Back to Journals » Clinical Interventions in Aging » Volume 18
Future Perspectives to Improve CHA2DS2VASc Score: The Role of Left Atrium Remodelling, Inflammation and Genetics in Anticoagulation of Atrial Fibrillation
Authors Rachieru C , Luca CT, Văcărescu C, Petrescu L, Cirin L , Cozma D
Received 26 June 2023
Accepted for publication 7 October 2023
Published 18 October 2023 Volume 2023:18 Pages 1737—1748
DOI https://doi.org/10.2147/CIA.S427748
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Nandu Goswami
Ciprian Rachieru,1– 3 Constantin-Tudor Luca,4– 6 Cristina Văcărescu,4– 6 Lucian Petrescu,4 Liviu Cirin,4 Dragos Cozma4– 6
1Faculty of Medicine, Department of Internal Medicine I, Discipline of Medical Semiology I “Victor Babes” University of Medicine and Pharmacy, Timisoara, 300041, Romania; 2Internal Medicine Department, County Emergency Hospital, Timisoara, 300079, Romania; 3Center for Advanced Research in Cardiovascular Pathology and Hemostaseology, “Victor Babes” University of Medicine and Pharmacy, Timisoara, 300041, Romania; 4Cardiology Department, “Victor Babes” University of Medicine and Pharmacy, Timisoara, 300041, Romania; 5Institute of Cardiovascular Diseases Timisoara, Timisoara, 300310, Romania; 6Research Center of the Institute of Cardiovascular Diseases Timisoara, Timisoara, 300310, Romania
Correspondence: Cristina Văcărescu, Cardiology Department, Institute of Cardiovascular Diseases, 13A Gheorghe Adam Street, Timișoara, 300310, Romania, Tel +40 722 956 370, Fax +40 256 207 362, Email [email protected]
Abstract: In 10% of ischemic strokes, non-valvular atrial fibrillation (NVAF) is detected retroactively. Milder, or even asymptomatic forms of NVAF have shown high mortality, thrombotic risk, and deterioration of cognitive function. The current guidelines for the diagnosis and treatment of AF contain “grey areas”, such as the one related to anticoagulant treatment in men with CHA2DS2-VASc score 1 and women with score 2. Moreover, parameters such as renal function, patient weight or left atrium remodelling are missing from the recommended guidelines scores. Vulnerable categories of patients including the elderly population, high hemorrhagic risk patients or patients with newly diagnosed paroxysmal episodes of atrial high rate at device interrogation are at risk of underestimation of the thrombotic risk. This review presents a systematic exposure of the most important gaps in evaluation of thrombotic and hemorrhagic risk in patients with NVAF. The authors propose new algorithms and risk factors that should be taken into consideration for an accurate thrombotic and hemorrhagic risk estimation, especially in vulnerable categories of patients.
Keywords: atrial fibrillation, thrombotic risk, hemorrhagic risk, new risk scores, left atrium remodelling, genetics and inflammation in atrial fibrillation
Introduction
In 10% of ischemic strokes, non-valvular atrial fibrillation (NVAF) is detected retroactively.1 Looking back, even the European register (EORP-AF) notes some surprising aspects, after only 1 year of follow-up. First, the apparently milder forms – asymptomatic or very recently detected – show the highest mortality and thrombotic complication score.2,3 Moreover, the absence of thrombotic risk assessment of renal function (in CHA2DS2-VASc score) is accompanied by a relatively constant deterioration of renal function, even over the short time interval specified.4 Not to be neglected is also the percentage of deterioration of cognitive function (not only by endothelial and implicit hemodynamic mechanisms, but also by repeated micro-embolic phenomena), as well as that of progressive deterioration of the contractile capacity of the heart, with significant hemodynamic impairment.
This review presents a systematic exposure of the most important gaps in the evaluation of thrombotic and hemorrhagic risk in patients with NVAF. The authors highlight the gray zones of the current guidelines and propose new algorithms and risk factors that should be taken into consideration for an accurate thrombotic and hemorrhagic risk estimation, especially in vulnerable categories of patients.
Grey Areas in Atrial Fibrillation Anticoagulation
The latest ESC guideline on the diagnosis and treatment of AF5 better frames the aims of the management of this arrhythmia in the context of the etiopathogenesis and the comorbidities installed, insisting not only on prolonging the duration of life but above all on the quality of life, starting from the correction of rhythm vs frequency, hemodynamic, motor, and psychological tolerance, and so on, the correct therapy of comorbidities naturally occupying a central place.
A more recent problem is the active detection of arrhythmia, making use of genetic peculiarities that can lead to the sometimes-surprising appearance of AF, even beyond the systemic pathological background.6 In addition, the appropriate specification, ie, the duration of an AF access for risk category acceptance of at least 30 s, complemented this requirement. Thus, some studies have imposed more consistent criteria for deciding whether to institute anticoagulant treatment in these patients with relatively occult arrhythmias, such as the PROACTIA7,7 study and score, undertaken mainly in patients with cryptogenic stroke, in whom some devices (eg, implantable cardiac monitors or interrogatable pacemakers) detected occult episodes of AF. True, newer and newer arrhythmia detection devices – even asymptomatic ones – smart watches-phones, telemetry capabilities, longer-term 72-hr Holter examinations, and so on have gradually increased the possibility of timely diagnosis of the most common human cardiac arrhythmia. However, beyond the thromboembolic risk manifested not by the type of NVAF but by the duration of the attacks, the tolerance of patients to attacks of up to 1 hr must also be considered, with the patient’s quality of life always being put first.8
Moreover, even the ESC guidelines for the diagnosis and treatment of AF contain enough “grey areas”, such as the one related to anticoagulant treatment in men with CHA2DS2-VASc score 1 and women with score 2. There are still issues of interpretation and effectiveness of a consistent prophylactic approach to thrombotic vs hemorrhagic events in both European, US, Canadian, Australian, etc. guidelines. This gives rise to the need to standardize and demonstrate the cost-effectiveness of active screening of the population, especially the elderly, and not only, as in the experience of the STROKESTOP study.9 A basic description of the structure in the decision analytic Markov model is used for this purpose. The study demonstrated that active screening could mean not only an increase in life expectancy but more importantly an increase in QUALYs, with clearly proven cost-effectiveness. Of course, the cost of a life saved or of avoiding severe and permanent disability is difficult to quantify. Oral anticoagulation and, in particular, the judicious use of direct oral anticoagulants (DOACs) instead of antivitamin K (aVKs), is in all these models the touchstone for increasing the quality of health-care services.
Some new opinions demonstrate the ineffectiveness of the classical approach to anticoagulation with aVK. Lowe, I. states that a more accurate protocol for loading, monitoring, and administering aVK would make this type of anticoagulation more effective.10 However, as patients grow older and more likely to develop arrhythmia, the risk of iatrogenic side events – especially intracerebral hemorrhages – increases. Benefit vs risk is increasingly difficult to demonstrate in the population over 80 years of age, where even conventional CHA2DS2-VASc – HAS BLED scores seem to have extremely close figures in these individuals, ie, embolic risk is almost identical to hemorrhagic risk, which should not make us give up anticoagulation, as hemorrhagic risk contains many controllable and variable factors and is not extremely restrictive but indicative. Certainly, we have enough reasons to be anxious about the routine use of limited risk scores, both ischemic (native) and hemorrhagic (iatrogenic). Moreover, studies undertaken with various DOACs (anti-factors Xa or IIa) have shown great variability in the results of undifferentiated applications based on ethnicity, age, standard doses, and body weight.11
New and Alternative Thrombotic/Hemorrhagic Risk Factors and Scores
The guidelines tell us that CHA2DS2-VASc – HAS BLED scores are sufficient for routine practices, although from medical practice as well as from research work and studies undertaken, their shortcomings when it comes to individualizing indications are known. Alternative risk scores – for ischaemia – ORBIT, ATRIA, ABC, ABCD-SD, AS5F12 may prove useful for certain groups of patients, even if this makes the work of individualizing risk more difficult. No less important is the reporting of ischemic strokes by NIHSS score, not only for the assessed severity but also for the most effective follow-up antithrombotic treatment.
Also, hemorrhagic risk scores can mean advantages in their individualization, beyond the uniform practicality of the HAS-BLED score. HEMORR₂HAGES, ORBIT, ATRIA, GARFIELD‐AF scores seem to further complicate individualized calculations.13 With all these advances and studies, a unifying attitude is still difficult to adopt without reservations. Where, in ischemic risk scores, for example, are data on left atrial geometry and remodeling,14 participation of inflammatory-prothrombogenic factors, beyond the biological markers in the ABC score, impact of epicardial inflammatory fat, etc.? Furthermore, where are some new data on markers that increase (even independently of correct OAC use) thrombotic risk, such as bone morphogenetic protein 10 (BMP10), a protein expressed in the atrial myocardium?15 However, in-depth studies of left atrial systolic function, epicardium, left atrial endocardium, cardio-metabolic risk, and electrophysiological mechanisms, coupled with DNA sequencing for specific genetic risk (increasingly available for screening) seem to bring many new insights into not only the usefulness of anticoagulation and appropriate dosages but also the particularity of efficacy (also genetically determined), depending on specific parameters of the individual concerned. Changes in atrial geometry from physiological ellipsoid shape and size to truncated conical shapes or other distortions with increased volume and altered left atrial systolic performance leads not only to increased risk of AF but also to increased risk of atrial thrombosis, even in the absence of evidence of AF attacks or other arrhythmias.
Of course, an exciting prospect is the advanced studies with anti-factor XI-a16 – Asundexian (PACIFIC-AF study), very possibly an answer to many of the current questions about the efficacy and safety of anticoagulation with aVK vs DOAC (anti-factor X-a or II-a), but also a new challenge, both in terms of the pharmacological-biochemical rationale for intervening in the “coagulation cascade” and, in terms of direct clinical evidence obtained in randomized trials, with strong efficacy and safety targets. As can be seen in recent decades, for new, relatively abundant antithrombotic medication, the competition is driven by safety goals, with no significant difference in efficacy between competitors.
Another large and severe risk category is acute or chronic patients with NVAF and coronary artery disease, especially those requiring revascularization. Protocols of studies such as PIONEER-AF PCI, RE DUAL-AF PCI, ARISTOTLE-AF PCI, or ENTRUST-AF PCI have shown that the relatively uncomfortable accompaniment of an anticoagulant (especially a DOAC) with antiplatelet therapy – necessary in native coronary artery disease, but also in iatrogenic post-PCI with a pharmacologically active stent can be controlled in the hope of minimizing the risk of bleeding by a minimal period of triple therapy (COCA+Aspirin+P2Y12 inhibitor) – from 1 week to a maximum of 1 month and preferential association with Clopidogrel (from the P2Y12 inhibitor category), especially in elderly and frail patients – frailty, instead of more potent inhibitors, over a period of 3–6 months of double antithrombotic therapy, followed by DOAC, without any other antithrombotic combination. Of course, this therapeutic scheme must consider not only fragility and multiple comorbidities but also anatomical-interventional particularities, surface and location of coronary stents, and so on. This may lead to an increase in the period of double association to 12 months.
The relatively recently completed 15-month surveillance trial – MASTER DAPT – in the section of coronary patients anticoagulated for AF states that a shorter period of double combination (3 months) can give good results in most patients, both for thrombotic risk and especially for hemorrhagic risk. As mentioned above, thrombotic risk figures often increase in tandem with hemorrhagic risk figures, especially with age.17 In general, the problem of choosing the right approach in elderly and inactive patients with obvious frailty criteria is a difficult challenge for all clinicians. On the one hand, the need for anticoagulation increases with age. The risk of hemorrhages, and in particular, of the most threatening form – intracerebral hemorrhages – increases similarly. Many studies and opinions have shown that in this category of patients, not only multiple comorbidities but also the ability of drugs to be absorbed and metabolized, protein binding, bioavailability, and pharmacodynamics are affected, together with renal, hepatic, and visceral disorders, but also polypragmaemia, which often occurs in these cases, leads to an increased risk of hemorrhage, through association with other self-administered drugs (especially non-steroidal anti-inflammatory drugs for rheumatic alginitis), which can increase this risk.18 Moreover, the issue of anticoagulant treatment after an intracerebral hemorrhagic stroke poses multiple problems, but this does not mean that anticoagulant therapy should be abandoned and correctly reinstated after 7–8 weeks.19
The circumstances of the new invasive therapeutic attitudes also need to be clarified. Catheter-ablation in AF has made great strides in the last decade. However, the decision to stop anticoagulation remains controversial, even in the case of seemingly certain therapeutic success, given arrhythmic relapses, and atrial endocardial scarring, as well as the case history of pulmonary vein thrombosis.20 The HRS/EHRA/ECAS expert consensus statement suggests at least 2–3 months of anticoagulation following AF ablation, with some studies indicating that longer durations may be warranted for high-risk patients.5 Post-ablation anticoagulation decisions should be individualized based on patient characteristics and the procedural outcome. If the procedure is deemed successful, with no evidence of arrhythmia recurrence during follow-up, the risk of thromboembolism may be reduced, and anticoagulation therapy can be reconsidered. Certain factors may increase the risk of thromboembolic events post-ablation, necessitating continued anticoagulation. These include a history of prior embolic events, persistently enlarged left atrium, presence of significant comorbidities, and the presence of residual arrhythmia or incomplete ablation. Another factor that may increase the risk of thromboembolic events post-ablation is a persistent left atrial reservoir strain impairment as assessed by two-dimensional speckle tracking echocardiography” which might suggest maintaining anticoagulation treatment.21 Shared decision-making between the patient and the health-care provider is essential in weighing the potential risks and benefits of anticoagulation therapy.
Likewise, in the case of left auricular occlusion (LAA occlusion), the decision to stop anticoagulation permanently is difficult, especially in the elderly, with increased bleeding and thrombotic risk.22 Although LAAO significantly reduces the risk of stroke by eliminating the source of blood clot formation, it does not eliminate the risk entirely. Despite successful occlusion, there is a possibility of residual LAA function or incomplete closure, which may result in clot formation and subsequent embolism. It is also important to keep in mind that even if the typical thrombus formation is in the LAA, the location of atrial thrombus in NVAF patients could also be extra-appendage, that is, attached to the left atrial free wall or atrial roof or interatrial septum. Notably, a left atrial cavity thrombus may be detected in some high-risk NVAF patients, such as elderly with heart failure and/or left ventricular dysfunction, and larger left atrial volumes, who are not adequately anticoagulated. Therefore, LAA closure alone will not eliminate the chances of thromboembolism in this cohort of patients.23,24 Currently, there is no consensus or established guidelines regarding the duration of anticoagulation post-LAAO. The decision on the duration of anticoagulation should be based on individual patient characteristics, the procedural outcome, and the overall risk-benefit assessment.
Several factors influence the decision-making process, including the patient’s CHA₂DS₂-VASc score, which assesses stroke risk factors such as age, hypertension, diabetes, and prior stroke. Additionally, the procedural success, presence of residual leaks, and LAA thrombus at the time of occlusion play a role in determining the need for and duration of anticoagulation.22 Collaboration between the patient, cardiologist, and stroke specialist is crucial in making informed decisions regarding anticoagulation therapy.
The decision to continue or discontinue anticoagulation after LAAO should be individualized based on the patient’s risk profile and the procedural outcome. Patients with a high CHA₂DS₂-VASc score, residual LAA leaks, or documented LAA thrombus at the time of occlusion may benefit from continued anticoagulation therapy. However, patients with low CHA₂DS₂-VASc scores, successful occlusion, and no evidence of LAA thrombus may be considered for anticoagulation discontinuation.
It is important to consider the potential bleeding risks associated with long-term anticoagulation therapy, especially in patients who underwent LAAO due to high bleeding risk. The decision to continue or discontinue anticoagulation should involve a comprehensive evaluation of both thromboembolic and bleeding risks, considering the individual patient’s overall health status and preferences. In some cases, alternative antithrombotic strategies may be considered in place of long-term anticoagulation therapy. Dual antiplatelet therapy (DAPT) with aspirin and a P2Y12 inhibitor, such as clopidogrel, has been explored as an option for stroke prevention following LAAO. This approach aims to provide a balance between reducing thromboembolic events and minimizing bleeding risk. However, the efficacy and safety of DAPT in this context are still under investigation, and it is not currently recommended as a standard practice.
Anticoagulation and LA Remodelling Importance in Clinical Practice
A special role in assessing thrombotic risk should normally play in left atrium remodelling, since fibrotic atrial cardiomyopathy is correlated with embolic strokes of undetermined origin even in non-AF patients.25 However, left atrium enlargement should not be overlapped with LA cardiomyopathy, since AF electroconversion seems safe even in patients with LA dilatation.26 LA remodelling can be described as a time-dependent process involving structural, functional, and/or electrical alterations in response to pressure and/or volume overload, metabolic, or electrical stressors. Heart failure, arterial hypertension, and valvular heart disease are the main conditions impairing LA function. Nevertheless, subclinical LA remodelling was demonstrated even in patients with frequent premature ventricular contractions.27,28
LA size is to be assessed at the end of LV systole, when the LA chamber is maximal, and care should be taken to avoid foreshortening of the LA. Because the long axes of the left ventricle and LA are situated in different plans, the 4-chamber view may be obtained in several angulations. The base of the left atrium should be at its maximal size, and LA length should also be maximized (provide positioning along the true LA long axis).
The LA assessment guideline29 stated that when tracing the borders of the left atrium, the confluence of the pulmonary veins and the LA appendage should be excluded. The 2019 guidelines recommendation is compulsory: LA volume assessment for the best evaluation of LA size evaluation. However, the real border between the left atrium (LA) and pulmonary veins (PV) is yet to be defined, and PV antrum implication/importance in the geometry of LA dilatation is not even mentioned and has not been completely investigated. Therefore, there is an important unexplored field to redefine a more accurate echocardiographic evaluation of LA, which is inexpensive and accessible but it is not even close in preciseness to gold standard CT/RMO. Direct comparative evaluation can be easily done for patients undergoing AF ablation (indication of 3D CT evaluation of LA and complete echocardiographic evaluation of LA).
This committee of guidelines in chamber evaluation did not mention recommending the use of 3D echocardiographic evaluation for LA due to the lack of a standardized methodology and limited normative data. Angulation of transducer importance and apparent LA shape modification may mislead a precise LA volume evaluation (Figure 1a and b).
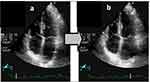 |
Figure 1 (a and b) Example of LA shape changing due to 2 different angulations while searching for PV visualisation. |
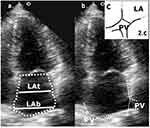
The LA endocardial borders can be traced in apical four-chamber view. A single-plane approach can be used, but the guidelines affirm that this method is based on the geometric assumption that the left atrium is circular and this is often not accurate. PV origin and visualisation should be taken into consideration while assessing LA size apical four chamber view. Figure 2 presents a suggestive image for a trapezoidal LA shape and a practical example of LA tracing after pulmonary veins ostia definition and consecutive evaluation of shape remodelling (Figure 2a–c).
LA and LV remodelling should be evaluated as a pathophysiological continuum, in both ways, for better or worse. In HF patients with cardiac resynchronization therapy, in particular fusion pacing CRT, left atrium reverse remodelling was associated with a lower incidence of atrial fibrillation.30–32 The relevance of sinus rhythm preservation in patients undergoing CRT pacing is linked to events free of hospitalisation and superior survival. LA function is an important determinant of outcome in these patients. The better the function, the better the survival.
Structural remodelling of the LA is a complex expression of underlying atrial cardiomyopathy, associated with an increased risk of developing AF even in healthy individuals. Accurate imaging of LA structural remodeling provides prognostic information and influences therapeutic choices and, perhaps, should be involved in the anticoagulation decision in special categories of patients.
Bayes syndrome, associated with LA electrical and mechanical remodelling, has recently been identified as a novel risk factor for non-lacunar cardioembolic ischemic stroke and vascular dementia. Bayes syndrome is characterized by advanced interatrial block and supraventricular arrhythmias such as atrial flutter and atrial fibrillation; the main electrocardiographically manifestation for the subclinical form is a prolonged P wave duration >120 ms with biphasic morphology ± in the inferior leads. Although a poorly recognized disease, Bayes syndrome could have important cardiologic and neurologic implications.33
The Interplay Between Inflammation and Thrombosis
Although strong literature data advocate that AF is related to augmented systemic inflammatory status and platelet activation, the relation of stroke risk to LA inflammation is not considered as a parameter of risk calculator for systemic embolism.
Inflammation and thrombosis are two interconnected processes that play crucial roles in our body’s immune response and maintenance of vascular health. These two processes are closely linked, and the dysregulation of either can lead to a heightened thrombotic risk.30 Understanding the relationship between inflammation and thrombotic risk is essential in developing effective preventive strategies.
Inflammation can directly trigger a pro-thrombotic state by promoting the activation of platelets and clotting factors, increasing the expression of adhesion molecules, and altering the balance between procoagulant and anticoagulant factors. Furthermore, inflammatory cells release extracellular traps called neutrophil extracellular traps (NETs), which can contribute to the formation of blood clots.34 Conversely, thrombosis can also induce and sustain an inflammatory response. When blood clots form within blood vessels, they can obstruct blood flow, leading to tissue ischemia and damage. This can result in the release of damage-associated molecular patterns (DAMPs) and the activation of immune cells, triggering an inflammatory response. Inflammatory cells recruited to the site of thrombosis can further exacerbate clot formation, perpetuating a vicious cycle of inflammation and thrombosis.35,36
Inflammatory status plays a significant role in the pathogenesis of various cardiovascular diseases, including atrial fibrillation. Emerging evidence suggests that inflammation contributes to left atrial remodeling, highlighting the intricate interplay between inflammation and cardiovascular health.37 Inflammation promotes the activation and infiltration of immune cells, such as macrophages and lymphocytes, into the left atrial tissue. These immune cells release pro-inflammatory cytokines, chemokines, and growth factors, which trigger a cascade of molecular and cellular events leading to fibrosis, hypertrophy, and electrical remodeling of the atrial tissue. These changes contribute to atrial structural abnormalities and electrical instability, and predispose to the development and maintenance of AF.38
Inflammatory mediators, such as C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α), have been associated with left atrial enlargement and fibrosis, both of which are markers of left atrial remodeling.39 Moreover, inflammation-induced oxidative stress and the production of reactive oxygen species (ROS) can promote fibroblast activation and collagen deposition in the atrial tissue, further contributing to left atrial remodeling.40,41
Additionally, inflammation-targeted therapies, such as anti-inflammatory medications or immunomodulatory agents, are being investigated as potential interventions to mitigate left atrial remodeling and prevent AF progression.42,43 These interventions aim to suppress the inflammatory response, reduce immune cell infiltration, and inhibit the release of pro-inflammatory cytokines and chemokines.
In conclusion, inflammation plays a crucial role in the pathogenesis of left atrial remodeling, contributing to the development and progression of AF. The activation of immune cells and the release of pro-inflammatory mediators initiate a cascade of events leading to structural and functional changes in the left atrium. Understanding the interplay between inflammation and left atrial remodeling may provide insights into novel therapeutic targets and strategies for the prevention and management of AF. Further research is warranted to elucidate the underlying mechanisms and to evaluate the clinical efficacy of inflammation-targeted interventions in attenuating left atrial remodeling and improving cardiovascular outcomes.
Genetics and Atrial Fibrillation
Atrial fibrillation has a multifactorial etiology, with genetic factors estimated to account for approximately 30% of the disease’s heritability. Advances in genetic research, including genome-wide association studies (GWAS) and familial studies, have identified various genetic variants associated with AF development.44
One of the most commonly implicated genes in AF is the KCNH2 gene, which encodes the alpha subunit of the potassium channel responsible for repolarization in cardiac cells. Mutations in this gene can lead to prolonged action potentials and abnormal electrical activity, increasing the risk of AF. Other genes involved in ion channel function, such as KCNQ1 and SCN5A, have also been associated with AF susceptibility.45,46 Additionally, genetic variations in structural proteins of the heart, such as MYH6 and MYH7, have been linked to an increased risk of AF. These proteins play a crucial role in the organization and function of cardiac muscle cells, and alterations in their structure or function can disrupt normal electrical signaling.47
Traditionally, the decision to anticoagulate patients has been based on clinical risk factors, such as age, hypertension, diabetes, and prior stroke or transient ischemic attack. However, emerging evidence suggests that genetic factors may also play a role in determining the appropriate anticoagulation therapy. Certain genetic variations, particularly those related to clotting factors and platelet function, have been associated with an increased risk of thromboembolic events in AF patients. For example, variations in the genes encoding clotting factors II, V, VII, and X, as well as von Willebrand factor, have been linked to a higher risk of stroke in individuals with AF.48,49 Genetic variants affecting platelet function, such as P2Y12 receptor polymorphisms, may also influence the efficacy of antiplatelet therapy in preventing stroke.50
Pharmacogenomic considerations are also relevant when selecting oral anticoagulants. Genetic variations in the cytochrome P450 enzyme system, specifically CYP2C9 and VKORC1 genes, can affect metabolism and response to warfarin.51,52 Knowledge of these genetic factors can aid in determining the appropriate initial dose and subsequent monitoring of warfarin therapy to achieve therapeutic anticoagulation.
As a future perspective, genetic testing could identify individuals at increased risk of developing AF and help stratify patients according to their thromboembolic risk. This information could then be used to inform decisions regarding anticoagulation therapy, dosage adjustments, and the selection of specific anticoagulant agents.
However, it is important to consider that genetic factors are only one piece of the puzzle, and the decision to anticoagulate should still be made in conjunction with clinical risk assessments and guidelines. Collaborative efforts between cardiologists, geneticists, and researchers are necessary to continue unraveling the complex genetic underpinnings of AF and develop tailored approaches to patient management.
The role of emerging and less extensively documented stroke risk factors, as previously described, remains uncertain. However, through meticulous clinical studies, we can gain a deeper understanding and enhance our ability to formulate more customized primary and secondary stroke prevention strategies. An essential line of future research should be the assessment of the impact of left atrium remodelling, inflammation, and genetics on anticoagulation of atrial fibrillation in a subgroup of very old patients (aged 80 or older), since demographics and risk factors are quite different in this age segment of stroke patients.53
Table 1 presents a simple and concise review of the main findings of several recent studies in the literature regarding the role of left atrium remodelling, inflammation and genetics in the anticoagulation of atrial fibrillation. Nevertheless, this data should be integrated extensively, along with the knowledge and clinical judgment individualized for each patient.
 |
Table 1 Brief Review of the Main Findings Regarding the Role of Left Atrium Remodeling, Inflammation and Genetics in the Anticoagulation of Atrial Fibrillation |
Conclusions and Future Perspectives
With all these studies and expert opinions, there are still differences in therapeutic protocols in the administration of some DOACs (Dabigatran dosages – USA vs Europe, Apixaban administration in severe renal failure USA, etc.). Low dosages according to three criteria – age, weight status, and renal function (2 out of 3 criteria) remain somewhat controversial, with insufficient clinical evidence of efficacy for low doses for some DOACs. Moreover, it appears that the use of OAC, including DOAC in the severely obese or severely underweight, is still a matter of dispute.
However, AF remains a multifactorial pathology that is still sufficiently hidden, both in terms of patient perception, the perception of medical staff towards a consistent preventive anticoagulation attitude and the still high morbidity and mortality figures recorded, especially in the population over 70. Loss of compliance, adherence, and persistence to these radical treatments can mean a bitter failure. For this reason, educating health-care networks, and especially patients, intra-family supervision of medication administration and prevention of life-threatening attitudes leads to substantial gains in lives and quality of life.
Further research is needed to validate and compare the performance of the new thrombotic risk scores in different patient populations. Additionally, ongoing advancements in precision medicine and the integration of genetic and biomarker data may further refine and personalize thrombotic risk assessment in AF.
This is a challenge for any modern health system.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lyth J, Svennberg E, Bernfort L, et al. Cost-effectiveness of population screening for atrial fibrillation: the STROKESTOP study. Europ Heart J. 2022;00:1–10.
2. Boriani G, Laroche C, Diemberger I, et al. Asymptomatic atrial fibrillation: clinical correlates, management, and outcomes in the EORP-AF Pilot General Registry. Am J Med. 2015;128(5):509–18.e2. PMID: 25534423. doi:10.1016/j.amjmed.2014.11.026
3. Lip GYH, Laroche C, Popescu MI, et al. Prognosis and treatment of atrial fibrillation patients by European cardiologists: one year follow-up of the EURObservational research programme-atrial fibrillation general registry pilot phase (EORP-AF Pilot registry). Europ Heart J. 2014;35(47):3365. doi:10.1093/eurheartj/ehu374
4. Boriani G, Laroche C, Diemberger I, et al. Glomerular filtration rate in patients with atrial fibrillation and 1-year outcomes. Sci Rep. 2016;6:30271. doi:10.1038/srep30271
5. Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for cardio-thoracic surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European society of cardiology (ESC) developed with the special contribution of the European heart rhythm association (EHRA) of the ESC. Europ Heart J. 2021;42(5):373–498. doi:10.1093/eurheartj/ehaa612
6. Marston NA, Garfinkel AC, Kamanu FK, et al. A polygenic risk score predicts atrial fibrillation in cardiovascular disease. Europ Heart J. 2023;44(3):221–231. doi:10.1093/eurheartj/ehac460
7. Skrebelyte-Strøm L, Morten Rønning O, DahL FA, Steine K, Kjekshus H. Prediction of occult atrial fibrillation in patients after cryptogenic stroke and transient ischaemic attack: PROACTIA. Europace. 2022;24:1881–1888. doi:10.1093/europace/eu
8. Andrade JG, Deyell MW, Macle L, et al. Healthcare utilization and quality of life for atrial fibrillation burden: the CIRCA-DOSE study. Europ Heart J. 2023;44(9):765–776. doi:10.1093/eurheartj/ehac692
9. Svennberg E, Friberg L, Frykman V, Al-Khalili F, Engdahl J, Rosenqvist M. Clinical outcomes in systematic screening for atrial fibrillation (STROKESTOP): a multicentre, parallel group, unmasked, randomised controlled trial. Lancet. 2021;398(10310):1498–1506. PMID: 34469764. doi:10.1016/S0140-6736(21)01637-8
10. Lowe I. Critique of warfarin dosing: the use of nudging to adjust the load. Academia Letters. 2022;2022:2–8.
11. Thomas FL. Unresolved issues of anticoagulation in atrial fibrillation: age, BMI, reduced dose, and ethnicity. Europ Heart J. 2019;40:1477–1481. doi:10.1093/eurheartj/ehz302
12. Lee J-D, Kuo Y-W, Lee C-P, Huang Y-C, Lee M, Lee T-H. Development and validation of a novel score for predicting paroxysmal atrial fibrillation in acute ischemic stroke. Int J Environ Res. 2022;19:7277. doi:10.3390/ijerph19127277
13. Edmiston MK, Lewis WR. Bleeding risk scores in atrial fibrillation: helpful or harmful? J Am Heart Assoc. 2018;7(18):e010582. PMID: 30371216; PMCID: PMC6222951. doi:10.1161/JAHA.118.010582
14. Zheng Y, Li TYW, Goh FQ, et al. Abnormal left atrial strain is associated with eventual diagnosis of atrial fibrillation in patients with embolic stroke of undetermined source. Europ Heart J. 2023;44:ehac779–002. doi:10.1093/eurheartj/ehac779.002
15. Hijazi Z, Benz AP, Lindbäck J, et al. Bone morphogenetic protein 10: a novel risk marker of ischaemic stroke in patients with atrial fibrillation. Europ Heart J. 2023;44(3):208–218. doi:10.1093/eurheartj/ehac632
16. De Caterina R, Prisco D, Eikelboom JW. Factor XI inhibitors: cardiovascular perspectives. Europ Heart J. 2023;44(4):280–292. doi:10.1093/eurheartj/ehac464
17. Andreotti F, Geisler T, Collet J-P, et al. Acute, periprocedural and long term antithrombotic therapy in older adults: 2022 Update by the ESC working group on thrombosis. Europ Heart J. 2023;44(4):262–279. doi:10.1093/eurheartj/ehac515
18. Andreotti F, Rocca B, Husted S, et al. Antithrombotic therapy in the elderly: expert position paper of the European society of cardiology working group on thrombosis. Europ Heart J. 2015;36:3238–3249. doi:10.1093/eurheartj/ehv304
19. Pennlert J, Overholser R, Asplund K, et al. Optimal timing of anticoagulant treatment after intracerebral hemorrhage in patients with atrial fibrillation. Stroke. 2016;48:314–320. doi:10.1161/STROKEAHA.116.014643
20. Al-Kaisey AM, Kalman JM. Atrial arrhythmia recurrence post-catheter ablation: when perfect is the enemy of good. Europ Heart J. 2023;44(9):777–779. doi:10.1093/eurheartj/ehac736
21. Vincenti A, Genovesi S, Sonaglioni A, et al. Mechanical atrial recovery after cardioversion in persistent atrial fibrillation evaluated by bidimensional speckle tracking echocardiography. J Cardiovasc Med. 2019;20(11):745–751. PMID: 31483328. doi:10.2459/JCM.0000000000000864
22. Freeman JV, Varosy P, Price MJ, et al. The NCDR left atrial appendage occlusion registry. J Am Coll Cardiol. 2020;75(13):1503–1518. PMID: 32238316; PMCID: PMC7205034. doi:10.1016/j.jacc.2019.12.040
23. Manjunath CN, Srinivasa KH, Ravindranath KS, et al. Balloon mitral valvotomy in patients with mitral stenosis and left atrial thrombus. Catheter Cardiovasc Interv. 2009;74(4):653–661. PMID: 19777604. doi:10.1002/ccd.22176
24. Sonaglioni A, Vincenti A, Lombardo M, Anzà C. Left atrial cavity thrombus and fatal systemic embolization in a stroke patient with nonvalvular atrial fibrillation: a caveat against left atrial appendage closure for stroke prevention. J Cardiovasc Echogr. 2020;30(1):41–43. PMID: 32766107; PMCID: PMC7307615. doi:10.4103/jcecho.jcecho_46_19
25. Floria M, Radu S, Gosav EM, et al. Left Atrial structural remodelling in non-valvular atrial fibrillation: what have we learnt from CMR? Diagnostics. 2020;10(3):137. PMID: 32131455; PMCID: PMC7151417. doi:10.3390/diagnostics10030137
26. Cozma D, Streian CG, Vacarescu C, Mornos C. Back to sinus rhythm from atrial flutter or fibrillation: dabigatran is safe without transoesophageal control. Kardiol Pol. 2016;74(5):425–430. PMID: 26502941. doi:10.5603/KP.a2015.0209
27. Luca CT, Crisan S, Cozma D, et al. Arterial hypertension: individual therapeutic approaches-from DNA sequencing to gender differentiation and new therapeutic targets. Pharmaceutics. 2021;13(6):856. PMID: 34207606; PMCID: PMC8229802. doi:10.3390/pharmaceutics13060856
28. Cozma D, Streian CG, Petrescu L, Mornos C. Subclinical left atrium remodelling in patients with frequent premature ventricular contractions. Kardiol Pol. 2014;72(11):1141–1147. PMID: 25522754. doi:10.5603/KP.a2014.0133
29. Douglas PS, Carabello BA, Lang RM, et al. 2019 ACC/AHA/ASE key data elements and definitions for transthoracic echocardiography: a report of the American college of cardiology/American heart association task force on clinical data standards (writing committee to develop cardiovascular endpoints data standards) and the American society of echocardiography. Circ Cardiovasc Imaging. 2019;12(7):e000027. PMID: 31233331. doi:10.1161/HCI.0000000000000027
30. Birnie D, Hudnall H, Lemke B, et al. Continuous optimization of cardiac resynchronization therapy reduces atrial fibrillation in heart failure patients: results of the adaptive cardiac resynchronization therapy trial. Heart Rhythm. 2017;14(12):1820–1825. PMID: 28893549. doi:10.1016/j.hrthm.2017.08.017
31. Vacarescu C, Luca CT, Feier H, et al. Beta blockers and ivabradine titration according to exercise test in LV only fusion CRT pacing. Diagnostics. 2022;12(5):1096. PMID: 35626251; PMCID: PMC9139204. doi:10.3390/diagnostics12051096
32. Goanță EV, Luca CT, Vacarescu C, et al. Non-ischemic super-responders in fusion CRT pacing with normal atrioventricular conduction. Diagnostics. 2022;12(9):2032. PMID: 36140434; PMCID: PMC9497644. doi:10.3390/diagnostics12092032
33. Arboix A, Martí L, Dorison S, Sánchez MJ. Bayés syndrome and acute cardioembolic ischemic stroke. World J Clin Cases. 2017;5(3):93–101. PMID: 28352633. doi:10.12998/wjcc.v5.i3.93
34. Guo Y, Lip GY, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol. 2012;60(22):2263–2270. PMID: 23194937. doi:10.1016/j.jacc.2012.04.063
35. Nso N, Bookani KR, Metzl M, Radparvar F. Role of inflammation in atrial fibrillation: a comprehensive review of current knowledge. J Arrhythm. 2020;37(1):1–10. PMID: 33664879; PMCID: PMC7896450. doi:10.1002/joa3.12473
36. Schnabel RB, Larson MG, Yamamoto JF, et al. Relation of multiple inflammatory biomarkers to incident atrial fibrillation. Am J Cardiol. 2009;104(1):92–96. doi:10.1016/j.amjcard.2009.02.053
37. Goette A, Honeycutt C, Langberg JJ. Electrical remodeling in atrial fibrillation. Time course and mechanisms. Circulation. 1996;94(11):2968–2974. doi:10.1161/01.CIR.94.11.2968
38. Karam BS, Chavez‐Moreno A, Koh W, Akar JG, Akar FG. Oxidative stress and inflammation as central mediators of atrial fibrillation in obesity and diabetes. Cardiovasc Diabetol. 2017;16(1):1–9. doi:10.1186/s12933-017-0604-9
39. Korantzopoulos P, Letsas KP, Tse G, Fragakis N, Goudis CA, Liu T. Inflammation and atrial fibrillation: a comprehensive review. J Arrhythm. 2018;34(4):394–401. PMID: 30167010; PMCID: PMC6111477. doi:10.1002/joa3.12077
40. Acampa M, Lazzerini PE, Guideri F, Tassi R, Lo monaco A, Martini G. Inflammation and atrial electrical remodelling in patients with embolic strokes of undetermined source. Heart Lung Circ. 2019;28(6):917–922. PMID: 29887417. doi:10.1016/j.hlc.2018.04.294
41. Lazzerini PE, Laghi-Pasini F, Acampa M, et al. Systemic inflammation rapidly induces reversible atrial electrical remodeling: the role of interleukin-6-mediated changes in connexin expression. J Am Heart Assoc. 2019;8(16):e011006. PMID: 31423933; PMCID: PMC6759884. doi:10.1161/JAHA.118.011006
42. Varghese B, Feldman DI, Chew C, et al. Inflammation, atrial fibrillation, and the potential role for colchicine therapy. Heart Rhythm. 2021;2(3):298–303. PMID: 34337581; PMCID: PMC8322795. doi:10.1016/j.hroo.2021.03.011
43. Ang YS, Rajamani S, Haldar SM, Hüser J. A new therapeutic framework for atrial fibrillation drug development. Circ Res. 2020;127(1):184–201. PMID: 32717173. doi:10.1161/CIRCRESAHA.120.316576
44. Roselli C, Rienstra M, Ellinor PT. Genetics of atrial fibrillation in 2020: GWAS, genome sequencing, polygenic risk, and beyond. Circ Res. 2020;127(1):21–33. PMID: 32716721; PMCID: PMC7388073. doi:10.1161/CIRCRESAHA.120.316575
45. Andersen JH, Andreasen L, Olesen MS. Atrial fibrillation-a complex polygenetic disease. Eur J Hum Genet. 2021;29(7):1051–1060. PMID: 33279945; PMCID: PMC8298566. doi:10.1038/s41431-020-00784-8
46. Lee SP, Ashley EA, Homburger J, et al. SHaRe investigators. incident atrial fibrillation is associated with myh7 sarcomeric gene variation in hypertrophic cardiomyopathy. Circ Heart Fail. 2018;11(9):e005191. PMID: 30354366. doi:10.1161/CIRCHEARTFAILURE.118.005191
47. Buchtele N, Schwameis M, Gilbert JC, Schörgenhofer C, Jilma B. Targeting von Willebrand factor in ischaemic stroke: focus on clinical evidence. Thromb Haemost. 2018;118(6):959–978. PMID: 29847840; PMCID: PMC6193403. doi:10.1055/s-0038-1648251
48. Wysokinski WE, Cohoon KP, Konik EA, et al. Effect of atrial fibrillation duration on plasma von Willebrand factor level. Eur J Haematol. 2017;99(6):569–576. PMID: 28952167; PMCID: PMC6310225. doi:10.1111/ejh.12975
49. Ding WY, Harrison S, Gupta D, Lip GYH, Lane DA. Stroke and bleeding risk assessments in patients with atrial fibrillation: concepts and controversies. Front Med. 2020;7:54. PMID: 32154260; PMCID: PMC7047213. doi:10.3389/fmed.2020.00054
50. Gairolla J, Ahluwalia J, Khullar M, et al. Clopidogrel response in ischemic stroke patients: is polymorphism or gender more important? Results of the CRISP study. J Clin Neurosci. 2020;76:81–86. PMID: 32317191. doi:10.1016/j.jocn.2020.04.038
51. Al-Eitan LN, Almasri AY, Khasawneh RH. Impact of CYP2C9 and VKORC1 polymorphisms on warfarin sensitivity and responsiveness in Jordanian cardiovascular patients during the initiation therapy. Genes. 2018;9(12):578. PMID: 30486437; PMCID: PMC6316567. doi:10.3390/genes9120578
52. Jorgensen AL, FitzGerald RJ, Oyee J, Pirmohamed M, Williamson PR. Influence of CYP2C9 and VKORC1 on patient response to warfarin: a systematic review and meta-analysis. PLoS One. 2012;7(8):e44064. PMID: 22952875; PMCID: PMC3430615. doi:10.1371/journal.pone.0044064
53. Arboix A, García-Eroles L, Massons J, Oliveres M, Targa C. Lacunar infarcts in patients aged 85 years and older. Acta Neurol Scand. 2000;101(1):25–29. PMID: 10660148. doi:10.1034/j.1600-0404.2000.00005.x
54. Chung H, Lee JM. Left atrial remodeling and thromboembolic risk in patients with atrial fibrillation. Int J Heart Fail. 2022;4(1):26–28. PMID: 36262196; PMCID: PMC9383338. doi:10.36628/ijhf.2022.0006
55. Sahin T, Acar E, Celikyurt U, et al. Relation of hs-CRP and BNP levels with the atrial spontaneous echo contrast and thrombi in permanent atrial fibrillation patients with different etiologies. Med Sci Monit. 2012;18(2):CR78–87. PMID: 22293881; PMCID: PMC3560580. doi:10.12659/msm.882461
56. Christophersen IE, Rienstra M, Roselli C, et al. Large-scale analyses of common and rare variants identify 12 new loci associated with atrial fibrillation. Nat Genet. 2017;49(6):946–952. PMID: 28416818; PMCID: PMC5585859. doi:10.1038/ng.3843
57. Jansen HJ, Bohne LJ, Gillis AM, Rose RA. Atrial remodeling and atrial fibrillation in acquired forms of cardiovascular disease. Heart Rhythm. 2020;1(2):147–159. PMID: 34113869; PMCID: PMC8183954. doi:10.1016/j.hroo.2020.05.002
58. Chen YC, Voskoboinik A, Gerche A, Marwick TH, McMullen JR. Prevention of pathological atrial remodeling and atrial fibrillation: JACC state-of-the-art review. J Am Coll Cardiol. 2021;77(22):2846–2864. PMID: 34082914. doi:10.1016/j.jacc.2021.04.012
59. Jumeau C, Rupin A, Chieng-Yane P, et al. Direct thrombin inhibitors prevent left atrial remodeling associated with heart failure in rats. JACC Basic Transl Sci. 2016;1(5):328–339. PMID: 27642643; PMCID: PMC5012373. doi:10.1016/j.jacbts.2016.05.002
60. Marott SC, Nordestgaard BG, Zacho J, et al. Does elevated C-reactive protein increase atrial fibrillation risk? A Mendelian randomization of 47,000 individuals from the general population. J Am Coll Cardiol. 2010;56(10):789–795. PMID: 20797493. doi:10.1016/j.jacc.2010.02.066
61. Fu T, Chen M, Xu L, et al. Association of the MYH6 gene polymorphism with the risk of atrial fibrillation and warfarin anticoagulation therapy. Genet Test Mol Biomarkers. 2021;25(9):590–599. PMID: 34515533. doi:10.1089/gtmb.2021.0025
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.