Back to Journals » OncoTargets and Therapy » Volume 12
GADD45α-targeted suicide gene therapy driven by synthetic CArG promoter E9NS sensitizes NSCLC cells to cisplatin, resveratrol, and radiation regardless of p53 status
Authors Shi Q, Sutariya V, Varghese Gupta S, Bhatia D
Received 24 October 2018
Accepted for publication 12 March 2019
Published 26 April 2019 Volume 2019:12 Pages 3161—3170
DOI https://doi.org/10.2147/OTT.S192061
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr XuYu Yang
Qiwen Shi,1 Vijaykumar Sutariya,2 Sheeba Varghese Gupta,2 Deepak Bhatia3
1Collaborative Innovation Center of Yangtze River Delta Region Green Pharmaceuticals, Zhejiang University of Technology, Hangzhou, Zhejiang, People’s Republic of China; 2College of Pharmacy, University of South Florida, Tampa, FL, USA; 3Bernard J. Dunn School of Pharmacy, Shenandoah University, Ashburn, VA, USA
Background: GADD45α is a tumor suppressor protein often upregulated by environmental stresses and DNA-damage agents to cause growth arrest, apoptosis, tumor growth inhibition, and anti-angiogenesis. A novel suicide gene therapy vector pE9NS.G45α was engineered by cloning GADD45α opening reading frame downstream to the synthetic CArG promoter E9NS, which contains nine repeats of CArG element with modified core A/T sequence and functions as a molecular switch to drive the expression of GADD45α. The current study aims to determine the efficacy of this suicide gene therapy vector in combination with cisplatin, resveratrol, and radiation in NSCLC cell lines with various p53 statuses.
Methods: Three NSCLC cell lines, H1299 (deleted p53), A549 (wild-type p53), and H23 (mutated p53), were examined in the present investigation to represent NSCLC with different p53 functions. MTT assay was conducted to select suitable doses of cisplatin, resveratrol, and radiation for gene therapy, and dual luciferase assay was performed to validate the activation of promoter E9NS. The efficacy of gene therapy combinations was evaluated by the amount of GADD45α expression, cell survival, and apoptosis.
Results: All the combinations successfully activated promoter E9NS to elevate intracellular GADD45α protein levels and subsequently enhanced cell viability reduction and apoptosis induction regardless of p53 status.
Conclusion: Our study demonstrates that GADD45α-targeted suicide gene therapy controlled by synthetic promoter E9NS sensitizes NSCLC cells to cisplatin, resveratrol, and radiation and is effective against NSCLC at least in vitro.
Keywords: GADD45α, gene therapy, inducible promoter, CArG element, apoptosis
Introduction
As chemo- and radio-resistance becomes a persistent problem to cancer treatment, the development of other approaches is urgent. Suicide gene therapy is the delivery of pro-apoptotic genes or enzyme genes that convert non-toxic prodrugs into lethal agents to kill cancerous cells. This strategy has achieved success in a large number of in vitro and in vivo studies, but its clinical effectiveness has been limited by identification of the appropriate therapeutic gene and optimal transgene expression.1 Therefore, a novel vector that carries an effective suicide gene under the control of an inducible promoter may bring big breakthroughs in gene therapy research.
Growth arrest and DNA damage inducible 45 alpha (GADD45α) is an ubiquitously expressed nuclear protein physically interacting with cellular proteins that are implicated in DNA repair, cell cycle regulation, apoptosis, senescence, and autophagy.2 For example, it interacts with elongation factor 1α to disrupt cytoskeletal stability, consequently inducing the release of pro-apoptotic protein Bim from mitochondria to initiate apoptosis.3,4 The transcription of GADD45α is directly regulated by multiple tumor suppressors including p53, FOXO3a, and BRCA1 in response to environmental and/or physiological stresses.5 The absence of GADD45α exhibits genomic instability and increased sensitivity to carcinogens, facilitating the process of tumorigenesis.6 In fact, dysregulation of GADD45α has been observed in various types of cancer, particularly in osteosarcoma, lung, and prostate cancer.7–9 Upregulation of GADD45α is an essential step for the anti-tumor activity of chemotherapeutic agents including docetaxel, trichostatin A, and curcumin, and silence of GADD45α may weaken the therapeutic effects of these drugs.10
Several inducible systems have been investigated to control the expression of transgene, such as tetracycline-controlled transcriptional activation, artificial riboswitches, and ecdysone-regulated gene switch.11–13 CArG element has been identified as a 10-nucleotide motif of the consensus sequence CC(A/T)6GG in the Egr-1 promoter that is responsible for the inducibility of Egr-1 promoter by radiation and chemotherapeutic agents. Both synthetic CArG promoter based on isolated CArG elements and nature Egr-1 promoter have been successfully applied for the spatial and temporal control of transgene expression.14,15 They can be induced by radiation, chemotherapeutic agents, and non-toxic compounds to express the targeted gene, while remaining minimal intrinsic activity when not activated.16–18
The purpose of the present study is to evaluate the in vitro therapeutic efficacy of our suicide gene therapy vector in combination with cisplatin, resveratrol, or radiation in non-small cell lung cancer (NSCLC). We first determined the cytotoxic effects of cisplatin, resveratrol, and radiation in three NSCLC cell lines with different p53 statuses, and confirmed the responsiveness of synthetic CArG promoter that contains nine tandem-repeat copies of the new CArG sequence (CCATATAAGG) to cisplatin, resveratrol and radiation. Then, we constructed suicide gene therapy vector pE9NS.G45α that expresses GADD45α when the inducible promoter is activated. Each suicide gene therapy combination (pE9NS.G45α with cisplatin, resveratrol or radiation) successfully achieved cell survival inhibition and apoptosis induction in tested NSCLC cell lines.
Material and methods
Cell culture and chemical reagents
Human NSCLC cell lines H1299, A549, and H23 were purchased from American Type Culture Collection (ATCC), and maintained in RPMI 1640 medium (Invitrogen Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin and 100 µg/mL streptomycin (Sigma Aldrich, St Louis, MO, USA). All the cells were incubated in a humidified incubator at 37ºC with 5% CO2. Cisplatin was obtained from AdipoGen (San Diego, CA, USA) and resveratrol was manufactured by ChromaDex (Irvine, CA, USA).
Construction of plasmids
The pTargetTM Vector (Promega, Madison, WI, USA) was used as the basis for pE9NS.G45α. The human GADD45α opening reading frame (ORF) was amplified from GADD45α cDNA (Origene, MD, USA) using PCR. The fusion primers were designed by Clontech bioinformatics (Clontech Laboratories, Inc., Mountain View, CA, USA): 5ʹ-GGGCGAATTCGGATCCGCCACCATGACTTTGGAGGAATTCTCG-3ʹ and 5ʹ-TTGGAATTCGCGGCCGCTCACCGTTCAGGGAGATTAAT-3ʹ. Amplification was performed for five cycles of 15 s at 94ºC, 60 s at 53ºC and 60 s at 72ºC, with a further 25 cycles of 15 s at 94ºC, 60 s at 60ºC and 60 s at 72ºC. pTarget vector was linearized by Fast Digest BamHI and NotI (Life Technologies, Carlsbad, CA, USA) at 37ºC for 10 mins and 80 ºC for 5 mins and purified by gel purification Qiagen Kit (Qiagen Sciences, Germantown, MD, USA) after running on GTG 0.5% agarose gel. Linearized pTarget was infused with GADD45α ORF by infusion dry HD cloning kit (Clontech Laboratories, Inc., Mountain View, CA, USA) to create pT.G45α. Synthetic CArG promoter E9NS was cloned as double-strand linker molecules derived from complementary single-stranded oligonucleotides (ODN): 5ʹ-GATCT(CCATATAAGG)9GCGAT-3ʹ and 5ʹ-CGC(CCTTATATGG)9A-3ʹ. Linkers were produced by mixing 1 nm of each ODN in 20 µL volume of oligo annealing buffer (10 mM Tris.HCl, 100 mM NaCl and 10 mM EDTA), heating to 95ºC for 4 mins then cooling to room temperature. pT.G45α was linearized by Fastdigest BgI II and AsiSI (Life Technologies, Carlsbad, CA, USA) at 37ºC for 20 mins and gel purified. Linearized pT.G45α was mixed with E9NS dODN and T4 ligase in T4 ligase buffer at 22ºC for 1 hr and 70ºC for 5 mins to construct pE9NS.G45α.
Transfection of DNA
Transient transfection with plasmids was performed by using LipofectamineTM 2000 (Invitrogen Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s protocol. Cells were seeded in Opti-MEM® I Reduced Serum Medium (Life Technologies, CA, USA) containing 10% FBS and 1% Non-Essential Amino Acid (NEAA) (Lonza, Allendale, NJ, USA) without antibiotics. Complexes in a DNA (µg) to LipofectamineTM 2000 (µL) ratio of 1:2 were added directly to 70–90% confluent cells on the second day and incubated at least 24 hrs before further treatment.
Western blot analysis
Whole cell lysates were prepared as described previously.30 Protein concentration was determined using PierceTM BCA Protein Assay Kit (Thermo Scientific, Waltham, MA, USA). Cell lysates (50 µg) were electrophoresed through 4–20% precast polyacrylamide gels (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and transferred to nitrocellulose membranes (GE healthcare, USA). The blots were blocked in Tris-Buffered Saline and Tween 20 (TBST) (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) containing 4% milk and probed with the antibody GADD45α (H-165), β-actin (C4) (Santa Cruz Biotechnology, Inc., Dallas, TX, USA), caspase-3 (9662), cleaved caspase-3 (9661) or cleaved PARP (5625) (Cell Signaling Technology, MA, USA) diluted according to manufacturer’s recommendation in TBST with 5% Bovine Serum Albumin (Boston BioProducts, Ashland, MA, USA) plus 0.02% sodium azide (NaN3) overnight at 4ºC. After washed with TBST, the blots were incubated with horseradish peroxidase-conjugated anti-mouse/rabbit antibody for 1 hr, and then washed and detected by the West Pico from SuperSignal (Thermo Scientific, Waltham, MA, USA).
Cell viability assay
Cells were seeded in 96-well tissue culture plates at a density of 7,000 cells/well and treated with different doses of cisplatin, resveratrol, or radiation on the next day, or transfected with pTarget or pE9NS.G45α before exposed to 3 µM of cisplatin, 25 µM of resveratrol, and 5 Gy of radiation. Forty-eight hours later, the culture medium was replaced by a medium containing 1% FBS and 1 mg/mL of MTT (Boston BioProducts, Ashland, MA, USA). At the end of 4-hr incubation, the medium was removed carefully and the converted dye was solubilized with 150 µL of DMSO. Absorbance was measured at a wavelength of 570 nm with background subtraction at 690 nm. Cell viability was calculated by using the equation: cell viability (%) = (Atest/Acontrol) × 100%.
Dual luciferase assay
Cells were seeded in 96-well plates and cotransfected with firefly luciferase construct pE9NS. Luc and Renilla luciferase plasmid pRL-TK on the second day. Forty-eight hours after transfection, cells were stimulated by cisplatin, resveratrol or radiation and incubated overnight before lysed with M-PER® Mammalian Protein Extraction Reagent (Thermo Scientific, Waltham, MA, USA). Luciferase activity was measured by using Dual Luciferase Report Assay System (Promega, Madison, WI, USA).
Measurement of apoptotic cells by flow cytometry
The quantitative analysis of apoptosis was done by using Dead Cell Apoptosis Kit with Annexin V FITC and PI following the manufacturer's protocol (Life Technologies, Carlsbad, CA, USA). After transfection with pE9NS.G45α or pTarget and exposure to cisplatin, resveratrol or radiation for additional 48 hrs, cells were harvested with trypsin, washed with cold phosphate buffered saline and subjected to Annexin V-FITC and propidium iodide staining in binding buffer at room temperature for 15 mins in the dark. Stained cells were proceeded to flow cytometry using BD AccuriTM C6, and the data were analyzed by CFlow Plus (BD Biosciences, Franklin Lakes, NJ, USA).
Statistical analysis
Data are presented as mean ± SD or mean ± SEM. Statistical analysis was performed using an independent t-test for two-group comparison and one-way ANOVA for multiple comparisons.
Results
NSCLC cell viability is reduced by cisplatin, resveratrol, and radiation
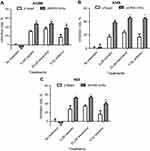
The cytotoxic effect of a certain drug may vary among different cell lines. Therefore, in order to select a reasonable dose for each combination of our suicide gene therapy, the effects of cisplatin, resveratrol, and radiation on H1299, A549 and H23 cell viability were determined by MTT assay. Cisplatin, resveratrol, and radiation reduced NSCLC cell viability in a dose-dependent manner. H23 cells were more susceptible to cisplatin and high dose of resveratrol compared with H1299 and A549 cells (Figure 1A and B). The three NSCLC cell lines had a similar dose–response curve in viability in response to radiation, and the viability dropped significantly from 2 to 5 Gy (Figure 1C).
Synthetic CArG promoter is sensitive to cisplatin, resveratrol, and radiation
Previous studies showed that synthetic CArG promoter E9NS effectively responded to radiation, chemotherapy, and resveratrol in different types of cancer cells.14,17 To confirm and compare the inducibility of E9NS promoter by cisplatin, resveratrol, and radiation in NSCLC cell lines with different p53 statuses, cells were transiently co-transfected with firefly luciferase construct containing promoter E9NS and Renilla luciferase internal control reporter plasmid pRL-TK for 48 hrs followed by the stimulation of cisplatin (1 and 5 µM), resveratrol (10 and 50 µM) or radiation (2 and 8 Gy). All the treatments increased E9NS promoter activity with a dose-dependent trend in all the tested cell lines regardless of p53 function (Figure 2A–C). Taken together, promoter E9NS is suitable to work as the inducible promoter in our suicide gene therapy.
Gadd45α expression is induced by activated pE9NS.G45α in NSCLC cells
Western blot analysis was used to assess cellular GADD45α protein levels after cells were transfected with pE9NS.G45α or pTarget for 24 hrs and then exposed to cisplatin, resveratrol, or radiation for an additional 24 hrs. None of these cell lines expressed detectable level of GADD45α protein after transfected with control vector and treated with 3 µM of cisplatin, 25 µM of resveratrol or 5 Gy of radiation. In pE9NS.G45α-transfected cells, no increase in GADD45α protein expression was observed when the suicide gene vector was inactive, however, when activated by cisplatin, resveratrol or radiation, the vector started to express GADD45α protein, and a significant elevation in GADD45α protein level was observed (Figure 3). Our data indicate that cisplatin, resveratrol, and radiation at applied doses fail to upregulate GADD45α expression in NSCLC cells, but through the suicide gene therapy vector, they are able to achieve a high protein level of GADD45α.
Inhibition of NSCLC cell survival by activated pE9NS.G45α
MTT assay was performed to evaluate cell survival after suicide gene therapy treatment. The cells were transfected with control or therapeutic vector, and on the second day exposed to 3 µM of cisplatin, 25 µM of resveratrol or 5 Gy of radiation for additional 48 hrs. As shown in Figure 4A–C, each therapeutic combination significantly decreased cell viability compared with control vector plus the same treatment. For example, a dose of 5 Gy radiation with control vector reduced A549 cell viability to 83%, but the number dropped to 56% in pE9NS.G45α-transfected A549 cells followed by irradiation (Figure 4B). In general, activated suicide gene therapy vector inhibited cell survival by 13–27%. Therefore, it is confirmed that the expression of transgene GADD45α can enhance the cytotoxicity of cisplatin, resveratrol, and radiation in NSCLC cells independent of p53.
Apoptosis is induced by activated pE9NS.G45α
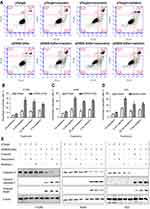
To test whether overexpression of GADD45α sensitizes NSCLC cells to cisplatin, resveratrol, and radiation by inducing apoptosis, H1299, A549, and H23 cells were transfected with control vector or pE9NS.G45α for 24 hrs, and then treated with 3 µM of cisplatin, 25 µM of resveratrol or 5 Gy of radiation for additional 2 days before subjected to Annexin V-FITC/PI staining and flow cytometry. Figure 5A is the data from H1299 cells exemplifying the results from the other two cell lines. The percentages of apoptotic cells were recorded as the sum of the percentages of early-phase apoptotic cells (Annexin V-FITC+/PI–) and late-phase apoptotic cells (Annexin V-FITC+/PI+). A significantly higher percentage of apoptosis was observed in cells transfected with pE9NS.G45α and stimulated. For instance, 3 µM of cisplatin alone increased the apoptotic rate by 10% in A549 cells, while combined with suicide gene therapy vector pE9NS.G45α, the percentage was increased to 48% (Figure 5C). Other combinations exhibited similar results. All the combinations successfully enhanced apoptosis in H1299, A549, and H23 cells and no significant differences were noticed among three NSCLC cell lines (Figure 5B–D). We also detected the levels of apoptosis-related proteins by western blot analysis, and found that the level of caspase-3 was decreased upon gene therapy treatment, while the levels of cleaved caspase-3 and cleaved PARP were increased (Figure 5E). In sum, combining our suicide gene therapy with cisplatin, resveratrol or radiation is able to induce apoptosis in NSCLC cells with various p53 statuses.
Discussion
Our study has supported the role of GADD45α as a suitable target for NSCLC therapeutics by showing that transcriptionally targeting GADD45α could reduce cell survival and induce apoptosis (Figure 6). GADD45α upregulation is involved in the anti-tumor activity of a number of cancer therapeutic agents, and overexpression of GADD45α has achieved success in several pre-clinical anti-cancer studies.19 Li et al, transfected pancreatic cells with the adenoviral construct of GADD45α and observed growth suppression caused by apoptosis and cell cycle arrest.20 Tong et al, found that upregulation of GADD45α was positively correlated with cell growth inhibition and activation of apoptosis in colon carcinoma cells.3 High expression of GADD45α is associated with good prognosis in lung squamous cell carcinoma (LSCC) patients, and heterogeneous nuclear ribonucleoprotein U-mediated GADD45α upregulation can sensitize LSCC cells to cisplatin.21 However, the function of GADD45α in cancer may be paradoxical. Knockdown of GADD45α facilitated temozolomide (TMZ)-induced cell growth arrest and programmed death in both TMZ-sensitive and TMZ-resistant glioblastoma multiforme partially by reducing TMZ-induced p53 expression.22 Blocking GADD45α expression facilitated the cytotoxic effect of ultraviolet (UV) irradiation and cisplatin in RKO cells by decreasing DNA repair, indicating that intrinsic increase of GADD45α protein may also play a role in cell survival depending on cell type, stimulus and possibly the intensity of stimulus.23 Therefore, it would be also interesting to determine whether antisense of GADD45α could benefit DNA-damage based therapy for NSCLC.
Our results demonstrate that high levels of GADD45α protein sensitize NSCLC cells to cisplatin, resveratrol, and radiation regardless of p53 status, and further reinforce that GADD45α-targeted gene therapy can be utilized for cancer prevention or treatment without concern of p53 function. p53 is a well-identified tumor suppressor, and its dysregulation including mutation and deletion is highly related to tumorigenesis and poor prognosis. As a regulator of GADD45α expression, p53 can mediate GADD45α protein levels through both transcriptional and post-transcriptional ways. The p53 binding sites on GADD45α have been well recognized, and the post-transcriptional regulation involves miR-138 and miR-130b.21 The necessity of p53 in GADD45α regulation depends on the type of stress. For example, the presence of p53 is mandatory for the induction of GADD45α by ionizing radiation, but histone deacetylase inhibitors can activate GADD45α expression without functional p53.24,25 On the other hand, p53 may modulate GADD45α-associated cellular changes. GADD45α silencing represses skin squamous cell carcinoma (SCC) cell apoptosis and senescence through p53 signaling pathway.26 Overexpression of GADD45α suppressed the proliferation of HeLa cells that are p53-inactivated through inducing apoptosis not G2/M arrest, suggesting that p53 is required for GADD45α-induced cell cycle G2/M arrest, but is unimportant in GADD45α-induced cell growth inhibition.3,27
Besides inducing apoptosis and growth arrest, GADD45α has other anti-tumor properties. It suppresses angiogenesis through blocking mTOR/STAT pathway and alters gene expression through unknown mechanisms against cancer cell migration and invasion.28,29 In our laboratory, a lower ability in wound-healing was observed after cells were transfected with GADD45α cDNA (data not shown), indicating the possibility of our suicide gene therapy in anti-invasion and anti-metastasis. A recent report revealed the inhibitory action of GADD45α in autophagy by impairing the interaction between BECN1 and PIK3C3, thereby repression of autophagy might be another mechanism contributing to GADD45α-operated programmed cell death.2
Previously, we optimized synthetic CArG promoter in response to resveratrol by changing the number and sequence of CArG elements and found that E9NS, which contains nine repeats of new CArG sequence (CCATATAAGG), had the maximal induction.14 In addition to cisplatin, resveratrol, and radiation, which have been proven in this report as appropriate candidates to combine with our suicide gene therapy vector, previous research has provided an additional tool for the candidates. Lopez et al, have affirmed that cyclophosphamide, doxorubicin, 5-FU, and gemcitabine are all able to initiate CArG element-controlled gene therapy, and reactive oxygen species (ROS) plays a vital role in the activation of CArG elements.15 Hence, other chemotherapeutic agents and natural compounds that increase ROS level may be evaluated in combination with our suicide gene therapy vector in the future.
Conclusion
In conclusion, our suicide gene therapy is potentially applicable to patients with NSCLC as an adjunctive therapy to increase the sensitivity of tumor to chemotherapy and/or radiotherapy. The suicide gene therapy vector can be combined with chemotherapeutic agents, radiation or natural compounds alone or in combination according to the tolerance of patients and the characteristics of tumors.
Acknowledgment
This work was supported in part by grants from the National Natural Science Foundation of China (No 81703181).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Scanlon KJ. Cancer gene therapy: challenges and opportunities. Anticancer Res. 2004;24(2A):501–504.
2. Zhang D, Zhang W, Li D, Fu M, Chen R, Zhan Q. GADD45A inhibits autophagy by regulating the interaction between BECN1 and PIK3C3. Autophagy. 2015;11(12):2247–2258. doi:10.1080/15548627.2015.1112484
3. Tong T, Ji J, Jin S, et al. Gadd45a expression induces Bim dissociation from the cytoskeleton and translocation to mitochondria. Mol Cell Biol. 2005;25(11):4488–4500. doi:10.1128/MCB.25.11.4488-4500.2005
4. Gao M, Guo N, Huang C, Song L. Diverse roles of GADD45alpha in stress signaling. Curr Protein Pept Sci. 2009;10(4):388–394.
5. Salvador JM, Brown-Clay JD, Fornace AJ
6. Moskalev AA, Smit-McBride Z, Shaposhnikov MV, et al. Gadd45 proteins: relevance to aging, longevity and age-related pathologies. Ageing Res Rev. 2012;11(1):51–66. doi:10.1016/j.arr.2011.09.003
7. Al-Romaih K, Sadikovic B, Yoshimoto M, Wang Y, Zielenska M, Squire JA. Decitabine-induced demethylation of 5ʹ CpG island in GADD45A leads to apoptosis in osteosarcoma cells. Neoplasia. 2008;10(5):471–480.
8. Higashi H, Vallbohmer D, Warnecke-Eberz U, et al. Down-regulation of Gadd45 expression is associated with tumor differentiation in non-small cell lung cancer. Anticancer Res. 2006;26(3A):2143–2147.
9. Ramachandran K, Gopisetty G, Gordian E, et al. Methylation-mediated repression of GADD45alpha in prostate cancer and its role as a potential therapeutic target. Cancer Res. 2009;69(4):1527–1535. doi:10.1158/0008-5472.CAN-08-3609
10. Tamura RE, de Vasconcellos JF, Sarkar D, Libermann TA, Fisher PB, Zerbini LF. GADD45 proteins: central players in tumorigenesis. Curr Mol Med. 2012;12(5):634–651.
11. Zhu Z, Zheng T, Lee CG, Homer RJ, Elias JA. Tetracycline-controlled transcriptional regulation systems: advances and application in transgenic animal modeling. Semin Cell Dev Biol. 2002;13(2):121–128.
12. Ketzer P, Kaufmann JK, Engelhardt S, et al. Artificial riboswitches for gene expression and replication control of DNA and RNA viruses. Proc Natl Acad Sci U S A. 2014;111(5):E554–E562. doi:10.1073/pnas.1318563111
13. Saez E, Nelson MC, Eshelman B, et al. Identification of ligands and coligands for the ecdysone-regulated gene switch. Proc Natl Acad Sci U S A. 2000;97(26):14512–14517. doi:10.1073/pnas.260499497
14. Shi Q, Geldenhuys W, Sutariya V, Bishayee A, Patel I, Bhatia D. CArG-driven GADD45alpha activated by resveratrol inhibits lung cancer cells. Genes Cancer. 2015;6(5–6):220–230. doi:10.18632/genesandcancer.62
15. Lopez CA, Kimchi ET, Mauceri HJ, et al. Chemoinducible gene therapy: a strategy to enhance doxorubicin antitumor activity. Mol Cancer Ther. 2004;3(9):1167–1175.
16. Weichselbaum RR, Kufe D. Translation of the radio- and chemo-inducible TNFerade vector to the treatment of human cancers. Cancer Gene Ther. 2009;16(8):609–619. doi:10.1038/cgt.2009.37
17. Greco O, Powell TM, Marples B, Joiner MC, Scott SD. Gene therapy vectors containing CArG elements from the Egr1 gene are activated by neutron irradiation, cisplatin and doxorubicin. Cancer Gene Ther. 2005;12(7):655–662. doi:10.1038/sj.cgt.7700834
18. Bickenbach KA, Veerapong J, Shao MY, et al. Resveratrol is an effective inducer of CArG-driven TNF-alpha gene therapy. Cancer Gene Ther. 2008;15(3):133–139. doi:10.1038/sj.cgt.7701103
19. Cretu A, Sha X, Tront J, Hoffman B, Liebermann DA. Stress sensor Gadd45 genes as therapeutic targets in cancer. Cancer Ther. 2009;7(A):268–276.
20. Li Y, Qian H, Li X, et al. Adenoviral-mediated gene transfer of Gadd45a results in suppression by inducing apoptosis and cell cycle arrest in pancreatic cancer cell. J Gene Med. 2009;11(1):3–13. doi:10.1002/jgm.1270
21. Li J, Dong J, Li S, et al. An alternative microRNA-mediated post-transcriptional regulation of GADD45A by p53 in human non-small-cell lung cancer cells. Sci Rep. 2017;7(1):7153. doi:10.1038/s41598-017-07332-3
22. Wang HH, Chang TY, Lin WC, Wei KC, Shin JW. GADD45A plays a protective role against temozolomide treatment in glioblastoma cells. Sci Rep. 2017;7(1):8814. doi:10.1038/s41598-017-06851-3
23. Smith ML, Kontny HU, Zhan Q, Sreenath A, O’Connor PM, Fornace AJ
24. Kastan MB, Zhan Q, el-Deiry WS, et al. A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992;71(4):587–597.
25. Hirose T, Sowa Y, Takahashi S, et al. p53-independent induction of Gadd45 by histone deacetylase inhibitor: coordinate regulation by transcription factors Oct- 1and NF-Y. Oncogene. 2003;22(49):7762–7773. doi:10.1038/sj.onc.1207091
26. Liu LQ, Tian FJ, Xiong Y, Zhao Y, Song JB. Gadd45a gene silencing by RNAi promotes cell proliferation and inhibits apoptosis and senescence in skin squamous cell carcinoma through the p53 signaling pathway. J Cell Physiol. 2018;233(9):7424–7434. doi:10.1002/jcp.26588
27. Jin S, Tong T, Fan W, et al. GADD45-induced cell cycle G2-M arrest associates with altered subcellular distribution of cyclin B1 and is independent of p38 kinase activity. Oncogene. 2002;21(57):8696–8704. doi:10.1038/sj.onc.1206034
28. Yang F, Zhang W, Li D, Zhan Q. Gadd45a suppresses tumor angiogenesis via inhibition of the mTOR/STAT3 protein pathway. J Biol Chem. 2013;288(9):6552–6560. doi:10.1074/jbc.M112.418335
29. Shan Z, Li G, Zhan Q, Li D. Gadd45a inhibits cell migration and invasion by altering the global RNA expression. Cancer Biol Ther. 2012;13(11):1112–1122. doi:10.4161/cbt.21186
30. Shi Q, Sutariya V, Bishayee A, Bhatia D. Sequential activation of Elk-1/Egr-1/GADD45alpha by arsenic. Oncotarget. 2014;5(11):3862–3870. doi:10.18632/oncotarget.1995
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.