Back to Journals » Pharmacogenomics and Personalized Medicine » Volume 15
GABRA1 and GABRB2 Polymorphisms are Associated with Propofol Susceptibility
Authors Zeng Y , Cao S, Chen M, Fang C , Ouyang W
Received 6 November 2021
Accepted for publication 24 January 2022
Published 9 February 2022 Volume 2022:15 Pages 105—117
DOI https://doi.org/10.2147/PGPM.S348170
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Youjie Zeng,1 Si Cao,1 Minghua Chen,1 Chao Fang,1,2 Wen Ouyang1
1Department of Anesthesiology, Third Xiangya Hospital, Central South University, Changsha, Hunan, 410013, People’s Republic of China; 2Hunan Cancer Hospital, The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, Hunan, 410013, People’s Republic of China
Correspondence: Wen Ouyang, Department of Anesthesiology, Third Xiangya Hospital, Central South University, Changsha, Hunan, 410013, People’s Republic of China, Email [email protected]
Purpose: To explore the effect of gene polymorphisms of propofol GABAA receptor and metabolic enzyme on drug susceptibility during the induction period of general anesthesia.
Patients and Methods: A total of 294 female patients aged 18– 55 years, ASA I–II, who underwent hysteroscopy with intravenous general anesthesia, were included in the study. Anesthesia was induced by continuous intravenous infusion of propofol at 40 mg·kg− 1·h− 1. Infusion of propofol was ended when both the Modified Observer’s Assessment of Awareness/Sedation scale (MOAA/S scale) decreased to 1 and the BIS index decreased to 60. The time when the MOAA/S scale decreased to 1 and the time when BIS index decreased to 60 was recorded to assess the susceptibility to the sedation effect. The maximum decreased percentage in mean arterial pressure (MAP) within 5 minutes was recorded to assess the susceptibility of cardiovascular response. Venous blood of each patient was collected to identify the presence of genetic variants in the GABRA1, GABRA2, GABRB2, GABRB3, GABRG2, CYP2B6, and UGT1A9 genes using the Sequenom MassARRAY® platform.
Results: After receiving propofol infusion, carriers of polymorphic GABRA1 rs4263535 G allele required significantly less time for BIS decreased to 60, while carriers of polymorphic GABRB2 rs3816596 T allele required significantly more time for BIS decreased to 60, carriers of polymorphic GABRA1 rs1157122 C allele and carriers of polymorphic GABRB2 rs76774144 T allele had a significantly less change in MAP.
Conclusion: GABRB2 rs3816596 and GABRA1 rs4263535 polymorphisms are associated with susceptibility to the sedation effect of propofol. GABRA1 rs1157122 and GABRB2 rs76774144 polymorphisms are associated with the degree of drop in blood pressure after propofol infusion.
Keywords: pharmacogenomics, drug susceptibility, GABAA receptor, CYP2B6, UGT1A9
Introduction
Propofol is an intravenous anesthetic that is frequently used for induction of general anesthesia, maintenance of general anesthesia, sedation in the intensive care unit (ICU), and various painless treatments due to its advantages of rapid onset, rapid awakening, and low incidence of postoperative nausea and vomiting.1
Despite the numerous advantages, propofol can still carry some side effects. Frequent adverse reactions are pain on injection, hypotension, and respiratory depression.2 In addition, the drug effect of propofol varies with different individuals, even when administered by the same standard.3 Deep anesthesia will excessively suppress the stress response, leading to severe hypotension and even disrupting the perfusion of vital organs, while inadequate depth of anesthesia will lead to an increased incidence of intraoperative awareness. Therefore, it is important to individualize the medication and give patients the most proper dose to maintain an appropriate depth of anesthesia.
Propofol is mainly metabolized in the liver.4 Seventy percent of propofol bound to uridine diphosphate glucuronosyltransferase 1A9 (UGT1A9) and transform into propofol glucuronide, 29% of propofol is transformed into propofol-4-hydroxypropophol by CYP2B6 and CYP2C9, in which CYP2B6 plays a primary role and CYP2C9 a secondary role.5 Propofol exerts its sedation effect mainly through activating the GABAA receptor (GABAAR), thereby enhancing the interaction between GABAA and GABAAR.6
Pharmacogenomics focuses on the relationship between genetic factors and drug response variability.7 Single nucleotide polymorphism (SNP) refers to a DNA sequence polymorphism caused by a single nucleotide variation that has a prevalence of more than 1% in the population.8 Pharmacogenomics studies often concern the SNPs related to the pharmacodynamic and pharmacokinetic of certain drugs.
Identifying SNPs associated with drug response can help reduce adverse drug reactions, particularly important in patients with poor general conditions.9 For instance, genotype-guided treatment can optimize the dosage of antithrombotic drugs and thus reduce the risk of bleeding complications.10 However, the application of pharmacogenomics in anesthetics is currently limited.11 Therefore, exploring potential SNPs associated with propofol susceptibility is necessary to achieve precision and personalized medicine during the perioperative period.
This study aims to explore the association between gene polymorphisms (CYP2B6, UGT1A9, GABRA1, GABRA2, GABRB2, GABRB3, and GABRG2) and propofol susceptibility.
Materials and Methods
Study Participants
From October 2020 to January 2021, 294 Chinese Han female patients who underwent hysteroscopy under general anesthesia were recruited. Every patient met the following criteria: 1) ASA I–II; 2) age 18–55 years; 3) body mass index (BMI) 18–28 kg/m2. Patients were excluded if they met one of these exclusion criteria: 1) abnormal liver and kidney function; 2) severe cardiopulmonary disease or hemodynamic instability; 3) pregnant; 4) mental disease; 5) allergy to propofol; 6) history of drug abuse. Our study was registered with the Chinese Clinical Trial Registry under registration number ChiCTR2000039432 (https://www.chictr.org.cn/index.aspx). The study was approved by the ethics committee of The Third Xiangya Hospital of Central South University (registration number: Fast I 20032). Written informed consent was signed by each patient. This study was conducted in accordance with the Declaration of Helsinki.
Treatments
Patients did not receive pre-operative medication. Non-invasive blood pressure (NIBP), electrocardiogram (ECG), pulse oxygen saturation (SPO2), and BIS index were routinely monitored. Propofol was administered 40 mg·kg−1·h−1 through the infusion pump. This pumping method provides adequate discrimination of individual dose requirements of propofol and possibly enables propofol to mix entirely in the central pharmacokinetic compartment.12 Infusion of propofol was ended when both MOAA/S scale decreased to 1 and BIS index decreased to 60. Blood pressure was monitored every minute for 5 minutes. The observation was ended after 5 minutes, and surgical operations were subsequently performed.
Assessment of Propofol Susceptibility
The time when the MOAA/S scale (score 5=patient responds rapidly to normal-sized tone calls to names; 4=patient responds dully to normal-sized tone calls to names; 3=patient responds only to loud or repeated name calls; 2=patient responds only to gentle shaking of the body; 1=patient does not respond to gentle shaking of the body and responds only to painful stimulation) decreased to 1 and the time when BIS index decreased to 60 were recorded to assess the susceptibility to the sedation susceptibility to propofol. The baseline mean arterial pressure (MAP) was recorded, and the maximal percentage decrease in MAP within 5 minutes was recorded to assess the susceptibility of cardiovascular response.
SNP Selection
We conducted an extensive literature study related to the metabolic pathways and receptor proteins of propofol. SNPs with minor allele frequencies (MAF) greater than 0.05 in Chinese Han nationality were screened through the Ensembl database (https://www.ensembl.org/). In total, 22 SNPs located in 7 different genes were selected (Table 1). Two of the investigated genes are involved in propofol pharmacokinetics (CYP2B6 and UGT1A9). Five genes participate in the anesthetic mechanism of propofol (GABRA1, GABRA2, GABRB2, GABRB3, and GABRG2).
 |
Table 1 Selected Genes and Polymorphisms |
Isolation of Genomic DNA
Genomic DNA was isolated from the patient’s peripheral blood using the TIANamp genomic DNA kit (Tiangen Biotech (Beijing) Co. Ltd., China). The concentration and quality of DNA were detected by UV spectrophotometer and agarose gel electrophoresis.
Primers Design for Detection of the SNP Site
According to the SNP site, primers were designed by the software of Assay Design 3.1 of Sequenom Company. PCR primers and extension primers are shown in Table 2.
 |
Table 2 The Sequences of PCR Primers and Extension Primers |
SNP Genotyping
DNA template containing SNP sites was amplified by PCR, using shrimp alkaline phosphatase to neutralize unincorporated dNTPs in amplification products, and then a single base extension was carried out. After being purified by resin, the extended products were transferred onto a SpectroCHIP by the MassARRAY nano dispenser. The SpectroCHIP was then analyzed by the MassARRAY analyzer compact. The mass spectrum peaks were detected by MassARRAY Typer 4.0 software, and the genotypes of target sites were interpreted according to the mass spectrum peaks.
Statistical Analysis
SPSS 25.0 statistical software was used for statistical analysis. Pearson χ2 test was adopted to assess Hardy–Weinberg equilibrium (HWE). The genotypes of each tested SNP were divided into two groups: 1) homozygotes of the major alleles and 2) combination of homozygotes and heterozygotes of the minor allele. As the sedation effect data did not follow a normal distribution, the results were presented in a median with an interquartile. Analysis of sedation effects between every two groups was performed by Mann–Whitney U-test. As the maximal percentage decrease in MAP followed a normal distribution, the results were presented as a mean value with standard deviation. Analysis of maximal percentage decrease in MAP between every two groups was performed by independent sample t-test. P values lower than 0.05 were considered statistically significant.
Results
Genotyping Results
In this study, 22 SNPs in 294 individuals were genotyped. The genotype distributions of the 22 selected SNPs are shown in Table 1. No useful results were obtained from testing on GABRB2 rs6556547. Except for GABRA1 rs77332276 and GABRA1 rs78446575, the frequencies of all other polymorphisms followed the Hardy–Weinberg equilibrium (HWE).
Propofol Susceptibility Results
Detailed information about the clinical characteristics of 294 included patients is given in Table 3. According to our result, after propofol infusion, the time MOAA/S scale decreased to 1 varied from 100 seconds to 300 seconds (3.0-fold), the time BIS index decreased to 60 varied from 115 seconds to 300 seconds (2.6-fold), and the maximal percentage decreased in MAP within 5 minutes varied from 7.51% to 38.98% (5.2-fold), indicating that propofol susceptibility varied from individuals.
 |
Table 3 Clinical Characteristics of 294 Patients |
Correlation Between Genotype and Propofol Susceptibility
The time MOAA/S scale decreased to 1 (s), the time BIS index decreased to 60 (s), and the maximal percentage decrease in MAP (%) were recorded to assess propofol susceptibility.
Excluding the two SNPs that did not follow Hardy–Weinberg’s equilibrium law (GABRA1 rs77332276 and GABRA1 rs78446575) and the SNP with no genotype result (GABRB2 rs6556547), the remaining 19 SNPs were continued to be analyzed.
Based on genotypes of each SNP, patients were divided into two groups: 1) homozygotes of the major alleles and 2) combination of homozygotes and heterozygotes of the minor allele. Propofol susceptibility was compared between two groups of each SNP. There were significant differences in the time BIS index decreased to 60 between two groups of GABRA1 rs4263535 (AA group vs AG+GG group) and GABRB2 rs3816596 (CC group vs CT+TT group) (Table 4). In addition, there were significant differences in the percentage of maximal decrease in MAP between two groups of GABRA1 rs1157122 (TT group vs CT+CC group) and GABRB2 rs76774144 (CC group vs CT+TT group) (Table 4). The remaining 15 SNPs showed no significant difference in propofol susceptibility (Table 5).
 |
Table 4 SNPs with Detected Significant Differences in Propofol Susceptibility |
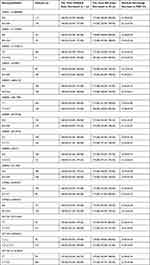 |
Table 5 SNPs with No Significant Difference in Propofol Susceptibility |
Comparison of Clinical Characteristics Between Groups
To eliminate the effect of clinical features of the patients, we further analyzed the clinical characteristics (age, height, weight, and BMI) between different genotype groups of significant SNPs (Table 6). No statistical differences were shown in the general clinical characteristics between the groups.
 |
Table 6 Clinical Characteristics of Each Genotype Group of GABRA1 rs1157122, GABRA1 rs4263535, GABRB2 rs3816596, and GABRB2 rs76774144 |
GABRA1 rs4263535 and GABRB2 rs3816596 are Associated with Sedation Effect of Propofol
In our study, carriers of polymorphic GABRA1 rs4263535 G allele required significantly less time for BIS decreased to 60 (180.00 [160.00–200.00] vs 172.50 [150.25–190.75], Z = −1.984, p = 0.047) (Table 4). The results indicate that G carriers of GABRA1 rs4263535 are more susceptible to the sedation effect of propofol.
In addition, carriers of polymorphic GABRB2 rs3816596 T allele required significantly more time for BIS decreased to 60 (170.00[150.00–188.00] vs 180.00[158.00–199.00], Z = −2.212, p = 0.027) (Table 4). The results indicate that T carriers of GABRB2 rs3816596 are less susceptible to the sedation effect of propofol.
GABRA1 rs1157122 and GABRB2 rs76774144 are Associated with Percentage of Maximal Decrease in MAP After Propofol Infusion
Since the dose of propofol can affect the degree of drop in blood pressure, analyses of the total dose of propofol between two genotype groups of GABRA1 rs1157122 and GABRB2 rs76774144 are necessary. The results showed no statistical difference in dose between two groups (p>0.05) (Table 7).
 |
Table 7 Dose of Propofol of Each Genotype Group of GABRA1 rs1157122 and GABRB2 rs76774144 |
Carriers of polymorphic GABRA1 rs1157122 C allele had a less change in MAP within 5 minutes after receiving propofol infusion ([24.51%±5.81]% vs [22.74%±5.45%], t = −2.569, p = 0.011) (Table 4). Likewise, carriers of polymorphic GABRB2 rs76774144 T allele had a less change of MAP within 5 minutes after receiving propofol infusion ([23.95%±5.69%] vs [22.25%±5.46%], t = 1.992, p = 0.047) (Table 4).
Discussion
Precision dosing aims to provide individualized dosing regimens based on the variability of the patient’s response to the drug, which is particularly relevant in the case of drugs with a narrow therapeutic window and severe side effects.13 With the development of pharmacogenomics, researchers have revealed that genetic factors can affect an individual’s sensitivity to drugs.14 SNPs on metabolic enzyme genes and receptor protein genes of drugs widely present in the human genome.15 However, only a minority of SNPs were significantly associated with drug effects. Thus, identifying SNPs that have an enormous impact on the pharmacodynamics or pharmacokinetics of drugs is significant.
The precise control of the anesthetics dose helps achieve precision medicine during the perioperative period.16 Adjusting the depth of anesthesia is an essential portion of the perioperative period, as anesthetic depth influences the outcome of patients.17 Propofol is one of the most frequently used intravenous general anesthetics, but the drug effect varies among individuals.3 A previous study has reported that ethnicity affects the required dose of propofol,18 indicating genetic factors as a cause of variation in propofol susceptibility. There remain numerous SNPs that are associated with propofol susceptibility to be explored.
We examined the drug effects of propofol in 294 Chinese female patients during the induction period of general anesthesia. The sedation susceptibility to propofol (The time MOAA/S scale decreased to 1 and the time BIS index decreased to 60) and MAP decrease were recorded. Twenty-two SNPs were genotyped for each patient. The result showed that both the sedation effect and decrease of MAP vary from individuals during the induction period of anesthesia.
CYP2B6 is a hepatic cytochrome P450 enzyme with exceptionally high inter-individual variability.19 CYP2B6 plays an important role in the metabolism of propofol, participating in the hydroxylation process.20 CYP2B6 rs3745274 (c.516G>T) and rs2279343 (c.785A>G) are two missense mutations that occur in exons. Several studies have demonstrated that CYP2B6 rs3745274 affects the metabolic rate of propofol and influences the total propofol dose during the perioperative period.21–25 CYP2B6 rs2279343 has also been reported to affect the metabolism of propofol.26 In contrast, some studies have reported that CYP2B6 rs3745274 contributes little to the variation of drug effects of propofol.3,27–30 Our current results suggest that CYP2B6 rs3745274 and rs2279343 do not influence propofol susceptibility during the induction period of anesthesia.
UGT1A9 is involved in the glucuronidation process in propofol metabolism.31 UGT1A9 c.98T>C30 and UGT1A9 –440C>T32 have been reported to be associated with the required dose of propofol. UGT1A9 rs2741049 (I399C> T) is a high-frequency mutation that occurs in an intron and increases glucuronidation activity.33 rs13418420 (−1818T > C) and rs3832043 (−118 > insT, T9 > T10) locate near to the 5’ end of gene UGT1A9, which may affect the transcription of UGT1A9 mRNA. However, our results suggest that none of the three UGT1A9 SNPs examined were associated with propofol drug sensitivity during anesthesia induction.
Although some SNPs in CYP2B6 and UGT1A9 have been reported to be potential impact factors of propofol susceptibility in some previous studies, none of the metabolic enzyme SNPs detected in our study is significantly associated with anesthetic sensitivity. This may be attributed to the experimental design. Since the observation duration was limited to the anesthetic induction period, the SNPs are challenging to influence the effects of the function of metabolic enzymes significantly.
GABAA receptor plays a vital role in the anesthetic effects of propofol and is composed of a very broad species of subunits.34 Most GABAARs are composed of two α1 subunits, two β2 subunits, and one γ2 subunit.35 The research on the influence of SNPs of GABAA receptor genes in propofol susceptibility is currently scarce. According to Zhong et al, GABRA1 rs2279020 is associated with sedation susceptibility to propofol.36 In our study, 17 SNPs in GABAA receptors (GABRA1, GABRA2, GABRB2, GABRB3, and GABRG2) were investigated. In conclusion, our results show that GABRA1 rs4263535 and GABRB2 rs381659 significantly correlate with the individual variation of the propofol sedation effect.
Hypotension is one of the most frequent adverse effects of propofol, which may cause inadequate perfusion of vital organs, leading to serious complications.37 Zhong et al first explored the relationship between SNPs of GABAA receptor genes and hypotension after propofol infusion. Their results indicated that GABRA1 rs2279020 and GABRA2 rs11503014 influence the cardiovascular response after propofol infusion.36 Contrary to their previous research, our results did not show the correlation between GABRA2 rs11503014 and the degree of MAP decrease. However, our results still show the same trend (GABRA2 rs11503014 - CC vs CG+GG = [23.33±5.63] vs [25.43±5.76], p = 0.053). This insignificant result may be attributed to the insufficient of patients included. Nevertheless, our present study showed that GABRA1 rs115712 and GABRB2 rs76774144 impacted the degree of MAP decrease after propofol infusion. It has been reported that GABAA plays an essential role in regulating the cardiovascular system and sympathetic activity by affecting the hypothalamic paraventricular nucleus (PVN) and rostral ventrolateral medulla (RVLM).38 The SNPs of the GABAA receptor may influence the sympathetic activity regulated by GABAA and thus affects the degree of blood pressure decrease after propofol infusion.
Limitations
The sample size of this study was relatively small, and the observations in this study were limited to the induction period of anesthesia rather than the entire perioperative period. Additional studies are required with more participants and more extended observation for drug response.
Conclusion
This study suggests that GABRA1 rs4263535 and GABRB2 rs3816596 are associated with susceptibility to the sedation effect of propofol. In addition, GABRA1 rs1157122 and GABRB2 rs76774144 polymorphisms are associated with the degree of drop in blood pressure after propofol infusion.
Data Sharing Statement
Individual deidentified participant data used to support the results of this study are available from the corresponding author based on reasonable demand. Data will be available within 1 year of publication.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sahinovic MM, Struys M, Absalom AR. Clinical pharmacokinetics and pharmacodynamics of propofol. Clin Pharmacokinet. 2018;57(12):1539–1558.
2. Marik PE. Propofol: therapeutic indications and side-effects. Curr Pharm Des. 2004;10(29):3639–3649.
3. Iohom G, Ni Chonghaile M, O’Brien JK, Cunningham AJ, Fitzgerald DF, Shields DC. An investigation of potential genetic determinants of propofol requirements and recovery from anaesthesia. Eur J Anaesthesiol. 2007;24(11):912–919.
4. Takizawa D, Hiraoka H, Goto F, Yamamoto K, Horiuchi R. Human kidneys play an important role in the elimination of propofol. Anesthesiology. 2005;102(2):327–330.
5. Mikstacki A, Skrzypczak-Zielinska M, Tamowicz B, Zakerska-Banaszak O, Szalata M, Slomski R. The impact of genetic factors on response to anaesthetics. Adv Med Sci. 2013;58(1):9–14.
6. Rudolph U, Antkowiak B. Molecular and neuronal substrates for general anaesthetics. Nat Rev Neurosci. 2004;5(9):709–720.
7. Guo C, Xie X, Li J, et al. Pharmacogenomics guidelines: current status and future development. Clin Exp Pharmacol Physiol. 2019;46(8):689–693.
8. Gardiner SJ, Begg EJ. Pharmacogenetics, drug-metabolizing enzymes, and clinical practice. Pharmacol Rev. 2006;58(3):521–590.
9. Lozupone M, Panza F, Stella E, et al. Pharmacogenetics of neurological and psychiatric diseases at older age: has the time come? Expert Opin Drug Metab Toxicol. 2017;13(3):259–277.
10. Conti V, Corbi G, Manzo V, et al. The role of pharmacogenetics in antithrombotic therapy management: new achievements and barriers yet to overcome. Curr Med Chem. 2021;28(32):6675–6703.
11. Xie S, Ma W, Guo Q, et al. The pharmacogenetics of medications used in general anesthesia. Pharmacogenomics. 2018;19(3):285–298.
12. Morley AP, Papageorgiou CH, Marinaki AM, Cooper DJ, Lewis CM. The effect of pre-operative anxiety on induction of anaesthesia with propofol. Anaesthesia. 2008;63(5):467–473.
13. Polasek TM, Shakib S, Rostami-Hodjegan A. Precision dosing in clinical medicine: present and future. Expert Rev Clin Pharmacol. 2018;11(8):743–746.
14. Roden DM, McLeod HL, Relling MV, et al. Pharmacogenomics. Lancet. 2019;394(10197):521–532.
15. Robert F, Pelletier J. Exploring the impact of single-nucleotide polymorphisms on translation. Front Genet. 2018;9:507.
16. Pal N, Kertai MD. Perioperative precision medicine: where are we in 2020? Curr Opin Anaesthesiol. 2020;33(3):463–474.
17. Zorrilla-Vaca A, Healy RJ, Wu CL, Grant MC. Relation between bispectral index measurements of anesthetic depth and postoperative mortality: a meta-analysis of observational studies. Can J Anaesth. 2017;64(6):597–607.
18. Natarajan A, Strandvik GF, Pattanayak R, et al. Effect of ethnicity on the hypnotic and cardiovascular characteristics of propofol induction. Anaesthesia. 2011;66(1):15–19.
19. Desta Z, El-Boraie A, Gong L, et al. PharmVar GeneFocus: CYP2B6. Clin Pharmacol Ther. 2021;110(1):82–97.
20. Bach-Rojecky L, Vadunec D, Lozic M, et al. Challenges in anesthesia personalization: resolving the pharmacogenomic puzzle. Per Med. 2019;16(6):511–525.
21. Kansaku F, Kumai T, Sasaki K, et al. Individual differences in pharmacokinetics and pharmacodynamics of anesthetic agent propofol with regard to CYP2B6 and UGT1A9 genotype and patient age. Drug Metab Pharmacokinet. 2011;26(5):532–537.
22. Mastrogianni O, Gbandi E, Orphanidis A, et al. Association of the CYP2B6 c.516G>T polymorphism with high blood propofol concentrations in women from northern Greece. Drug Metab Pharmacokinet. 2014;29(2):215–218.
23. Kobayashi Y, Yokozuka M, Miyakawa H, Watanabe M, Kumai T, Tateda T. Effects of genetic polymorphism of CYP2B6 and UGT1A9 and sex differences on pharmacokinetics of propofol. J St Marianna Univ. 2015;6(2):183–193.
24. Mourao AL, de Abreu FG, Fiegenbaum M. Impact of the Cytochrome P450 2B6 (CYP2B6) gene polymorphism c.516G>T (rs3745274) on propofol dose variability. Eur J Drug Metab Pharmacokinet. 2016;41(5):511–515.
25. Mikstacki A, Zakerska-Banaszak O, Skrzypczak-Zielinska M, et al. The effect of UGT1A9, CYP2B6 and CYP2C9 genes polymorphism on individual differences in propofol pharmacokinetics among Polish patients undergoing general anaesthesia. J Appl Genet. 2017;58(2):213–220.
26. Eugene AR. CYP2B6 genotype guided dosing of propofol anesthesia in the elderly based on nonparametric population pharmacokinetic modeling and simulations. Int J Clin Pharmacol Toxicol. 2017;6(1):242–249.
27. Loryan I, Lindqvist M, Johansson I, et al. Influence of sex on propofol metabolism, a pilot study: implications for propofol anesthesia. Eur J Clin Pharmacol. 2012;68(4):397–406.
28. Choong E, Loryan I, Lindqvist M, et al. Sex difference in formation of propofol metabolites: a replication study. Basic Clin Pharmacol Toxicol. 2013;113(2):126–131.
29. Kanaya A, Sato T, Fuse N, Yamaguchi H, Mano N, Yamauchi M. Impact of clinical factors and UGT1A9 and CYP2B6 genotype on inter-individual differences in propofol pharmacokinetics. J Anesth. 2018;32(2):236–243.
30. Pavlovic D, Budic I, Jevtovic Stoimenov T, et al. The effect of UGT1A9, CYP2B6 and CYP2C9 genes polymorphism on propofol pharmacokinetics in children. Pharmgenomics Pers Med. 2020;13:13–27.
31. Dinis-Oliveira RJ. Metabolic profiles of propofol and fospropofol: clinical and forensic interpretative aspects. Biomed Res Int. 2018;2018:6852857.
32. Wang YB, Zhang RZ, Huang SH, Wang SB, Xie JQ. Relationship between UGT1A9 gene polymorphisms, efficacy, and safety of propofol in induced abortions amongst Chinese population: a population-based study. Biosci Rep. 2017;37(5):BSR20170722.
33. Girard H, Villeneuve L, Court MH, et al. The novel UGT1A9 intronic I399 polymorphism appears as a predictor of 7-ethyl-10-hydroxycamptothecin glucuronidation levels in the liver. Drug Metab Dispos. 2006;34(7):1220–1228.
34. Solomon VR, Tallapragada VJ, Chebib M, Johnston GAR, Hanrahan JR. GABA allosteric modulators: an overview of recent developments in non-benzodiazepine rs. Eur J Med Chem. 2019;171:434–461.
35. Zhu S, Noviello CM, Teng J, Walsh RM, Kim JJ, Hibbs RE. Structure of a human synaptic GABA receptor. Nature. 2018;559(7712):67–72.
36. Zhong Q, Chen X, Zhao Y, Liu R, Yao S. Association of polymorphisms in pharmacogenetic candidate genes with propofol susceptibility. Sci Rep. 2017;7(1):3343.
37. Bijker JB, van Klei WA, Kappen TH, van Wolfswinkel L, Moons KGM, Kalkman CJ. Incidence of intraoperative hypotension as a function of the chosen definition: literature definitions applied to a retrospective cohort using automated data collection. Anesthesiology. 2007;107(2):213–220.
38. Milanez MIO, Silva AM, Perry JC, et al. Pattern of sympathetic vasomotor activity induced by GABAergic inhibition in the brain and spinal cord. Pharmacol Rep. 2020;72(1):67–79.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
