnt/β-catenin signaling is frequently dysregulated in acute myeloid leukemia (AML) and has been implicated in leukemogenesis. γ-catenin was previously demonstrated to be associated with the nuclear localization of β-catenin, the central mediator, and to exert oncogenic effects in AML; however, the underlying mechanisms remain unclear. Our study aimed to investigate the expression characteristics of γ-catenin in AML patients, explore the mechanisms by which γ-catenin regulates β-catenin, and discuss the feasibility of targeting γ-catenin for AML treatment.
Methods: The mRNA expression levels of γ-catenin in AML patients were measured by qRT-PCR. Cell proliferation was examined via Cell Counting Kit-8 (CCK-8) assays. The expression levels of related proteins were measured via Western blotting. Specific siRNA was used to modulate the expression level of the γ-catenin gene. Apoptosis and cell cycle distribution were quantified by flow cytometry. The subcellular localization of γ-catenin and β-catenin was examined via immunofluorescence with a confocal laser scanning microscope.
Results: Overexpression of γ-catenin was frequently observed in AML and correlated with poor prognosis. Consistent with this finding, suppression of γ-catenin in the AML cell line THP-1 induced growth inhibition, promoted apoptosis and blocked β-catenin nuclear translocation. Interestingly, γ-catenin knockdown sensitized THP-1 cells to cytotoxic chemotherapeutic agents such as cytarabine and homoharringtonine and further inhibited β-catenin nuclear localization. Moreover, our data implied the relationship between γ-catenin and GSK3β (whose effect on β-catenin is mediated by its own phosphorylation), which may be the principal mechanism underlying the anti-AML effect of γ-catenin inhibition.
Conclusion: Taken together, our results revealed a potential role of γ-catenin in AML pathogenesis–mainly through the inhibition of GSK3β-mediated nuclear localization of β-catenin–and indicate that targeting γ-catenin might offer new AML treatments.
Keywords: acute myeloid leukemia, γ-catenin, β-catenin, chemotherapy
Introduction
Acute myeloid leukemia (AML) is the most common form of leukemia and arises from the clonal expansion of transformed pluripotent hematopoietic stem cells that cannot differentiate. AML is also a heterogeneous disease with a remarkable array of genomic alterations.1,2 Despite the major advances in understanding the genetic landscape of AML, current standard therapies, which are based on intensive chemotherapy and allogeneic hematopoietic stem cell transplantation, have not significantly improved clinical outcomes.3,4 Thus, most AML patients experience relapse.4 Thus, more effective and less toxic treatment strategies for AML patients are urgently required.
Wnt signaling has been demonstrated to play an essential role in regulating cell proliferation, survival, and differentiation. The canonical Wnt signaling pathway is maintained in a suppressed state under basal conditions through constitutive degradation of the central mediator, β-catenin. A destruction complex comprising CK-1, GSK3β, axin-1 and APC mediates the proteasomal degradation of β-catenin in the cytoplasm.5 Activation of Wnt signaling dephosphorylates and stabilizes β-catenin, resulting in its nuclear translocation. After translocation to the nucleus, unphosphorylated β-catenin can associate with the co-transcriptional regulator T-cell factor (TCF)/lymphoid enhancer factor (LEF) and promote the overexpression and activation of proto-oncogenic Wnt target genes such as c-Myc, Cyclin D1 and survivin.6,7 Accumulating evidence shows that leukemia stem cells (LSCs) drive the initiation and perpetuation of AML as well as chemotherapeutic resistance. LSCs are also the major clinical factors in disease progression and relapse.8 Aberrant Wnt/β-catenin pathway activity has been demonstrated to be essential for AML initiation and progression and is required for LSC self-renewal and survival.9,10 Recent preclinical studies indicate that inhibiting the Wnt pathway is promising for AML treatment,6,11 which implies that targeting the Wnt pathway may also eliminate the LSC population in AML.
As the central mediator of Wnt signaling, β-catenin is frequently overexpressed in AML,12 and its expression correlates with inferior survival.13 Wnt signaling activation is dependent on the nuclear translocation of β-catenin, which is also frequently observed in AML.14 However, some patients exhibit little or no nuclear β-catenin, even when cytosolic β-catenin is abundant. Therefore, control of the subcellular localization of β-catenin is an alternative mechanism regulating abnormal Wnt pathway activation. Previous studies have demonstrated that elevated γ-catenin expression promotes the stabilization and nuclear localization of β-catenin and exerts oncogenic effects in AML.12,15 γ-catenin (also known as plakoglobin) is a member of the catenin family that shares high structural and functional homology with β-catenin. Abnormal expression of the γ-catenin gene reportedly occurs in various hematologic malignancies and numerous solid tumors. However, the roles of γ-catenin in different types of tumors are varied or even contradictory.16 γ-catenin was found to be upregulated in AML via AML-specific fusion proteins such as AML1-ETO, PML-RARα, and PLZF-RARα.15,17 In addition, ectopic expression of γ-catenin was found to accelerate cell cycle progression in murine hematopoietic stem/progenitor cells (HSPCs) and to promote their self-renewal and leukemogenic capacities.17 Thus, γ-catenin regulation could be an alternative or additional mechanism of Wnt activation in AML. These observations also highlighted the importance of γ-catenin as a target for the selective killing of AML cells. Our current study investigated the expression levels of γ-catenin in AML patients and evaluated the prognostic significance of γ-catenin. Then, we explored the function of γ-catenin in the pathogenesis of AML, hypothesizing that γ-catenin is a potential therapeutic target in AML.
Materials and Methods
Patients and Samples
Between March 2009 and December 2014, bone marrow (BM) mononuclear cells (MCs) were obtained from 98 untreated patients with primary AML and 21 normal persons at the First Affiliated Hospital of Zhejiang University. Informed consent was provided by all patients or their legally authorized representatives according to the Declaration of Helsinki principles for cryopreservation and medical research. All methods used in this study were in accordance with the guidelines approved by the Institutional Review Board of the First Affiliated Hospital of Zhejiang University. Detailed information about the patients is listed in Table 1. A total of 21 healthy Chinese volunteers of Han nationality who were unrelated residents of Zhejiang Province, the People’s Republic of China, were also recruited. MCs were isolated from the BM samples via Ficoll-Paque (Haoyang Biotech, Tianjin, China) density gradient centrifugation according to the manufacturer’s instructions and stored at −80°C until further use.
 |
Table 1 The Characteristics of the Two Subgroups of Patients Stratified by the γ-Catenin mRNA Expression Level (n=98) |
Risk Classifications
The traditional cytogenetic analysis of AML was performed in patients at the time of diagnosis. According to the criteria of the NCCN Guidelines Version 2.2016 for AML, all patients were allocated to three risk statuses as follows: low risk, inv(16) or t(16;16) or t(8;21) or t(15;17); intermediate risk, normal cytogenetics or +8 alone or t(9;11) or other undefined cytogenetics; and high risk, complex aberrations (≥3 clonal chromosomal abnormalities), monosomal karyotype, or −5, 5q-, −7, 7q-, 11q23, - non t(9;11), inv(3), t(3;3), t(6;9), t(9;22).
Cell Culture and Agents
The human AML cell line THP-1 was obtained from the China Center for Type Culture Collection (CCTCC, Wuhan, China). THP-1 cells and human primary cells were cultured in RPMI 1640 medium (Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS; Gibco) and maintained at 37°C in a humidified atmosphere containing 5% CO2. The chemotherapeutic drugs cytarabine (Ara-C), homoharringtonine (HHT) and daunorubicin (DNR) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
RNA Isolation and Semiquantitative RT-PCR
Total RNA extraction was carried out with RNAiso Plus (Takara Shuzo, Kyoto, Japan). Agarose gel electrophoresis and spectrophotometric analysis (A260:280 nm ratio) were used to evaluate RNA quality and quantity. RNA was stored at −80°C prior to use. cDNA templates were generated from total RNA using an M-MLV reverse transcriptase kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Assessment of γ-catenin mRNA expression was then carried out by quantitative RT-PCR (qRT-PCR). GAPDH served as a control to determine the relative levels of γ-catenin. The following specific primers were used to amplify the indicated genes: γ-catenin, 5′-GCCTGGAGAGTGTGCTGAAG-3′ (forward) and-5′-GCGTCTTGT TCTTGCTGTTG-3′ (reverse); and GAPDH, 5′-AAGGTGAAGGTCGGAGTCA-3′ (forward) and 5′-GGAAGATGGTGATGGGATTT-3′ (reverse). Experiments were performed in triplicate. The relative expression level was calculated using the 2−ΔΔCT method.
Knockdown of γ-Catenin via siRNA
A specific siRNA against γ-catenin and negative control (NC) siRNA were purchased from Hanheng Biotechnology (Shanghai, China). Before transfection, THP-1 cells were cultured in RPMI 1640 medium containing 10% FBS. Transient transfection was performed using Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Cellular γ-catenin expression was evaluated via Western blot analysis, and cells with γ-catenin knockdown were then collected for further experiments. The siRNA sequences were as follows: γ-catenin, 5ʹ-AGCUGAUCAUCCUGGCCAATT-3ʹ; and NC siRNA, 5ʹ-UUCUCCGAACGUGUCACGUTT-3ʹ.
Cell Viability Assay
Cell viability was measured with a Cell Counting Kit-8 (CCK-8; Dojindo, Tokyo, Japan). THP-1 cells were plated in 96-well plates (1×105 cells/mL) and treated for 24 h with the following reagents: γ-catenin siRNA, NC siRNA, γ-catenin siRNA + Ara-C, γ-catenin siRNA + HHT and γ-catenin siRNA + DNR. The absorbance at 450 nm was measured with a microplate spectrophotometer (Bio-Rad, Hercules, CA, USA).
Flow Cytometry
Cells were treated with different reagents and cultured at 37°C for 24 h. Cells were then collected, fixed with 70% ethanol on ice for 20 min, and centrifuged. After the cells were subjected to propidium iodide (PI) and Annexin V-FITC double staining using a detection kit (BD Pharmingen, San Diego, CA, USA) according to the manufacturer’s instructions, the number of apoptotic cells was quantified. The cell cycle distribution was determined by quantitation of cellular DNA content via PI (MultiSciences Biotech, China) staining. The stained cells were analyzed on a flow cytometer (Accuri C6; BD, Franklin Lakes, NJ, USA), and the data were analyzed with FlowJo software (TreeStar, Ashland, OR, USA).
Western Blot Analysis
Total protein was extracted from the collected cells. Cytoplasmic and nuclear proteins were extracted using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher Scientific, Rockford, IL, USA) according to the manufacturer’s instructions. Equal amounts of protein were separated via sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and were then transferred onto polyvinylidene fluoride (PVDF) membranes (Merck Millipore). Membranes were subsequently blocked for 2 h in Tris-buffered saline containing 0.1% Tween and 5% nonfat dry milk followed by incubation with primary antibodies overnight at 4°C. After a second incubation with horseradish peroxidase-conjugated secondary antibodies (1:5000; Multi Sciences Biotech, Hangzhou, China), the bands on the PVDF membranes were developed using enhanced chemiluminescence (ECL; Biological Industries, Beit HaEmek, Israel). All primary antibodies (anti-γ-catenin, anti-β-catenin, anti-Cyclin D1, anti-c-Myc, anti-GSK3β, anti-phospho-GSK3β, anti-Histone H1 and anti-β-actin) were obtained from Cell Signaling Technology (Beverly, MA, USA).
Detection of Caspase-3 Activity
Cells were treated with the indicated agents and cultured at 37°C for 24 h. Caspase-3 activity was measured using a caspase-3/CPP32 colorimetric assay kit (BioVision, Mountain View, CA) according to the manufacturer’s instructions. The absorbance at 405 nm was assessed with a microplate spectrophotometer (Bio-Rad), and the results are expressed as a fold increase over the basal level (THP-1 NC cells).
Immunofluorescence Studies
Cells were treated with different agents and then fixed with 4% formaldehyde. After permeabilization with 0.3% Triton X-100 and blocking with goat serum (Dawen Biotech, Hangzhou, China), cells were incubated with the indicated primary antibodies overnight at 4°C. Next, cells were incubated with AlexaFluor 488 (green)- or 647 (red)-labeled secondary antibodies (Abcam). After the cells were washed with PBS, the nuclei were counterstained with DAPI (Southern Biotech, AL, USA). Fluorescence was observed under a Nikon S1 confocal laser scanning microscope.
Statistical Analysis
Data are presented as the means ± standard deviations (SDs). Statistical analysis was performed with χ2 tests and Student’s t-tests. The cumulative survival rate was calculated with the Kaplan-Meier method, and the statistical significance of the rates was analyzed by the Log rank test. Single-sample t-test(1) was used to find a suitable cut-off to distinguish between “high” and “low” γ-catenin expression. A P-value of <0.05 was considered statistically significant. All statistical analyses were performed using SPSS software version 22.0 (SPSS Inc., Chicago, IL, USA). Graphs were plotted using GraphPad Prism software version 7.0 (GraphPad Software, Inc., La Jolla, CA, USA).
(1)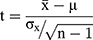
(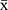 : sample mean;
: sample mean; 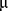 : population mean;
: population mean; 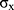 : sample variance;
: sample variance;  : sample size)
: sample size)
Results
Expression of γ-Catenin in AML Patients
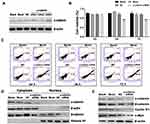
The mRNA levels of γ-catenin in BMMCs from 98 AML (non-M3) patients and 21 healthy persons were measured by qRT-PCR. Compared with healthy individuals (mean relative mRNA expression = 0.0326), AML patients (mean relative mRNA expression = 0.0486) exhibited significantly higher expression levels of γ-catenin mRNA (**P<0.01, Figure 1A).
Correlation Analysis Between γ-Catenin mRNA Expression and AML Clinical Characteristics
In this study, we used single-sample t-test to find the minimum mean value which can distinguish between the normal and abnormal γ-catenin expression level. The γ-catenin expression of 21 healthy individuals were regarded as the control sample, and then 0.0408 was determined to be the cut-off value, which was significant at the 5% level. Thus, patients with γ-catenin mRNA levels higher than 0.0408 were regarded as having a “high level”. Those with γ-catenin mRNA levels lower than 0.0408 were considered to be similar to healthy individuals and were regarded as having a “low level”. Therefore, the 98 AML patients were divided into two subgroups: a low γ-catenin expression group (52/98; relative γ-catenin mRNA expression < 0.0408) and a high γ-catenin expression group (46/98; relative γ-catenin mRNA expression ≥ 0.0408). The clinical characteristics of the two subgroups are summarized in Table 1. Significant correlations with the γ-catenin expression level were established (P<0.05) for the presence of the fusion gene AML1-ETO and FLT3-ITD and NPM1 mutations. However, γ-catenin expression was not correlated with age, sex, blast count, white blood cell (WBC) count, hemoglobin (Hb) level, platelet (Plt) count, French-American-British (FAB) type or chromosome anomaly (all P>0.05). Additionally, no difference was revealed in the AML risk classifications of patients with high or low γ-catenin expression (all P>0.05).
We further analyzed the prognosis of the 98 non-M3 AML patients with low or high γ-catenin expression levels. The median overall survival (OS) in the low and high γ-catenin expression groups was 27.4 months (ranging from 3 to 63 months) and 12.6 months (ranging from 1 to 38 months), respectively. The survival rate of these patients was 27.8% in the high γ-catenin expression group and 49.7% in the low γ-catenin expression group at the end of the follow-up period, with significant differences observed between the two subgroups (P<0.05, Figure 1B).
Knockdown of γ-Catenin Inhibited Proliferation and Induced Apoptosis of the AML Cell Line THP-1 While Blocking β-Catenin Nuclear Translocation
To examine the effect of γ-catenin knockdown in AML cells, THP-1 cells were transfected with a γ-catenin-specific siRNA to robustly decrease the γ-catenin protein level. The siRNA with the highest interference efficiency (siRNA2) was selected for subsequent experiments (Figure 2A). Based on the results of the CCK-8 assays and flow cytometric analysis, γ-catenin knockdown clearly inhibited proliferation and induced apoptosis in THP-1 cells in a time-dependent manner (Figure 2B and C). Previous studies demonstrated that elevated expression of γ-catenin promotes the stabilization and nuclear localization of β-catenin, which subsequently associates with the co-transcriptional regulator TCF/LEF to mediate the activation of Wnt target genes such as c-Myc and Cyclin D1 and exerts oncogenic effects in AML.12,15 In our study, a significant reduction in β-catenin protein expression was observed in both the cytosolic (C) and nuclear (N) fractions of THP-1 cells with γ-catenin knockdown (Figure 2D). In addition, the expression levels of downstream targets of β-catenin, including c-Myc and Cyclin D1, were decreased (Figure 2E). Specifically, downregulation of γ-catenin inhibited the nuclear translocation and carcinogenic activities of β-catenin in AML cells.
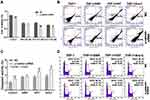
Knockdown of γ-Catenin Sensitized THP-1 Cells to the Antileukemic Effects of Chemotherapeutic Agents
Currently, chemotherapy remains the cornerstone of clinical AML treatment. Thus, we investigated whether the strategy of combining traditional chemotherapeutic agents with γ-catenin knockdown enhanced the effectiveness of the agents in treating AML. In the current study, γ-catenin knockdown THP-1 cells were treated with appropriate concentrations of Ara-C (20 μM), HHT (2.5 nM) and DNR (40 nM). As shown in Figure 3A, the combination of γ-catenin silencing and Ara-C or HHT treatment was more efficacious than either drug alone in inhibiting the viability of THP-1 cells. In addition, apoptosis and the cell cycle distribution were quantified via PI staining and/or Annexin V binding. The combination of γ-catenin silencing with HHT and Ara-C elicited apoptosis in 39.6% and 48.6% of THP-1 cells, respectively, in contrast to treatment with either agent alone (13.3% and 32.7%, respectively; P<0.05) (Figure 3B). However, cotreatment with DNR did not show an enhanced effect in γ-catenin knockdown AML cells (Figure 3A and B). We further evaluated apoptosis using the caspase-3 activation assay. As illustrated in Figure 3C, treatment of γ-catenin knockdown THP-1 cells with Ara-C or HHT resulted in enhanced caspase-3 cleavage, which was consistent with the above observations. Cell cycle analysis showed G2/M phase arrest in THP-1 cells treated with DNR or Ara-C alone and showed S phase arrest in cells treated with HHT. In γ-catenin knockdown THP-1 cells treated with HHT or Ara-C, the cell cycle was arrested at G0/G1 phase. However, the cell cycle distribution was unchanged in γ-catenin knockdown cells treated with DNR (Figure 3D). Therefore, our data suggested that γ-catenin downregulation can exert a chemosensitizing effect on THP-1 cells to chemotherapeutic drugs such as HHT or Ara-C.
Cotreatment with Chemotherapeutic Agents Further Suppressed the Activity and Nuclear Translocation of β-Catenin in THP-1 Cells
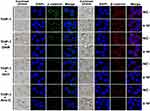
To further explore the molecular mechanisms by which γ-catenin inhibition improves the effect of chemotherapeutic agents, we conducted Western blotting and immunofluorescence analyses. Our data suggested a further decrease in β-catenin protein levels in both the cytoplasm and nucleus of γ-catenin knockdown THP-1 cells treated with HHT or Ara-C; this decrease was especially apparent in the nucleus (Figure 4A). Previous studies have shown that cytoplasmic β-catenin, the central mediator of the “canonical” Wnt pathway, can be degraded by the APC/Axin-GSK3β complex. In this complex, GSK3β functions to facilitate the phosphorylation of β-catenin and ubiquitin-mediated degradation of β-catenin, and GSK3β activity is abrogated by its phosphorylation. Generally, activated Wnt signaling can lead to the dissociation of the APC/Axin-GSK-3β complex through various mechanisms, thus inhibiting the phosphorylation and degradation of β-catenin.5 In the current study, downregulation of γ-catenin in THP-1 cells significantly increased GSK3β expression and inhibited its phosphorylation, suggesting that GSK3β inactivation, which is the prerequisite and foundation for the oncogenicity of β-catenin, is driven by γ-catenin (Figure 4B). The above data imply the relationship between γ-catenin and GSK3β, which may be the principal mechanism underlying the anti-AML effect of γ-catenin inhibition. Next, we found that treatment of γ-catenin knockdown THP-1 cells with HHT or Ara-C also resulted in enhanced downregulation of molecules downstream of β-catenin (c-Myc and Cyclin D1) (Figure 4B). However, we found that among cells with γ-catenin knockdown, the THP-1+DNR group showed decreased levels of γ-catenin in the nucleus compared to those of THP-1 alone, and c-Myc and cyclin D1 also further decreased these levels. These effects may relate to the nonsignificant effects of DNR. To further verify our finding, we performed an immunofluorescence assay, which showed that downregulation of γ-catenin (green) can greatly limit the nuclear translocation ability of β-catenin (red) in THP-1 cells. Similarly, the nuclear translocation ability of β-catenin was decreased further when cells were incubated with HHT or Ara-C (Figure 5).
Discussion
The Wnt signaling pathway plays a critical role in the regulation of early embryogenesis and is progressively deactivated in differentiated mature cells.18 This pathway is also active during normal hematopoietic development. Wnt signaling pathways are generally termed “canonical” pathways, are β-catenin-dependent and are well defined. Multiple Wnt genes have been identified as expressed in human hematopoiesis, and these genes can influence the proliferation and differentiation of HSPCs.19,20 Dysregulation of Wnt signaling has been reported to play a crucial role in the pathological progression of numerous hematological malignancies, including AML.10
As the central mediator of Wnt signaling, β-catenin has received much attention because of its leukemogenic role,9,21 prognostic influence13,22 and functional redundancy during normal hematopoietic development.23 Recently, it has been thoroughly demonstrated that the key to Wnt signaling is the translocation of β-catenin into the nucleus; moreover, this process is frequently observed in AML.14
To further investigate the mechanisms that affect the nuclear localization of β-catenin, we focused on γ-catenin, which shares high structural and functional homology with β-catenin and is one of the most important molecules that interacts with β-catenin in the canonical Wnt cascade.24 Unlike the clearly defined oncogenic role of β-catenin, the role of γ-catenin in Wnt signaling and AML development/progression remains controversial. Some reports suggest that γ-catenin is dysregulated in AML and is linked to the AML pathogenesis.17,25 However, to date, few systematic studies have addressed the role and therapeutic potential of γ-catenin in AML.
In this study, we compared γ-catenin mRNA levels in BMMCs from primary AML patients and healthy donors, investigated the potential correlation between γ-catenin and various clinical parameters, and analyzed the clinical significance of γ-catenin in AML. Our results suggested that the γ-catenin mRNA expression level is significantly higher in AML patients than in healthy donors, consistent with findings of previous studies.17,24,25 Our data further showed that AML patients with high γ-catenin expression levels had significantly shorter OS times than did patients with low γ-catenin expression levels. The data reported by Xu et al also indicated that AML patients with lower γ-catenin levels were more likely to achieve complete remission than were patients who have higher γ-catenin levels.16 Therefore, γ-catenin overexpression is important in AML and is associated with poor clinical prognosis. Interestingly, more patients in the low γ-catenin expression group had NPM1 mutations, while FLT3-ITD mutations and AML1-ETO fusion genes were more common in the high γ-catenin expression group. NPM1 mutations are associated with a favorable prognosis in AML when the ITD in the FLT3 gene is absent (FLT3-ITDneg) or present in a low allelic ratio (FLT3-ITDlow),26 and the presence of the AML1-ETO fusion gene usually indicates a better prognosis for patients with AML.27 In addition, a previous study also reported that patients with a CEBPα mutation (implying low risk) had higher γ-catenin levels than did those in the unmutated group.16 Thus, it is necessary to better clarify the specific relationship between γ-catenin expression and AML development and progression.
Furthermore, we conducted an in vitro study in the human AML cell line THP-1. Downregulation of γ-catenin significantly reduced cell viability and induced apoptosis and cell cycle arrest in THP-1 cells. Elevated γ-catenin expression has been demonstrated to promote the stabilization and nuclear localization of β-catenin.24 Our study also showed a significant decrease in β-catenin protein levels in both the cytoplasm and nucleus in γ-catenin knockdown THP-1 cells, which was accompanied by a reduction in the levels of the downstream oncogenes c-Myc and Cyclin D1, as suggested in a previous study.12 Moreover, the immunofluorescence assay clearly showed that β-catenin nuclear translocation can be blocked by downregulating γ-catenin. Therefore, γ-catenin may exert leukemogenic activities by affecting β-catenin in the Wnt pathway. Interestingly, downregulation of γ-catenin enhanced the toxicity of the chemotherapeutic agents Ara-C and HHT to THP-1 cells. As classical chemotherapeutic drugs, Ara-C and HHT are widely used in the clinical treatment of AML patients. Our study innovatively indicates that downregulating γ-catenin can sensitize AML cells to the cytotoxic effects of routinely used chemotherapeutic drugs.
GSK3β can function as a regulator to facilitate phosphorylation and the subsequent ubiquitin-mediated degradation of β-catenin, resulting in β-catenin inactivation.5 Through in-depth research, we found that γ-catenin downregulation in THP-1 cells can significantly reduce the levels of GSK3β, an important modulator of β-catenin. Treatment of these knockdown cells with HHT or Ara-C resulted in enhanced GSK3β inhibition, leading to a reduction in the nuclear translocation of β-catenin as well as in the levels of its downstream targets. Thus, it can be concluded that regulating β-catenin through GSK3β may be the principal mechanism by which γ-catenin affects the development and progression of AML.
Conclusion
Our study showed that γ-catenin was significantly overexpressed in AML patients compared to healthy donors. Patients with lower levels of γ-catenin expression were more likely to have better prognoses. Downregulation of γ-catenin significantly inhibited the growth and induced apoptosis of THP-1 AML cells mainly by limiting the GSK3β-mediated nuclear translocation of β-catenin. Additionally, this effect was enhanced by the chemotherapeutic drugs HHT and Ara-C. However, further investigation is required to more completely elucidate the mechanisms by which γ-catenin regulates AML cell survival and invasion as well as the mechanisms underlying its ability to enhance the effects of chemotherapeutic drugs, which could hold therapeutic interest.
Ethics Approval and Informed Consent
The Ethics Committee of the First Affiliated Hospital of Zhejiang University (Hangzhou, China) approved the use of human specimens in the current study. Written informed consent was obtained from all patients. All methods and experimental protocols were approved by the First Affiliated Hospital of Zhejiang University.
Acknowledgments
This research was supported by funds from the Natural Science Foundation of China (No. 81900152), the Natural Science Foundation of Zhejiang Province (No. LQ19H080005), and the Science Technology Department of Zhejiang Province (No. 2016C33137 and No. 2018C03016-1).
Author Contributions
WBQ and XJY designed the study; JJQ, XBH and YYZ conducted the study; XBH and JJQ analyzed the data; XBH drafted the manuscript. Critical revision of the manuscript was done by XJY and WBQ. All authors contributed to the data analysis and drafting or revising of the article; gave final approval of the version to be published; and agreed to be accountable for all aspects of this work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi:10.3322/caac.21442
2. Short NJ, Rytting ME, Cortes JE. Acute myeloid leukaemia. Lancet. 2018;392(10147):593–606. doi:10.1016/S0140-6736(18)31041-9
3. Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136–1152. doi:10.1056/NEJMra1406184
4. Thol F, Schlenk RF, Heuser M, Ganser A. How I treat refractory and early relapsed acute myeloid leukemia. Blood. 2015;126(3):319–327. doi:10.1182/blood-2014-10-551911
5. Nusse R, Clevers H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169(6):985–999. doi:10.1016/j.cell.2017.05.016
6. Fiskus W, Sharma S, Saha S, et al. Pre-clinical efficacy of combined therapy with novel β-catenin antagonist BC2059 and histone deacetylase inhibitor against AML cells. Leukemia. 2015;29(6):1267–1278. doi:10.1038/leu.2014.340
7. Morgan RG, Ridsdale J, Payne M, et al. LEF-1 drives aberrant β-catenin nuclear localization in myeloid leukemia cells. Haematologica. 2019;104(7):1365–1377. doi:10.3324/haematol.2018.202846
8. Tabe Y, Konopleva M. Advances in understanding the leukaemia microenvironment. Br J Haematol. 2014;164(6):767–778. doi:10.1111/bjh.12725
9. Wang Y, Krivtsov AV, Sinha AU, et al. The Wnt/beta-catenin pathway is required for the development of leukemia stem cells in AML. Science. 2010;327(5973):1650–1653. doi:10.1126/science.1186624
10. Lane SW, Wang YJ, Lo Celso C, et al. Differential niche and Wnt requirements during acute myeloid leukemia progression. Blood. 2011;118(10):2849–2856. doi:10.1182/blood-2011-03-345165
11. Ma S, Yang LL, Niu T, et al. SKLB-677, an FLT3 and Wnt/β-catenin signaling inhibitor, displays potent activity in models of FLT3-driven AML. Sci Rep. 2015;5:15646.
12. Simon M, Grandage VL, Linch DC, Khwaja A. Constitutive activation of the Wnt/beta-catenin signalling pathway in acute myeloid leukaemia. Oncogene. 2005;24(14):2410–2420. doi:10.1038/sj.onc.1208431
13. Ysebaert L, Chicanne G, Demur C, et al. Expression of beta-catenin by acute myeloid leukemia cells predicts enhanced clonogenic capacities and poor prognosis. Leukemia. 2006;20(7):1211–1216. doi:10.1038/sj.leu.2404239
14. Griffiths EA, Golding MC, Srivastava P, et al. Pharmacological targeting of β-catenin in normal karyotype acute myeloid leukemia blasts. Haematologica. 2015;100(2):e49–52. doi:10.3324/haematol.2014.113118
15. Majeti R, Becker MW, Tian Q, et al. Dysregulated gene expression networks in human acute myelogenous leukemia stem cells. Proc Natl Acad Sci U S A. 2009;106(9):3396–3401. doi:10.1073/pnas.0900089106
16. Xu J, Wu W, Shen W, Liu P. The clinical significance of γ-catenin in acute myeloid leukemia. Onco Targets Ther. 2016;9:3861–3871. doi:10.2147/OTT.S105514
17. Zheng X, Beissert T, Kukoc-Zivojnov N, et al. Gamma-catenin contributes to leukemogenesis induced by AML-associated translocation products by increasing the self-renewal of very primitive progenitor cells. Blood. 2004;103(9):3535–3543. doi:10.1182/blood-2003-09-3335
18. Staal FJ, Clevers HC. WNT signalling and haematopoiesis: a WNT-WNT situation. Nat Rev Immunol. 2005;5(1):21–30. doi:10.1038/nri1529
19. Staal FJ, Luis TC. Wnt signaling in hematopoiesis: crucial factors for self-renewal, proliferation, and cell fate decisions. J Cell Biochem. 2010;109(5):844–849. doi:10.1002/jcb.22467
20. Sturgeon CM, Ditadi A, Awong G, Kennedy M, Keller G. Wnt signaling controls the specification of definitive and primitive hematopoiesis from human pluripotent stem cells. Nat Biotechnol. 2014;32(6):554–561. doi:10.1038/nbt.2915
21. Zhao C, Blum J, Chen A, et al. Loss of beta-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell. 2007;12(6):528–541. doi:10.1016/j.ccr.2007.11.003
22. Xu J, Suzuki M, Niwa Y, et al. Clinical significance of nuclear non-phosphorylated beta-catenin in acute myeloid leukaemia and myelodysplastic syndrome. Br J Haematol. 2008;140(4):394–401. doi:10.1111/j.1365-2141.2007.06914.x
23. Koch U, Wilson A, Cobas M, Kemler R, Macdonald HR, Radtke F. Simultaneous loss of beta- and gamma-catenin does not perturb hematopoiesis or lymphopoiesis. Blood. 2008;111(1):160–164. doi:10.1182/blood-2007-07-099754
24. Morgan RG, Pearn L, Liddiard K, et al. γ-Catenin is overexpressed in acute myeloid leukemia and promotes the stabilization and nuclear localization of β-catenin. Leukemia. 2013;27(2):336–343. doi:10.1038/leu.2012.221
25. Müller-Tidow C, Steffen B, Cauvet T, et al. Translocation products in acute myeloid leukemia activate the Wnt signaling pathway in hematopoietic cells. Mol Cell Biol. 2004;24(7):2890–2904. doi:10.1128/MCB.24.7.2890-2904.2004
26. Angenendt L, Röllig C, Montesinos P, et al. Chromosomal abnormalities and prognosis in NPM1-mutated acute myeloid leukemia: a pooled analysis of individual patient data from nine international cohorts. J Clin Oncol. 2019;JCO1900416.
27. Tian Y, Wang G, Hu Q, Xiao X, Chen S. AML1/ETO trans-activates c-KIT expression through the long range interaction between promoter and intronic enhancer. J Cell Biochem. 2018;119(4):3706–3715. doi:10.1002/jcb.v119.4
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.