Back to Journals » Journal of Inflammation Research » Volume 13
Galangin Inhibits LPS-Induced MMP-9 Expression via Suppressing Protein Kinase-Dependent AP-1 and FoxO1 Activation in Rat Brain Astrocytes
Authors Yang CC , Hsiao LD, Yang CM
Received 24 August 2020
Accepted for publication 22 October 2020
Published 20 November 2020 Volume 2020:13 Pages 945—960
DOI https://doi.org/10.2147/JIR.S276925
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Chien-Chung Yang,1,2 Li-Der Hsiao,3 Chuen-Mao Yang3– 5
1Department of Traditional Chinese Medicine, Chang Gung Memorial Hospital at Tao-Yuan, Kwei-San, Tao-Yuan 33302, Taiwan; 2School of Traditional Chinese Medicine, College of Medicine, Chang Gung University, Kwei-San, Tao-Yuan 33302, Taiwan; 3Department of Pharmacology, College of Medicine, China Medical University, Taichung 40402, Taiwan; 4Program for Biotch Pharmaceutical Industry, China Medical University, Taichung 40402, Taiwan; 5Department of Post-Baccalaureate Veterinary Medicine, College of Medical and Health Science, Asia University, Wufeng, Taichung 41354, Taiwan
Correspondence: Chuen-Mao Yang Tel +886-4-22053366 (ext. 2229)
Email [email protected]
Purpose: Neuroinflammation, characterized by the increased expression of inflammatory proteins such as matrix metalloproteinases (MMPs), plays a critical role in neurodegenerative disorders. Lipopolysaccharide (LPS) has been shown to upregulate MMP-9 expression through the activation of various transcription factors, including activator protein 1 (AP-1) and forkhead box protein O1 (FoxO1). The flavonoid 3,5,7-trihydroxy-2-phenyl-4H-1-benzopyran-4-one (galangin) has been demonstrated to possess antioxidant and anti-inflammatory properties in various types of cells. Here, we investigated the mechanisms underlying the inhibitory effect of galangin on LPS-induced MMP-9 expression in rat brain astrocytes (RBA-1 cells).
Methods: Pharmacological inhibitors and siRNAs were employed to explore the effects of galangin on LPS-challenged RBA-1 cells. Gelatin zymography, Western blotting, real-time PCR, and a luciferase reporter assay were used to detect MMP-9 activity, protein expression, mRNA levels, and promoter activity, respectively. The protein kinases involved in the LPS-induced MMP-9 expression were determined by Western blot. A chromatin immunoprecipitation (ChIP) assay was employed to evaluate the activity of c-Jun at the MMP-9 promoter.
Results: Galangin treatment attenuated the LPS-mediated induction of MMP-9 protein and mRNA expression, as well as the activity at the MMP-9 promoter. In addition, galangin exerted its inhibitory effects on MMP-9 expression through suppressing the LPS-stimulated activation of proline-rich tyrosine kinase (Pyk2), platelet-derived growth factor receptor beta (PDGFRβ), phosphoinositide 3-kinase (PI3K), protein kinase B (Akt), mammalian target of rapamycin (mTOR), and mitogen-activated protein kinases (MAPKs). Pretreatment with galangin attenuated the LPS-induced phosphorylation of c-Jun and FoxO1. LPS-induced cell migration was also suppressed by galangin pretreatment.
Conclusion: Galangin attenuates the LPS-induced inflammatory responses, including the induction of MMP-9 expression and cell migration, via inhibiting Pyk2/PDGFRβ/PI3K/Akt/mTOR/JNK1/JNK2 and p44/p42 MAPK cascade-dependent AP-1 and FoxO1 activities. These results provide new insights into the mechanisms through which galangin mitigates LPS-induced inflammatory responses, and suggest novel strategies for the management of LPS-related brain diseases.
Keywords: neuroinflammation, astrocytes, LPS, matrix metalloproteinase, protein kinases, Chinese herbal medicine
Introduction
Galangin (3,5,7-trihydroxyflavone) is the major flavonol and bioactive component extracted from Alpinia officinarum and has long been used as herbal medicine and a spice in South Africa and Asia.1 Galangin has attracted considerable attention owing to its multiple biological properties, including neurovascular protective, anti-inflammatory, antioxidant, and antiapoptotic activities. These effects are reported to be exerted through the modulation of the nuclear factor kappa B (NF-κB), nuclear factor erythroid 2-related factor 2 (Nrf2), and cAMP response element-binding protein (CREB) signaling pathways.2–5 Recent studies have indicated that galangin also has a neuroprotective role in ischemic stroke,6 likely through improving regional cortical blood flow (rCBF), protecting mitochondria, and inhibiting the caspase-dependent mitochondrial cell death pathway. Moreover, galangin can regulate the levels of endogenous excitatory amino acid (EAAs) metabolites in the brain tissue of mice with cerebral ischemia.7 Galangin helps protect dopaminergic neurons by inhibiting microglial activation and the expression of proinflammatory mediators.8 Although the effects of galangin have been extensively studied, the detailed mechanisms through which galangin modulates inflammation-related responses in rat brain astrocytes (RBA-1 cells) remain unknown.
Lipopolysaccharide (LPS) is a potent inducer of inflammation, and can promote the expression of several inflammatory mediators, including tumor necrosis factor alpha (TNF-α), interleukin (IL)-6, IL-10, prostaglandin E2 (PGE2), and nitric oxide (NO).9 LPS can also induce the expression of matrix metalloproteinase 9 (MMP-9), which contributes to tissue inflammatory responses and disease development.10,11 We previously reported that LPS can induce MMP-9 expression via the TLR4/c-Src/Pyk2/PDGFR/PI3K/Akt/MAPKs/AP-1 pathway, and can also enhance the migratory ability of RBA-1 cells.12 MMPs are a family of zinc-dependent endopeptidases, and more than twenty MMP family members have been identified in humans to date. They are involved in blood–brain barrier (BBB) breakdown, demyelination, inflammation, and neurotoxicity in the central nervous system (CNS).13,14 Several studies have found that infection-mediated inflammatory responses can lead to the abnormal expression and activation of MMP-9, which causes the breakdown of the extracellular matrix (ECM), resulting in the disruption of the BBB, altered cellular signaling, and eventually cell death.15,16 In particular, MMP-9 plays a vital part in brain inflammation and neurodegenerative diseases such as Parkinson’s disease (PD),15 amyotrophic lateral sclerosis (ALS),17 Alzheimer’s disease (AD),18 stroke,19 and multiple sclerosis (MS).20 These observations suggest that adjuvant therapy with MMP inhibitors may be beneficial in reducing the neuroinflammation and neurodegeneration induced by inflammatory mediators such as LPS.
In the present study, we hypothesized that galangin may attenuate LPS-induced MMP-9 expression in RBA-1 cells via the modulation of related signaling components. We found that galangin blocked LPS-mediated inflammatory responses by inhibiting the phosphorylation of Pyk2, PDGFR, PI3K, Akt, mTOR, JNK1/2, and ERK1/2, leading to the downregulation of transcription factor activity, including that of c-Jun and FoxO1. To the best of our knowledge, this is the first report detailing the mechanisms through which galangin inhibits MMP-9 expression and cell migration in LPS-challenged RBA-1 cells. Our results indicated that galangin administration may be a promising therapeutic strategy for the management of neurodegenerative diseases, particularly those related to LPS.
Materials and Methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM)/Ham’s F-12 nutrient mixture and fetal bovine serum (FBS) were purchased from Invitrogen (Carlsbad, CA, USA). Hybond-C membranes and enhanced chemiluminescence (ECL) reagents were obtained from GE Healthcare Biosciences (Little Chalfont, Buckinghamshire, UK). Anti-phospho-Akt (Ser473, #9271), anti-phospho-Pyk2 (Tyr402, #3291), anti-phospho-PDGFRβ (Tyr751, #3161), anti-phospho-JNK1/2 (Thr183/Tyr185, #4668), anti-phospho-ERK1/2 (Thr202/Tyr204, #9101), anti-phospho-FoxO1 (Ser256, #9461), anti-phospho-mTOR (Ser2448, #5536), anti-mTOR (#2972), and anti-phospho-c-Jun (Ser63, #2361) antibodies were obtained from Cell Signaling Technology (Danvers, MA, USA). Anti-Pyk2 (ab32448) and anti-FoxO1A (ab52857) antibodies were purchased from Abcam (Cambridge, UK). The anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (#MCA-ID4) antibody was purchased from Encor (Gainesville, FL, USA). Anti-PDGFRβ (sc-374,573), anti-Akt (sc-8312), anti-ERK1 (sc-271,270), anti-ERK2 (sc-1647), anti-JNK1/2 (sc-7345), and anti-c-Jun (sc-44) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). All primary antibodies were diluted at 1:1000 in PBS with 1% bovine serum albumin (BSA). Galangin was purchased from Cayman Chemical (Ann Arbor, MI, USA). The bicinchoninic acid (BCA) protein assay reagent was purchased from Pierce (Rockford, IL, USA). Sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis (PAGE) reagents were purchased from MDBio, Inc. (Taipei, Taiwan). Dimethyl sulfoxide (DMSO), LPS (L2630), enzymes, TRIzol, a sodium 3′-[1-[(phenylamino)-carbony]-3,4-tetrazolium]-bis (4-methoxy-6-nitro) benzene-sulfonic acid hydrate (XTT) assay kit, and other chemicals were obtained from Sigma (St. Louis, MO, USA).
Cell Culture and Treatment
RBA-1 cells originated from a primary astrocyte culture of neonatal rat cerebrum and naturally developed through successive cell passages.12,21 The use of the cell line was approved by the Chang Gung University Institutional Animal Care and Use Committee (IACUC Approval No.: CGU16-081). Primary astrocyte culture purity was assessed using an anti-glial fibrillary acidic protein (GFAP) antibody (an astrocyte-specific marker), which showed that over 95% of the astrocytes were GFAP-positive. Experiments were performed with cells from passages 4 to 35. The cytotoxicity of galangin or LPS alone as well as that of the inhibitors used at the time of incubation was evaluated using an XTT assay kit. These treatments did not significantly affect cell viability (data not shown). The cells were plated onto 12-well culture plates, made quiescent at confluence by incubation in serum-free DMEM/F-12 for 24 h, and then incubated with LPS at 37°C for the indicated periods. When galangin or other inhibitors were used, the cells were pretreated for 1 h before exposure to LPS.
Protein Preparation and Western Blotting
Cells were washed with ice-cold PBS and harvested using an SDS-loading buffer (0.1 M Tris-HCl, pH 6.8; 1% SDS; 5% glycerol; 2.5% β-mercaptoethanol; and 0.02% bromophenol blue) to yield whole-cell extracts. The proteins were separated by SDS–PAGE and transferred by electrophoresis onto Hybond-C membranes. The membranes were incubated with antibodies diluted at 1:1000 in Tween 20–Tris-buffered saline (TTBS), with an anti-GAPDH antibody being used as the internal control. The membranes were washed four times with TTBS, 5 min each wash, and then incubated with a horseradish peroxidase-conjugated secondary antibody diluted at 1:1500 for 1 h. Following washing, the immunoreactive bands were detected by ECL and imaged using a UVP BioSpectrum 500 Imaging System (Upland, CA, USA). Densitometric analyses were performed using UN-SCAN-IT gel software (Orem, UT, USA).
MMP Gelatin Zymography
Growth-arrested cells were incubated with LPS for the indicated periods. The culture media were analyzed by gelatin zymography.22 The gelatinolytic activity was manifested as horizontal white bands on a blue background. Because cleaved MMPs were not reliably detectable, only pro-form zymogens were quantified.
Total RNA Extraction and Real-Time PCR Analysis
Total RNA was extracted from RBA-1 cells.22 The cDNA obtained from 0.5 μg of total RNA was used as a template for PCR amplification. Primers were designed based on GenBank entries for rat MMP-9 and GAPDH. The following primers were used for the amplification reaction: MMP-9, 5′-AGTTTGGTGTCGCGGAGCAC-3′ (sense), 5′-TACATGAGCGCTTCCGGCAC-3′ (antisense); GAPDH, 5′-AACTTTGGCATCGTGGAAGG-3′ (sense), 5′-GTGGATGCAGGGATGATGTTC-3′ (antisense). Real-time PCR was performed with the TaqMan Gene Expression assays using a 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). Relative gene expression levels were determined by the ΔΔCt method, where Ct represented the threshold cycle. All experiments were performed in triplicate.
Rat MMP-9 Promoter Construction, Transfection, and Luciferase Reporter Assays
The upstream region (−1280 to +19) of the rat MMP-9 promoter was cloned into a pGL3-basic vector containing the luciferase reporter gene.23,24 The plasmid was prepared using the QIAGEN plasmid DNA preparation kit (Hilden, Germany). The construct was transfected into RBA-1 cells using Lipofectamine reagent according to the manufacturer’s instructions. The transfection efficiency (~60%) was determined by transfection with an enhanced green fluorescent protein (GFP). After incubation with LPS, the cells were collected and disrupted by sonication in a lysis buffer (25 mM Tris, pH 7.8; 2 mM ethylenediaminetetraacetic acid [EDTA]; 1% Triton X-100; and 10% glycerol). After centrifugation, aliquots of the supernatants were tested for promoter activity using a luciferase assay system (Promega, Madison, WI, USA). Firefly luciferase activity was standardized to that of β-galactosidase.
Cell Migration Assay
RBA-1 cells were cultured to confluence in six-well culture plates and starved in serum-free DMEM/F-12 medium for 24 h. The monolayer of cells was manually scratched with a blue pipette tip, leaving a bright and clear field (∼2 mm) in the center of the plates. The detached cells were removed by washing once with PBS. Serum-free DMEM/F-12 medium with or without LPS (2 μg/mL) and containing the DNA synthesis inhibitor hydroxyurea (10 μM) was added to the respective wells during the period of observation after 1 h pretreatment with or without galangin. Cells migrating from the scratch boundary were observed under a light microscope and imaged with a digital camera (Olympus, Japan). The number of migrating cells was counted from the resulting four phase images for each point and then averaged for each experimental condition. The data presented are summarized from three separate assays.
Chromatin Immunoprecipitation (ChIP) Assay
To detect the association of nuclear proteins with the rat MMP-9 promoter, chromatin immunoprecipitation (ChIP) analysis was conducted as previously described.24 Briefly, RBA-1 cells were cross-linked in 1% formaldehyde for 10 min at 37 °C and washed three times with ice-cold PBS containing 1 mM phenylmethylsulfonyl fluoride (PMSF) and 1% aprotinin. The cell lysates were prepared using SDS-lysis buffer (1% SDS; 5 mM EDTA; 1 mM PMSF; and 50 mM Tris-HCl) and sonicated at 4 °C to yield 200–300-base pair DNA fragments. Next, the soluble chromatin was precleared by incubation with sheared salmon sperm DNA-protein agarose A; one portion of the sample was used as the DNA input control, while the other was immunoprecipitated with or without (control) an anti-c-Jun antibody and protein A beads. After washing and eluting, the precipitates were heated overnight at 65 °C to reverse the DNA/protein cross-linking. The DNA fragments were purified using phenol–chloroform extraction and ethanol precipitation. The purified DNA was subjected to PCR amplification using the primers specific for the region (−606 ~ −327, accession No: AF148065) containing the distal activator protein 1 (AP-1) binding site (−503 to −497) present in the MMP-9 promoter (sense primer: 5ʹ-AGAGCCTGCTCCCAGAGGGC-3ʹ; antisense primer: 5ʹ-GCCAAGTCAGGCAGGACCCC-3ʹ). The PCR fragments were analyzed on 3% agarose gels (1× TAE) containing ethidium bromide, and the size of the fragments (280 bp) was compared against that of a molecular weight marker. qPCR was performed using a Luna Universal qPCR Master Mix (M3003; New England BioLabs) on a StepOnePlus™ real-time PCR system (Applied Biosystems).
Statistical Analysis
All data were expressed as means or means ± S.E.M of three individual experiments performed in duplicate or triplicate. For Western blot data, the significance of the differences between two groups was determined by a paired two-tailed Student’s t-test. All other statistical analyses compared multiple groups using one-way analysis of variance (ANOVA), followed by Tukey’s post hoc test. GraphPad Prism (GraphPad Software, San Diego, CA, USA) was used to analyze the data. #p < 0.05 was considered significant.
Results
Galangin Attenuated LPS-Induced MMP-9 Expression and Cell Migration
We evaluated the effects of galangin on LPS-induced MMP-9 expression and cell migration. RBA-1 cells were pretreated with galangin (0.1, 1, or 10 µM) for 1 h and then incubated with LPS (2 µg/mL) for the indicated periods. As shown in Figure 1A, pretreatment with galangin significantly reduced the LPS-induced MMP-9 protein level in a concentration- and time-dependent manner, attenuated the LPS-induced MMP-9 mRNA level (Figure 1B), and time-dependently inhibited the LPS-induced MMP-9 promoter activity (Figure 1C). To further explore the inhibitory effect of galangin on MMP-9 activity, we evaluated how galangin affects the migratory capacity of RBA-1 cells after LPS challenge. The number of migrated RBA-1 cells was assessed 48 h after treatment with LPS/hydroxyurea in the presence or absence of galangin. The data showed that galangin treatment reduced the number of migrated RBA-1 cells following LPS stimulation (Figure 1D). These results suggested that galangin can inhibit the LPS-induced MMP-9 expression associated with cell migration of RBA-1 cells.
Galangin Attenuates the LPS-Induced Phosphorylation of Pyk2 and PDGFR
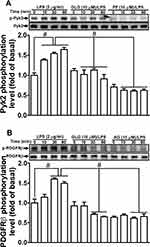
Several studies have demonstrated that receptor tyrosine kinases and nonreceptor tyrosine kinases, including PDGFR, EGFR, c-Src, and Pyk2, can regulate MMP-9 expression in various types of cells.25,26 We previously also found that LPS-induced expression of MMP-9 in RBA-1 cells was mediated by Pyk2, c-Src, and PDGFR.12 Therefore, we explored whether galangin can attenuate the LPS-stimulated activation of Pyk2 and PDGFR in RBA-1 cells. We found that the LPS-stimulated phosphorylation of Pyk2 was inhibited by pretreatment with either galangin (10 μM) or PF431396 (a dual focal adhesion kinase [FAK] and PYK2 inhibitor; 10 μM) (Figure 2A). In addition, the LPS-stimulated phosphorylation of PDGFRβ was reduced by pretreatment with either galangin (10 μM) or AG1296 (a selective PDGFR inhibitor; 10 μM) (Figure 2B). These results suggested that galangin reduced the LPS-mediated induction of MMP-9 expression in RBA-1 cells via suppressing Pyk2 and PDGFRβ activation.
Galangin Reduces the LPS-Stimulated Phosphorylation of Akt
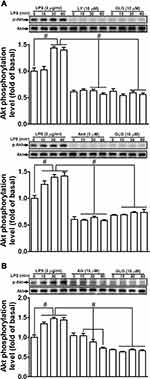
Several studies have demonstrated that Akt has a role in the activation of MMP-9 expression in various cell types.1,27 We previously also reported that PI3K/Akt participates in the LPS-mediated induction of MMP-9 expression in RBA-1 cells.12 Consequently, we investigated whether galangin can attenuate the LPS-stimulated PI3K/Akt-dependent pathway, thereby leading to the downregulation of MMP-9 expression in RBA-1 cells. We found that pretreatment with galangin (10 μM), LY294002 (an inhibitor of PI3K; 10 µM), or Akt inhibitor VIII (an inhibitor of Akt1 and Akt2; 3 µM) reduced the LPS-stimulated phosphorylation of Akt (Figure 3A). Moreover, the LPS-stimulated phosphorylation of Akt was also attenuated by pretreatment with AG1296 (10 µM) (Figure 3B). These results suggested that galangin reduces the LPS-stimulated induction of MMP-9 expression via attenuating the activation of the PDGFR-dependent PI3K/Akt pathway in RBA-1 cells.
Galangin Reduces the LPS-Stimulated Phosphorylation of Mammalian Target of Rapamycin (mTOR)
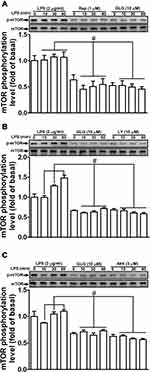
mTOR functions downstream of PI3K/Akt and has been shown to mediate the expression of MMP-9 in various cell types.1,27 Additionally, LPS can also activate mTOR-mediated responses.28,29 We, therefore, investigated whether galangin can suppress the LPS-induced mTOR-dependent pathway in RBA-1 cells. We found that pretreatment with either galangin (10 μM) or rapamycin (1 μM) reduced the LPS-induced levels of mTOR phosphorylation (Figure 4A). Moreover, the LPS-stimulated phosphorylation of mTOR was reduced by pretreatment with either LY294002 (10 µM) (Figure 4B) or Akt inhibitor VIII (3 µM) (Figure 4C). These results suggested that galangin reduces the LPS-mediated induction of MMP-9 expression via attenuating the PI3K/Akt-dependent activation of mTOR in RBA-1 cells.
Galangin Inhibits LPS-Stimulated MAPK Activation
Recent reports have indicated that MAPK-dependent pathways are involved in the LPS-induced expression of MMP-9 in several cell types,11,29,30 while we also recently revealed that MAPKs participate in the LPS-mediated induction of MMP-9 expression in RBA-1 cells.12 Therefore, we sought to determine whether galangin can inhibit the MAPK-dependent pathways associated with the LPS-stimulated induction of MMP-9 expression. We found that pretreatment with either galangin (10 μM) or SP600125 (a selective and reversible JNK inhibitor; 1 μM) suppressed the LPS-stimulated phosphorylation of JNK1/2 (Figure 5A) in RBA-1 cells, as did pretreatment with rapamycin (1 µM) (Figure 5B). Similarly, pretreatment with either galangin (10 μM) or U0126 (a MEK1/2 inhibitor; 1 μM) attenuated the LPS-stimulated phosphorylation of p44/p42 MAPK in RBA-1 cells (Figure 5C). The same effect was seen with rapamycin pretreatment (1 μM) (Figure 5D). These results indicated that galangin can reduce LPS-induced MMP-9 expression in RBA-1 cells by suppressing the activation of JNK1/2 and p44/p42 MAPK, kinases that function downstream of mTOR.
Galangin Inhibits LPS-Stimulated Transcription Factor Activation
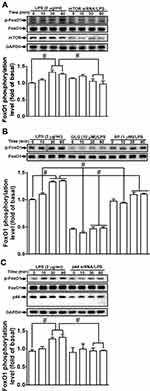
Several transcription factors, including NF-κB, AP-1, and FoxO1, are known to mediate the induction of MMP-9 expression following stimulation by different factors and in various types of cells.1,25,26,31,32 In our previous study, we also showed that NF-κB and AP-1 contribute to LPS-induced MMP-9 expression in RBA-1 cells.12,33 Here, we explored whether galangin blocked the activation of transcription factors such as NF-κB, AP-1, and FoxO1 that act in the LPS-stimulated induction of MMP-9 expression in RBA-1 cells. Our results showed that pretreatment with galangin significantly reduced the LPS-stimulated phosphorylation of c-Jun (Figure 6A–C) and FoxO1 (Figure 7B), but not that of NF-κB p65 (data not shown). Furthermore, pretreatment with rapamycin (1 μM) (Figure 6A), SP600125 (1 μM) (Figure 6B), or U0126 (1 μM) (Figure 6C) attenuated the LPS-stimulated phosphorylation of c-Jun in RBA-1 cells. Moreover, the ChIP assay data showed that the interaction between c-Jun and the MMP-9 promoter was blocked by galangin in LPS-challenged RBA-1 cells (Figure 6D). Additionally, transfection with mTOR siRNA (Figure 7A), pretreatment with SP600125 (1 μM) (Figure 7B), or transfection with p44 siRNA (Figure 7C) significantly attenuated the LPS-stimulated phosphorylation of FoxO1 in RBA-1 cells. These results indicated that, in RBA-1 cells, galangin can reduce the LPS-induced MMP-9 expression level by suppressing the activation of transcription factors such as AP-1 and FoxO1 that are activated by JNK1/2 and p44/p42 MAPK.
Discussion
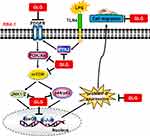
The elevated expression of MMP-9 plays a key role in the pathology of brain degenerative disorders, including stroke, PD, MS, and AD.15,17,18,20 LPS also participates in neuroinflammatory and neurodegenerative responses in these brain disorders via the regulation of proinflammatory signaling pathways,34,35 suggesting that it also has a role in neurodegenerative processes. This suggests that inhibitors of LPS-related signaling components and MMP-9 have therapeutic potential for the treatment of neuroinflammatory diseases.34,36 Galangin possesses various beneficial properties, including anti-inflammatory, antioxidant, antimutagenic, anticlastogenic, bactericidal, and antifibrotic effects37 in various disorders. This compound has been shown to inhibit MMP-9 activity and thrombin-mediated responses in human neuroblastoma cells.1 In the present study, we evaluated whether galangin also exerts inhibitory effects on the LPS-stimulated induction of MMP-9 expression mediated through related signaling components in RBA-1 cells. We found that galangin concentrations of up to 10 µM did not significantly affect cell viability, allowing the evaluation of how galangin attenuates the LPS-stimulated activation of protein kinases and transcription factors in RBA-1 cells. Our results confirmed that, in RBA-1 cells, galangin inhibited the LPS-stimulated induction of MMP-9 expression and cell migration through suppressing the Pyk2, PDGFR, PI3K, Akt, mTOR, JNK1/2, and p44/p42 MAPK signaling pathway-dependent activation of FoxO1 and AP-1 (Figure 8). To the best of our knowledge, this is the first report showing that galangin exerts anti-inflammatory effects on brain astrocytes by reducing the LPS-stimulated induction of MMP-9 expression and cell migration.
Pyk2 has a role in the regulation of cell polarization, adhesion, spreading, migration, and actin cytoskeleton organization. Pyk2 also plays an important role in inflammatory diseases. In AD patients, it is known that amyloid-β deposition is accompanied by innate immune system activation, and that this involves the inflammasome-dependent formation of ASC specks in microglia. There is evidence to support that downregulating Pyk2 expression by treatment with siRNAs or other inhibitors can abolish ASC oligomerization.38 PDGFR is a key regulator of cell growth, cell proliferation, cellular differentiation, wound healing, and inflammation, and has an important role in many diseases, including cancer. PDGF signaling is a therapeutic target in cardiovascular pathogeneses such as atherosclerosis, pulmonary arterial hypertension, angiogenesis, inflammation,39 and diabetes,40 as well as in several malignancies.41 Therefore, compounds with inhibitory effects against Pyk2 and PDGFR signaling represent a high-value therapeutic strategy for the treatment of inflammation-related disorders. We and others have previously reported that LPS-induced MMP-9 expression is mediated via Pyk2 and PDGFR in various types of cells.12,42 Additionally, galangin has been shown to inhibit protein tyrosine kinase activity.1 Lim et al,43 found that some flavonoids interfere with NLRP3 inflammasome activation via disrupting the Syk/Pyk2 pathway. Consistent with these results, we demonstrated that, in RBA-1 cells, galangin attenuated the LPS-mediated induction of MMP-9 expression via the suppression of Pyk2 and PDGFR phosphorylation.
The PI3K/Akt/mTOR signaling pathway plays a central role in a wide spectrum of cellular activities, including cell proliferation, survival, and differentiation. Accumulating evidence has revealed that the PI3K/Akt pathway is also involved in inflammatory responses.44 The PI3K/Akt/mTOR signaling pathway can be activated by several extracellular factors such as TLR ligands, neurotransmitters, amino acids, and growth factors, in addition to intracellular stimuli such as energy deprivation, all of which lead to the activation of a series of downstream effectors. These signaling pathways may also have a role in several brain disorders such as epilepsy,45 autism,46 and cognitive deficits.47 Increasing evidence has indicated that flavonoids can inhibit the development of brain pathology and exert ameliorative effects on cognitive dysfunction through the modulation of several molecular targets, including PI3K/Akt.48,49 This indicates that the PI3K/Akt/mTOR signaling pathway may be a promising therapeutic target for the treatment of these neurodegenerative disorders. In our previous study, we demonstrated that the induction of MMP-9 expression by LPS is mediated via PI3K/Akt signaling in RBA-1 cells.12 LPS can also activate Akt/mTOR signaling in various cell types.28,29 Zhu et al demonstrated that galangin can inhibit the migration and invasion of kidney cancer cells via suppressing the expression of the PI3K/Akt/mTOR signaling pathway.50 In our recent study, we also found that galangin reduced the thrombin-mediated induction of MMP-9 expression via inhibiting the Akt/mTOR pathway.1 Our results confirmed that galangin suppressed the LPS-induced expression of MMP-9 via suppressing the PI3K/Akt/mTOR pathway in RBA-1 cells. However, several studies have shown that galangin can also protect against neurotoxicity and improve insulin resistance via activating the Akt/GSK3β/mTOR pathway.51,52 These different effects may be due to the different cell types and experimental models used.
MAPKs are widely known to be involved in the inflammatory responses triggered by proinflammatory factors such as LPS, cytokines, and chemokines.11,53 We previously revealed that the LPS-stimulated induction of MMP-9 expression is mediated via MAPK signaling in RBA-1 cells,12 which was in agreement with the results of a previous report.11 Tomar et al demonstrated that galangin (100 mg/kg, p.o.) significantly ameliorated cisplatin-induced nephrotoxicity in rats by suppressing MAPK-mediated inflammation and apoptosis.54 Additionally, the p38 MAPK and JNK1/2 signaling pathways were shown to mediate the anti-inflammatory activity of galangin in an LPS-stimulated BV2 cell model.2 We previously also reported that galangin can reduce thrombin-induced MMP-9 expression via inhibiting the MAPK pathway in human neuroblastoma cells.1 In the present study, the results confirmed that galangin attenuated the LPS-induced expression of MMP-9 via suppressing the JNK1/2 and ERK1/2 pathways in RBA-1 cells.
Several transcription factors, such as NF-κB, AP-1, and FoxO1, have central roles in regulating the expression of numerous proinflammatory target genes, including MMP-9, that are associated with brain injury and inflammation during pathological events.1,25 We recently confirmed that NF-κB- and AP1-dependent transcription participates in the LPS-mediated induction of MMP-9 expression.12,33 The FoxO1 transcription factor has also been revealed to mediate LPS-elicited acute lung injury.55 Moreover, galangin was also shown to exert its anti-inflammatory effects via the inhibition of NF-κB-, FoxO1-, and AP-1-dependent pathways to downregulate MMP-9 expression.1 Several studies have also demonstrated that galangin exerts its anti-inflammatory effects via modulating the activity of NF-κB 2,54 and AP-156,57 under various experimental conditions. The present findings also showed that galangin can inhibit the LPS-stimulated phosphorylation of c-Jun and FoxO1, eventually leading to the downregulation of MMP-9 expression. However, in this study, galangin did not inhibit NF-κB activity, which may have been due to the different stimuli and types of cells used when compared with the other studies. We revealed that galangin inhibited the LPS-stimulated induction of MMP-9 expression via suppressing AP-1 and FoxO1 activities in RBA-1 cells.
Conclusions
Our results demonstrated that galangin attenuated the LPS-induced expression of MMP-9 via inhibiting the Pyk2, PDGFR, PI3K, Akt, mTOR, JNK1/2, and p44/p42 MAPK-dependent FoxO1 and AP-1 signaling pathways in RBA-1 cells. These findings shed further light on the detailed mechanisms by which galangin inhibits signaling components and attenuates MMP-9 expression and cell migration in LPS-challenged RBA-1 cells. However, in the present study, we only demonstrated that galangin has an inhibitory effect on the LPS-stimulated phosphorylation of protein kinases, leading to the downregulation of MMP-9 expression, and did not assess whether galangin can directly modulate the activities of these protein kinases. Thus, the inhibitory effects of galangin on these signaling components need further investigation. Nevertheless, our results indicated that galangin might be a potential therapeutic candidate for the treatment of brain inflammation and neurodegenerative diseases. However, the greatest limitation of this study was that we did not confirm the inhibitory effects of galangin in vivo. Moreover, the anti-inflammatory molecules that mediate the protective effects of galangin in neuroinflammation remain unknown and require further investigation, both in vivo and in vitro.
Abbreviations
AD, Alzheimer’s disease; AP-1, activator protein 1; BBB, blood-brain barrier; BCA, bicinchoninic acid; BSA, bovine serum albumin; ChIP, chromatin immunoprecipitation; CNS, central nervous system; DMEM, Dulbecco’s modified Eagle’s medium; DMSO, dimethyl sulfoxide; ECL, enhanced chemiluminescence; EDTA, ethylenediaminetetraacetic acid; ERK1/2, extracellular signal-regulated kinase ½; FBS, fetal bovine serum; FoxO1, Forkhead box protein O1; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GFP, green fluorescent protein; IL, interleukin; JNK, c-Jun amino-terminal kinase; MAPK, mitogen-activated protein kinase; MMP, matrix metalloproteinase; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor-κB; PD, Parkinson’s disease; PDGFR, platelet-derived growth factor receptor; PI3K, phosphoinositide 3-kinase; PMSF, phenylmethylsulfonyl fluoride; Pyk2, proline-rich tyrosine kinase; RBA-1, rat brain astrocytes; SEM, standard error of the mean; SDS, Sodium dodecyl sulfate; TNF, tumor necrosis factor; XTT, sodium 3ʹ-[1-[(phenylamino)-carbony]-3,4-tetrazolium]-bis (4-methoxy-6-nitro) benzene-sulfonic acid hydrate.
Data Sharing Statement
The data sets used and analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We appreciated Dr. Chen-yu Wang for his suggestions and construction of plasmids applied in this study and Ms. Ya-Fang Shih for her technical assistance. The manuscript was critically proofread by Dr. Jiro hasegawa Situmorang (Department of Pharmacology, China Medical University).
Author Contributions
CCY, LDH, and CMY designed and conducted the study. CCY, and LDH performed and collected the data. CCY, LDH, and CMY analyzed and interpreted the data. CCY and CMY prepared the manuscript. CCY, LDH, and CMY reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Science and Technology, Taiwan [Grant numbers: MOST107-2320-B-039-071-MY2, MOST108-2320-B-039-061, MOST109-2320-B-039-061, MOST109-2813-C-039-029-B, and MOST108-2320-B-182-014]; China Medical University, Taiwan [Grant number: CMU109-SR-24]; and Chang Gung Medical Research Foundation, Taiwan [Grant numbers: CMRPG5F0203, CMRPG5J0142, and CMRPG5J0143].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Yang CC, Lin CC, Hsiao LD, Yang CM. Galangin inhibits thrombin-induced MMP-9 expression in SK-N-SH cells via protein kinase-dependent NF-kappaB phosphorylation. Int J Mol Sci. 2018;19(12):4084. doi:10.3390/ijms19124084
2. Choi MJ, Lee EJ, Park JS, Kim SN, Park EM, Kim HS. Anti-inflammatory mechanism of galangin in lipopolysaccharide-stimulated microglia: critical role of PPAR-gamma signaling pathway. Biochem Pharmacol. 2017;144:120–131. doi:10.1016/j.bcp.2017.07.021
3. Huang YC, Tsai MS, Hsieh PC, et al. Galangin ameliorates cisplatin-induced nephrotoxicity by attenuating oxidative stress, inflammation and cell death in mice through inhibition of ERK and NF-kappaB signaling. Toxicol Appl Pharmacol. 2017;329:128–139. doi:10.1016/j.taap.2017.05.034
4. Lee HN, Shin SA, Choo GS, et al. Antiinflammatory effect of quercetin and galangin in LPS-stimulated RAW264.7 macrophages and DNCB-induced atopic dermatitis animal models. Int J Mol Med. 2018;41(2):888–898.
5. Fu Q, Gao Y, Zhao H, Wang Z, Wang J. Galangin protects human rheumatoid arthritis fibroblast-like synoviocytes via suppression of the NFkappaB/NLRP3 pathway. Mol Med Rep. 2018;18(4):3619–3624.
6. Li S, Wu C, Zhu L, et al. By improving regional cortical blood flow, attenuating mitochondrial dysfunction and sequential apoptosis galangin acts as a potential neuroprotective agent after acute ischemic stroke. Molecules. 2012;17(11):13403–13423. doi:10.3390/molecules171113403
7. Yang R, Chen K, Zhao Y, et al. Analysis of potential amino acid biomarkers in brain tissue and the effect of galangin on cerebral ischemia. Molecules. 2016;21(4):438. doi:10.3390/molecules21040438
8. Chen G, Liu J, Jiang L, et al. Galangin reduces the loss of dopaminergic neurons in an LPS-evoked model of Parkinson’s disease in rats. Int J Mol Sci. 2017;19(1).
9. Chae BS. Pretreatment of low-dose and super-low-dose LPS on the production of in vitro LPS-induced inflammatory mediators. Toxicol Res. 2018;34(1):65–73. doi:10.5487/TR.2018.34.1.065
10. Chen WY, Huang YC, Yang ML, et al. Protective effect of rutin on LPS-induced acute lung injury via down-regulation of MIP-2 expression and MMP-9 activation through inhibition of Akt phosphorylation. Int Immunopharmacol. 2014;22(2):409–413. doi:10.1016/j.intimp.2014.07.026
11. Tian X, Xie G, Ding F, Zhou X. LPS-induced MMP-9 expression is mediated through the MAPKs-AP-1 dependent mechanism in BEAS-2B and U937 cells. Exp Lung Res. 2018;44(4–5):217–225. doi:10.1080/01902148.2018.1493551
12. Yang CC, Lin CC, Hsiao LD, Kuo JM, Tseng HC, Yang CM. Lipopolysaccharide-induced matrix metalloproteinase-9 expression associated with cell migration in rat brain astrocytes. Int J Mol Sci. 2020;21(1).
13. Yong VW, Power C, Forsyth P, Edwards DR. Metalloproteinases in biology and pathology of the nervous system. Nat Rev Neurosci. 2001;2(7):502–511. doi:10.1038/35081571
14. Agrawal SM, Lau L, Yong VW. MMPs in the central nervous system: where the good guys go bad. Semin Cell Dev Biol. 2008;19(1):42–51. doi:10.1016/j.semcdb.2007.06.003
15. Hu J, Van den Steen PE, Sang QX, Opdenakker G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat Rev Drug Discov. 2007;6(6):480–498.
16. Candelario-Jalil E, Yang Y, Rosenberg GA. Diverse roles of matrix metalloproteinases and tissue inhibitors of metalloproteinases in neuroinflammation and cerebral ischemia. Neuroscience. 2009;158(3):983–994. doi:10.1016/j.neuroscience.2008.06.025
17. He X, Zhang L, Yao X, et al. Association studies of MMP-9 in Parkinson’s disease and amyotrophic lateral sclerosis. PLoS One. 2013;8(9):e73777. doi:10.1371/journal.pone.0073777
18. Lorenzl S, Albers DS, Relkin N, et al. Increased plasma levels of matrix metalloproteinase-9 in patients with Alzheimer’s disease. Neurochem Int. 2003;43(3):191–196. doi:10.1016/S0197-0186(03)00004-4
19. Turner RJ, Sharp FR. Implications of MMP9 for blood brain barrier disruption and hemorrhagic transformation following ischemic stroke. Front Cell Neurosci. 2016;10:56. doi:10.3389/fncel.2016.00056
20. Valado A, Leitao MJ, Martinho A, et al. Multiple sclerosis: association of gelatinase B/matrix metalloproteinase-9 with risk and clinical course the disease. Mult Scler Relat Disord. 2017;11:71–76. doi:10.1016/j.msard.2016.12.003
21. Jou TC, Jou MJ, Chen JY, Lee SY. [Properties of rat brain astrocytes in long-term culture]. Taiwan Yi Xue Hui Za Zhi. 1985;84(8):865–881. Chinese.
22. Wu CY, Hsieh HL, Jou MJ, Yang CM. Involvement of p42/p44 MAPK, p38 MAPK, JNK and nuclear factor-kappa B in interleukin-1beta-induced matrix metalloproteinase-9 expression in rat brain astrocytes. J Neurochem. 2004;90(6):1477–1488. doi:10.1111/j.1471-4159.2004.02682.x
23. Eberhardt W, Schulze M, Engels C, Klasmeier E, Pfeilschifter J. Glucocorticoid-mediated suppression of cytokine-induced matrix metalloproteinase-9 expression in rat mesangial cells: involvement of nuclear factor-kappaB and Ets transcription factors. Mol Endocrinol. 2002;16(8):1752–1766. doi:10.1210/me.2001-0278
24. Hsieh HL, Wu CY, Yang CM. Bradykinin induces matrix metalloproteinase-9 expression and cell migration through a PKC-delta-dependent ERK/Elk-1 pathway in astrocytes. Glia. 2008;56(6):619–632. doi:10.1002/glia.20637
25. Yang CC, Hsiao LD, Yang CM, Lin CC. Thrombin enhanced matrix metalloproteinase-9 expression and migration of SK-N-SH cells via PAR-1, c-Src, PYK2, EGFR, Erk1/2 and AP-1. Mol Neurobiol. 2017;54(5):3476–3491. doi:10.1007/s12035-016-9916-0
26. Lin CC, Lee IT, Chi PL, et al. C-Src/Jak2/PDGFR/PKCdelta-dependent MMP-9 induction is required for thrombin-stimulated rat brain astrocytes migration. Mol Neurobiol. 2014;49(2):658–672.
27. Tsao YT, Kuo CY, Cheng SP, Lee CH. Downregulations of AKT/mTOR signaling pathway for salmonella-mediated suppression of matrix metalloproteinases-9 expression in mouse tumor models. Int J Mol Sci. 2018;19(6):1630. doi:10.3390/ijms19061630
28. Xie T, Xu Q, Wan H, et al. Lipopolysaccharide promotes lung fibroblast proliferation through autophagy inhibition via activation of the PI3K-Akt-mTOR pathway. Lab Invest. 2019;99:625–633. doi:10.1038/s41374-018-0160-2
29. Kong F, Lee BH, Wei K. 5-Hydroxymethylfurfural mitigates lipopolysaccharide-stimulated inflammation via suppression of MAPK, NF-kappaB and mTOR activation in RAW 264.7 cells. Molecules. 2019;24(2):275. doi:10.3390/molecules24020275
30. Xu X, Kwon OK, Shin IS, et al. Novel benzofuran derivative DK-1014 attenuates lung inflammation via blocking of MAPK/AP-1 and AKT/mTOR signaling in vitro and in vivo. Sci Rep. 2019;9(1):862. doi:10.1038/s41598-018-36925-9
31. Yang CC, Lin CC, Chien PT, Hsiao LD, Yang CM. Thrombin/matrix metalloproteinase-9-dependent SK-N-SH cell migration is mediated through a PLC/PKC/MAPKs/NF-kappaB cascade. Mol Neurobiol. 2016;53(9):5833–5846. doi:10.1007/s12035-015-9485-7
32. Lin CC, Lee IT, Wu WB, et al. Thrombin mediates migration of rat brain astrocytes via PLC, Ca2+, CaMKII, PKCalpha, and AP-1-dependent matrix metalloproteinase-9 expression. Mol Neurobiol. 2013;48(3):616–630. doi:10.1007/s12035-013-8450-6
33. Yang CC, Hsiao LD, Tseng HC, Kuo CM, Yang CM. Pristimerin inhibits MMP-9 expression and cell migration through attenuating NOX/ROS-dependent NF-κB activation in rat brain astrocytes challenged with LPS. J Inflamm Res. 2020;13:325–341. doi:10.2147/JIR.S252659
34. Khan MS, Ali T, Kim MW, et al. Anthocyanins protect against LPS-induced oxidative stress-mediated neuroinflammation and neurodegeneration in the adult mouse cortex. Neurochem Int. 2016;100:1–10. doi:10.1016/j.neuint.2016.08.005
35. Shah SA, Khan M, Jo MH, Jo MG, Amin FU, Kim MO. Melatonin stimulates the SIRT1/Nrf2 signaling pathway counteracting lipopolysaccharide (LPS)-induced oxidative stress to rescue postnatal rat brain. CNS Neurosci Ther. 2017;23(1):33–44. doi:10.1111/cns.12588
36. Chaturvedi M, Kaczmarek L. MMP-9 inhibition: a therapeutic strategy in ischemic stroke. Mol Neurobiol. 2014;49(1):563–573. doi:10.1007/s12035-013-8538-z
37. Wang X, Gong G, Yang W, Li Y, Jiang M, Li L. Antifibrotic activity of galangin, a novel function evaluated in animal liver fibrosis model. Environ Toxicol Pharmacol. 2013;36(2):288–295. doi:10.1016/j.etap.2013.04.004
38. Chung IC, OuYang CN, Yuan SN, et al. Pyk2 activates the NLRP3 inflammasome by directly phosphorylating ASC and contributes to inflammasome-dependent peritonitis. Sci Rep. 2016;6:36214. doi:10.1038/srep36214
39. Hu W, Huang Y. Targeting the platelet-derived growth factor signalling in cardiovascular disease. Clin Exp Pharmacol Physiol. 2015;42(12):1221–1224. doi:10.1111/1440-1681.12478
40. Fountas A, Diamantopoulos LN, Tsatsoulis A. Tyrosine kinase inhibitors and diabetes: A novel treatment paradigm? Trends Endocrinol Metab. 2015;26(11):643–656. doi:10.1016/j.tem.2015.09.003
41. Jones AV, Cross NC. Oncogenic derivatives of platelet-derived growth factor receptors. Cell Mol Life Sci. 2004;61(23):2912–2923. doi:10.1007/s00018-004-4272-z
42. Yang H-L, Huang P-J, Liu Y-R, et al. Toona sinensis inhibits LPS-induced inflammation and migration in vascular smooth muscle cells via suppression of reactive oxygen species and NF-B signaling pathway. Oxid Med Cell Longev. 2014;2014.
43. Lim H, Min DS, Park H, Kim HP. Flavonoids interfere with NLRP3 inflammasome activation. Toxicol Appl Pharmacol. 2018;355:93–102. doi:10.1016/j.taap.2018.06.022
44. Xie S, Chen M, Yan B, He X, Chen X, Li D. Identification of a role for the PI3K/AKT/mTOR signaling pathway in innate immune cells. PLoS One. 2014;9(4):e94496. doi:10.1371/journal.pone.0094496
45. Hodges SL, Lugo JN. Therapeutic role of targeting mTOR signaling and neuroinflammation in epilepsy. Epilepsy Res. 2020;161:106282. doi:10.1016/j.eplepsyres.2020.106282
46. Sokol DK, Maloney B, Westmark CJ, Lahiri DK. Novel contribution of secreted amyloid-β precursor protein to white matter brain enlargement in autism spectrum disorder. Front Psychiatry. 2019;10:165. doi:10.3389/fpsyt.2019.00165
47. He JT, Li H, Yang L, Mao CY. Role of hydrogen sulfide in cognitive deficits: evidences and mechanisms. Eur J Pharmacol. 2019;849:146–153. doi:10.1016/j.ejphar.2019.01.072
48. Spencer JP, Vauzour D, Rendeiro C. Flavonoids and cognition: the molecular mechanisms underlying their behavioural effects. Arch Biochem Biophys. 2009;492(1–2):1–9. doi:10.1016/j.abb.2009.10.003
49. Williams RJ, Spencer JP. Flavonoids, cognition, and dementia: actions, mechanisms, and potential therapeutic utility for Alzheimer disease. Free Radic Biol Med. 2012;52(1):35–45. doi:10.1016/j.freeradbiomed.2011.09.010
50. Zhu Y, Rao Q, Zhang X, Zhou X. Galangin induced antitumor effects in human kidney tumor cells mediated via mitochondrial mediated apoptosis, inhibition of cell migration and invasion and targeting PI3K/AKT/mTOR signalling pathway. J BUON. 2018;23(3):795–799.
51. Huang L, Lin M, Zhong X, Yang H, Deng M. Galangin decreases p‑tau, Aβ42 and β‑secretase levels, and suppresses autophagy in okadaic acid‑induced PC12 cells via an Akt/GSK3β/mTOR signaling‑dependent mechanism. Mol Med Rep. 2019;19(3):1767–1774.
52. Liu Y, Liang X, Zhang G, Kong L, Peng W, Zhang H. Galangin and pinocembrin from propolis ameliorate insulin resistance in HepG2 cells via regulating Akt/mTOR signaling. Evid Based Complement Alternat Med. 2018;2018:7971842. doi:10.1155/2018/7971842
53. Guo C, Yang XG, Wang F, Ma XYIL. 1alpha induces apoptosis and inhibits the osteoblast differentiation of MC3T3-E1 cells through the JNK and p38 MAPK pathways. Int J Mol Med. 2016;38(1):319–327. doi:10.3892/ijmm.2016.2606
54. Tomar A, Vasisth S, Khan SI, et al. Galangin ameliorates cisplatin induced nephrotoxicity in vivo by modulation of oxidative stress, apoptosis and inflammation through interplay of MAPK signaling cascade. Phytomedicine. 2017;34:154–161. doi:10.1016/j.phymed.2017.05.007
55. Sun K, Huang R, Yan L, et al. Schisandrin attenuates lipopolysaccharide-induced lung injury by regulating TLR-4 and Akt/FoxO1 signaling pathways. Front Physiol. 2018;9:1104. doi:10.3389/fphys.2018.01104
56. Wang K, Ping S, Huang S, et al. Molecular mechanisms underlying the in vitro anti-inflammatory effects of a flavonoid-rich ethanol extract from chinese propolis (poplar type). Evid Based Complement Alternat Med. 2013;2013:127672.
57. Choi YJ, Lee YH, Lee ST. Galangin and kaempferol suppress phorbol-12-myristate-13-acetate-induced matrix metalloproteinase-9 expression in human fibrosarcoma HT-1080 cells. Mol Cells. 2015;38(2):151–155. doi:10.14348/molcells.2015.2229
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.