Back to Journals » Journal of Asthma and Allergy » Volume 15
Further Understanding of Neuro-Immune Interactions in Allergy: Implications in Pathophysiology and Role in Disease Progression
Authors Konstantinou GN , Konstantinou GN , Koulias C, Petalas K, Makris M
Received 21 January 2022
Accepted for publication 25 August 2022
Published 10 September 2022 Volume 2022:15 Pages 1273—1291
DOI https://doi.org/10.2147/JAA.S282039
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Amrita Dosanjh
George N Konstantinou,1 Gerasimos N Konstantinou,2,3,* Christopher Koulias,4,* Konstantinos Petalas,5,* Michael Makris4,*
1Department of Allergy and Clinical Immunology, 424 General Military Training Hospital, Thessaloniki, Greece; 2Department of Psychiatry, University of Toronto, Toronto, ON, Canada; 3Centre of Addiction and Mental Health (CAMH), Toronto, ON, Canada; 4Allergy Unit, 2nd Department of Dermatology and Venereology, National and Kapodistrian University of Athens, “Attikon” University Hospital, Athens, Greece; 5Department of Allergy, 251 General Air Force Hospital, Athens, Greece
*These authors contributed equally to this work
Correspondence: George N Konstantinou, Department of Allergy and Clinical Immunology, 424 General Military Training Hospital, Eleftheriou Venizelou 11, Kalamaria, Thessaloniki, 55 133, Greece, Tel/Fax +30 231 550 3444, Email [email protected]
Abstract: The complicated interaction between the central and the autonomic (sympathetic, parasympathetic, and enteric) nervous systems on the one hand and the immune system and its components, on the other hand, seems to substantially contribute to allergy pathophysiology, uncovering an under-recognized association that could have diagnostic and therapeutic potentials. Neurons connect directly with and regulate the function of many immune cells, including mast cells, the cells that have a leading role in allergic disorders. Proinflammatory mediators such as cytokines, neurotrophins, chemokines, and neuropeptides are released by immune cells, which stimulate sensory neurons. The release of neurotransmitters and neuropeptides caused by the activation of these neurons directly impacts the functional activity of immune cells and vice versa, playing a decisive role in this communication. Successful application of Pavlovian conditioning in allergic disorders supports the existence of a psychoneuroimmunological interplay in classical allergic hypersensitivity reactions. Activation of neuronal homeostatic reflexes, like sneezing in allergic rhinitis, coughing in allergic asthma, and vomiting in food allergy, offers additional evidence of a neuroimmunological interaction that aims to maintain homeostasis. Dysregulation of this interaction may cause overstimulation of the immune system that will produce profound symptoms and exaggerated hemodynamic responses that will lead to severe allergic pathophysiological events, including anaphylaxis. In this article, we have systematically reviewed and discussed the evidence regarding the role of the neuro-immune interactions in common allergic clinical modalities like allergic rhinitis, chronic rhinosinusitis, allergic asthma, food allergy, atopic dermatitis, and urticaria. It is essential to understand unknown – to most of the immunology and allergy experts – neurological networks that not only physiologically cooperate with the immune system to regulate homeostasis but also pathogenetically interact with more or less known immunological pathways, contribute to what is known as neuroimmunological inflammation, and shift homeostasis to instability and disease clinical expression. This understanding will provide recognition of new allergic phenotypes/endotypes and directions to focus on specialized treatments, as the era of personalized patient-centered medicine, is hastening apace.
Keywords: immunomodulation, allergic rhinitis, chronic rhinosinusitis, food allergy, allergic asthma, atopic dermatitis, urticaria, neuropeptides, neurotransmitters, neurotrophins, conditioning, behavioral, cognitive, brain
Introduction
The immune and nervous systems are physiologically in a continuous, uninterrupted, close interaction. Neuropeptides (slow-acting, large polypeptides producing prolonged action) and neurotransmitters (fast-acting smaller molecules causing short-term responses) play a decisive role in this communication by moderating interactions with the immune system components. The immune cells can also respond to neuropeptides by producing inflammatory mediators such as cytokines. This occurs not only between the immune cells that are hosting the Central Nervous System (CNS, namely the brain and the spinal cord), but also in the Peripheral Nervous System [PNS, namely the Somatic Nervous System, and the Autonomic Nervous System (ANS)]. The ANS contains three anatomically distinct divisions, the Sympathetic, the Parasympathetic and the Enteric nervous systems (ENS). The first two contain nerve fibers or axons that conduct information to and from the CNS [sensory (afferent), and motor (efferent), respectively]. The ENS functions independently and comprises reflex arcs that control the enteric smooth muscles’ contraction or relaxation and the digestion process (secretion, blood flow, and absorption).1
The sensory neurons and neurons form the ENS possess cytokine and neurotransmitter receptors and release neuropeptides. Therefore, mediators derived from immune cells, such as mast cells and macrophages, that show anatomical proximity with these peripheral neurons, induce neuronal activation that stimulates the nerve endings to produce impulses that are translated into information about the intensity of the stimulus in the CNS.2,3 This leads to further release of neuropeptides in a positive feedback loop.4,5 The neuropeptides and neurotransmitters secreted by the PNS have several regulatory effects on multiple immune cells, as well. This interchange is able to control homeostatic responses but may lead to abnormal, excessive, and sometimes deleterious inflammatory processes (infections, tissue injury, inflammatory or allergic disorders).6–8
Allergic diseases like allergic rhinitis (AR), asthma, and food and skin allergies are characterized by acute, intermittent or chronic type 2 (T2) inflammation that is responsible for recurrent and debilitating symptoms like sneezing in AR, coughing in asthma, vomiting in food allergy and pruritus in atopic dermatitis and urticaria. All these sensory responses at the epithelial or the epidermal barrier serve as protective mechanisms to mechanically remove noxious stimuli and are induced and facilitated by immune cells. For instance, mast cells, effector cells in allergic disorders, express receptors for neuropeptides and neurotransmitters and by this way they play a key role in this interplay.9–13 Moreover, IgE high-affinity receptors and other T2 receptors are expressed on sensory10 or enteric neurons and are functional and able to transmit signals to the CNS.14,15 Thus, these local neuroimmune interactions profoundly affect tissue homeostasis but may also play a role in chronic inflammation establishment and, therefore, in tissue reconstruction or remodeling. Notably, these effects are highly dependent by the local microenvironment.6–8
Bidirectional interactions between sensory neurons and T2 inflammatory cells and mediators facilitate both sensing and modulation of the neuroimmune response.16 Therefore, manipulating this neuroimmune regulation may be a promising therapeutic approach for these allergic conditions. Any intervention in this neuro-immunological crosstalk implies a deep understanding of the underlying physiology and pathophysiology, justifying the need for a shift in the focus of research in immune-neurobiology.
To appreciate this dynamic interaction in allergy there is a need: i) to define and understand the activation mechanisms of central and peripheral neurons in physiological status and in the context of T2 inflammation, ii) to examine the effects of neuropeptides and neurotransmitters on the immune system and T2 inflammation, and iii) to investigate the potentially different roles of neuroimmune crosstalk in different tissues, organs, and systems.
In this review, we discuss the recent evidence regarding the role of the neuro-immune interactions in six common clinical modalities with a high impact on patients’ quality of life: AR, chronic rhinosinusitis, allergic asthma, food allergy, atopic dermatitis, and urticaria.
Conditioned Immunomodulation and the First Experimental Evidence of Psycho-Neuro-Immune Interaction in Allergy
Pavlovian conditioning or classical conditioning is a type of conditioned learning which occurs because of the subject’s instinctive responses after hazardous environments or exposures. Immunological responses can also be learned and memorized by Pavlovian conditioning, a phenomenon that is based on mutual functional interactions between the brain, behavior, and peripheral immune functions. This interplay comprises psychoneuroimmunology.17
Classical conditioning has been shown in allergic disorders, as well. Sensitized subjects may experience allergic symptoms via behavioral conditioning independently of exposure to the allergen they are sensitized. Moreover, allergic symptoms may be controlled by conditioned suppression of the hypersensitivity reactions without administration of the immunosuppressive medication. Several such paradigms exist not only in animal models but also in humans.
The first such experimental evidence was reported in the middle of the previous century in allergic asthma.18 Two asthmatic patients sensitized to grass pollen and house dust mites had typical spontaneous asthmatic attacks when exposed to the culprit allergens or positive bronchoprovocation challenges to allergens they were sensitized to. Before the conditioning experiments, inhaling the neutral solvent of the allergen extracts had no effect. Their reaction pattern, however, changed in the course of the conditioning inhalation experiments. In the beginning, the patients started to react to the solvent. Later on, the inhalation of pure oxygen in the laboratory caused asthma exacerbations. Finally, just the introduction of the mouthpiece of the inhalation apparatus was enough to provoke severe asthma attacks that could not be distinguished from those that appeared with the allergen bronchoprovocation challenges. Interestingly, one of the two patients showed favoring effect in conditioned suppression only after long-term psychotherapy, suggesting the rigidity with which the patients remained attached to their conditioned behavior as opposed to the rapidity with which it was developed.18 This observation implied, at the same time, though, the existence of a type of cognitive “desensitization” intervention.
In another study, asthmatic guinea pigs were sensitized to bovine serum albumin. Bovine serum albumin was paired in a classical conditioning design with the odor dimethyl sulfide. Re-exposure only to the odor was enough to induce higher histamine levels in conditioned guinea pigs.19
A link between the CNS and the mast cells lining the nasal mucosa has been shown in a classical behavioral conditioning experiment in humans with AR. Challenge with a house dust mites (HDM) extract was paired in a classical conditioning design with a particular gustatory stimulus added in a drink during the acquisition process. After exposure to this taste, the tryptase levels in the nasal washings were significantly higher than those in allergic individuals exposed to the extract alone or the drink without adding the particular gustatory stimulus.20
Concerning the conditioned suppression of the allergic reactions without administration of antiallergic medication, a study with a similar design examined whether the effects of desloratadine in patients with AR to house-dust mites can be behaviorally conditioned. During the learning phase, a gustatory stimulus in a drink was associated with desloratadine in 30 patients. During the evocation trial, 11 patients were re-exposed to the drink with the gustatory stimulus together with a placebo pill (conditioned stimulus group), 10 patients received water with a placebo pill (water group), and 9 patients received water and desloratadine (drug group). The patients in the conditioned stimulus group showed a decrease in the subjective total symptom scores along with attenuated skin prick test reactions to histamine and reduced ex vivo basophil activation. These effects were comparable with the drug group. Interestingly, behaviorally conditioned effects modified the clinical symptomatology and the effector immune functions (basophil activation) only in the conditioned stimulus group and not in the water group, highlighting that the direct interplay between the CNS and allergy effector cells can be established only by the classical conditioning.21
Behavioral conditioning differs from cognitive factors that seem to mediate placebo responses in that autonomic orchestration (eg, peripheral immune functioning or hormone release) seems to be primarily affected by associative learning processes and not by mere cognitive expectations.22,23
A relevant experiment with conditioned suppression was conducted in an allergic contact dermatitis murine model. Cyclosporin injections were paired with saccharin. Re-exposure to saccharin alone suppressed contact hypersensitivity responses to the culprit contact antigen in the same manner as the suppression observed by cyclosporin.24
Allergic Rhinitis
Allergic rhinitis is an inflammatory disease characterized by congestion, watery discharge, sneezing, and pruritus. Patients with AR demonstrate these symptoms because of allergic airway inflammation due to exposure to the culprit allergens and hyperresponsiveness due to nonspecific stimuli, partially controlled by the autonomic nervous system. In various phenotypes and endotypes of rhinitis, exaggerated responses to environmental or endogenous stimuli occur because neural activity is upregulated due to an abnormal process, primarily of inflammatory nature. Theoretically, stimuli of any intensity can generate symptoms. Practically, this stimulation is regulated on a homeostatic basis. When the control is lost, the phenomenon of neural hyperresponsiveness occurs. This dysregulation is believed to play a critical role in the clinical expression of nasal symptoms.
The watery discharge can be produced by reflex activation of the submucosal glands. Various stimuli have been shown to provoke secretory responses. These include exogenous irritants like allergens, cold air, hypertonic solutions or capsaicin (the chemical irritant and the pungent ingredient in chili peppers), or the endogenous mediators histamine and bradykinin.25–30 Pruritus and sneezing can be generated if only the nervous system is involved. Pruritus results from a tactile sensation, and sneezing represents a classic central reflex, which targets various respiratory and laryngeal muscles.31–33 Nasal congestion and airflow limitation result from vasodilation and can also occur due to neural stimulation. This is a synergistic effect of a decreased sympathetic outflow, conceivably on top of an increased parasympathetic discharge.34
The basal sensory nerves of the nose arise from the olfactory, the ophthalmic and the trigeminal nerves. Nonolfactory sensory nerves consist of both myelinated and unmyelinated fibers. In the vast majority, unmyelinated afferent fibers belong to the nociceptor (from the Latin word nocere, which means “to harm or hurt”) C-fiber type and conduct slow signals of possible threats to the CNS. The dendrites of C fibers can be antidromically stimulated by action potentials originating at different terminals of the same neuron. This antidromic stimulation is also known as the axon reflex,35 and it is responsible for the release of inflammatory neuropeptides, including tachykinins (substance P, neurokinin A), calcitonin gene-related peptide (CGRP), and gastrin-releasing peptide. The sensory nerve endings release neuropeptides that can produce vasodilatation, increased vascular permeability, glandular activation, leukocyte differentiation and recruitment, and activation of various immune cells, including lymphocytes, eosinophils, mast cells, and macrophages.36–39
The parasympathetic function of the nasal airways is activated by preganglionic fibers, which synapse in the sphenopalatine ganglion, and postganglionic fibers, which innervate serous and mucous glands, arteries, veins, and arteriovenous anastomoses of this anatomical area. Preganglionic parasympathetic nerves release, among other neurotransmitters, acetylcholine, that act on nicotinic receptor ion channels on postganglionic neurons. Postganglionic fibers, in turn, release acetylcholine, which acts on muscarinic receptors and neuropeptides, such as vasoactive intestinal peptide (VIP). VIP controls various functions of the nasal cavity, including glandular discharge, vasodilation, and sinusoidal engorgement.40,41 VIP levels in nasal secretions are significantly higher in AR patients compared to control subjects. VIP competes with prostaglandin D2 (PGD2) for chemoattractant receptor-homologous molecule expressed on T helper 2 cells (CRTH2) signaling for eosinophil chemotaxis.42 CRTH2 is expressed on eosinophils, basophils, and T helper 2 (Th2) lymphocytes. CRTH2 gained much attention as a promoter for PGD2-induced eosinophilia in allergic airway diseases.43–45
In an AR murine model, it has been demonstrated that the PGD2-CRTH2 interaction is elevated following pollen sensitization.46 In the inflammatory process of AR, eosinophils play a pivotal role, being, along with lymphocytes, the chief inflammatory cell types infiltrating the nasal mucosa.47–50
In addition to neuropeptides, postganglionic nerve bodies contain nitric oxide (NO) synthase, an enzyme that contributes to NO production. As a consequence, postganglionic nerves indirectly exhibit a non-adrenergic, noncholinergic neural control of the nasal blood vessels.51 Stimulation of the sympathetic nervous system induces vasoconstriction and increased nasal airway patency.52,53 This input originates from preganglionic fibers, which rely on the superior cervical ganglion. The effects of sympathetic nerves are mediated mainly through stimulation of α1- and α2-adrenergic receptors on the smooth muscles of resistance vessels and venous sinusoids, leading to vasoconstriction and, consequently, reduced blood flow and less blood pooling. The β1- and β2- receptor effects are less pronounced than those induced by α-receptor stimulation.54
Neurotrophins were initially known for their primary activity, the growth of peripheral and central nerves.55 In the meantime, it has become evident that neurotrophins exert a variety of immunomodulatory effects on nonneuronal cells, including eosinophils and mast cells, which also produce neurotrophins.2,56 Nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3, and neurotrophin-4/neurotrophin-5 have been discovered. NGF is expressed in the glandular apparatus, nasal epithelium, and peripheral nerves in the nasal mucosa of patients with intermittent and chronic, persistent AR.57,58 Nasal BDNF expression was significantly increased after allergen provocation but only in AR and not in the control subjects.59
Eosinophils are functionally activated upon neurotrophin stimulation. NGF increases the release of IL-4 from peripheral blood eosinophils and inhibits their programmed cell death.60,61 Neurotrophins 3 and 4 significantly inhibited peripheral blood eosinophil apoptosis in AR compared with controls. BDNF and neurotrophin-3 increased the release of eosinophil protein X of peripheral blood eosinophils of AR patients.60 Eosinophils release neurotrophins with a functional effect on peripheral nerves.62 Neurotrophins serve as prime candidates for neuroimmune interactions between eosinophil granulocytes and neurons, underlining their bidirectional interaction in allergic diseases, including AR.
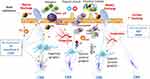
In humans, individuals with active AR were found to have a greater sensitivity and reactivity of the sneezing reflex, a higher secretory responsiveness to sensory nerve stimulation, and a more significant plasma extravasation indicated by albumin leakage following capsaicin nasal challenge compared with healthy individuals.29 As these patients were characterized by increased levels of NGF in nasal lavage, it was concluded that neurotrophins might be implicated in neural hyperresponsiveness. The complex neuroinflammation of AR is depicted in Figure 1.
Chronic Rhinosinusitis (CRS)
Chronic rhinosinusitis (CRS) is characterized as the inflammation that exists for at least 12 weeks in the nasal cavity mucosa and the paranasal sinuses. More than 10% of the adults in Europe and the USA may experience CRS. Common symptoms include nasal discharge, congestion, facial pain or pressure, and hyposmia or anosmia. Patients with CRS may also have other inflammatory airway conditions such as asthma and AR. Based on nasal endoscopic findings, CRS is divided, according to the presence of nasal polyps, into CRS without nasal polyps (CRSsNP) and CRS with nasal polyps (CRSwNP). Clinically, CRSwNP is characterized by greater morbidity than CRSsNP owing to more significant disease severity and a higher number of surgeries or medication exposure.63,64 The main risk factors are tobacco smoking, and other irritants like isocyanides, fumes from coal cooking, dust or smoke, hair-care products, and cleaning agents.65,66 Barrier dysfunction and inflammation are closely linked in CRS. Indeed, barrier loss is believed to lead to the entry of antigens/allergens, irritants, and pathogens that cause inflammation. On the other hand, inflammation itself, particularly T2 responses, can lead to barrier dysfunction and activation of sensory nerves, which are responsible for pruritus, pain, or the sensation of congestion, which contribute greatly to disease burden.67
Possible causes of polyp formation are pseudocyst, edema, and alterations in submucosal gland structure and function,68 phenomena can be explained by PNS and CNS stimulation in a dysregulated effort to maintain homeostasis. Vascular leak can be induced by autacoids (molecules with paracrine action released from inflammatory cells, neuroinflammatory responses or injury). This leak promotes the activation of thrombin and cleavage of fibrinogen in patients with CRS.69 The derived fibrin is crosslinked by factor XIIIa from alternatively activated macrophages. Most likely due to IL-13 exposure, polyp epithelium expresses low plasminogen activator levels, which reduces fibrinolysis, leading to fibrin accumulation and polyp growth.69,70 In Europe and the USA, eosinophils are found in up to 80% of polyps, in which they are located in a subepithelial distribution or dispersed throughout the polyp tissue.71
A study showed that the mean concentration of NGF in sinus mucosa was significantly higher in CRS than in control individuals. CRSsNP was associated with a 60% increase in sinus NGF over controls, and CRSwNP was associated with a 140% increase, but mean BDNF concentration was decreased in CRS, and especially in CRSwP patients compared with controls.72
Moreover, hemokinin-1 and neurokinin 1 receptor mRNA and protein expression were upregulated in eosinophilic and non-eosinophilic nasal polyps compared with control tissues, with eosinophilic polyps demonstrating a higher upregulation compared with that of non-eosinophilic polyps.73 This neurogenic inflammation may contribute to the pathophysiology of CRS; hence, any targeted intervention may provide alternative therapeutic options. The pathophysiological pathways involved in CRS neuroinflammation are depicted in Figure 1.
Allergic Asthma
At the end of the 19th century, the medical textbook of Sir William Osler referred to asthma as “a neurotic affection”. For a long time, even during the 20th century, asthma was considered a psychogenic disease primarily, and psychological factors were thought to be significant elicitors of asthma symptoms until the inflammatory basis of the disease became evident.74
Even from early life, persistent stress and its correlates (ie, maternal and childhood anxiety) have been associated with episodic wheezing and asthma. Furthermore, this tendency is extended to adolescence, as stressful environments and/or events during this period of life increase the likelihood of new asthma cases diagnosed in adulthood.75
Moreover, acute stress has been linked to both immediate and delayed asthma exacerbations, at least in children.76 Complex interactions among stressors, coping mechanisms, and well-known risk factors such as outdoor air pollution, exposure to tobacco smoke and aeroallergens are likely to result in prolonged physiological changes that influence subsequent disease risk.
The socio-economic model of life is clearly associated with asthma. Exposure to violence (ETV), including gun violence, affects stress responses, and both ETV and chronic stress have been shown to increase asthma morbidity.77 A recent study in a large urban city in the southwestern United States has shown that living in high-crime neighborhoods results in increased asthma diagnoses among children via the mechanism of toxic stress.78 Similarly, a screening survey in Chicago indicated that violent crime continued to be significantly associated with the neighborhood asthma prevalence, even after adjusting for several confounders (odds ratio, 1.27; 95% confidence interval, 1.04–1.55, p < 0.05). Therefore, crime as an inducer of chronic stress represents a socioenvironmental contributor to increased urban childhood asthma prevalence.79
Although naturalistic clinical assessments are not as common, in a small sample of adult asthmatics, it has been shown that everyday stressors, assessed across 10 consecutive days using preprogrammed watches, linked to peak expiratory flow rate values and symptoms.80 Ciprandi et al showed in a diverse Italian outpatient population, using a questionnaire quantifying levels of anxiety and depression, that a significant inverse correlation exists between anxiety scores and asthma control levels as reflected by subjective and objective measures like FEV1, fractional exhaled NO levels, Asthma Control Test scores, and physical findings.81
The widely accepted complexity of stress-related effects on asthma is further augmented by the fact that throughout childhood and adolescence, several changes occur in the neuroendocrine function, with a dramatic change in reactivity exhibited by the HPA axis in response to stressors. These responses are observed for both acute and chronic stressors, where evidence indicates more intense effects. Experimental models in youth with asthma and greater chronic family stress indicate enhanced in vitro stimulated production of asthma-related cytokines, including interleukin (IL)-5 and IL-13, and in vivo mobilization and eosinophils activation.82 The examination-related stress among asthmatic students potentiates IL-5 production and eosinophil mobilization in sputum after bronchial challenge.83
Different mechanisms such as mast cell activation, mediator release, inflammation, and impairment of respiratory tolerance have been identified in several asthma studies to contribute to the enhancement of bronchial hyperreactivity by psychological stress.84–86
Furthermore, psychosocial stress triggered by unpleasant visual stimulations can shortly increase airway resistance, as assessed by plethysmography and end-tidal PCO2 by capnometry, respectively. These findings are attributed to the stimulation of the cholinergic system and the subsequent vagal-mediated response. In certain asthmatic individuals, the enhanced basal cholinergic tone contributes to a greater extent to the resulting airway obstruction compared with other well-known elicitors as allergens and infections.87 The role of conditioning mechanisms in allergic asthma, as previously described, can explain the deterioration of pulmonary function independently of the exposure to the culprit allergens.18
A few studies have also evaluated the role of genetic or epigenetic mechanisms on stress-related asthma. In a cross-sectional study of three ethnically diverse cohorts, a meta-analysis of genotypic data from 2741 children and adolescents with asthma from seven different cohorts showed that a single nucleotide polymorphism (rs34548976) in the gene for adenylate cyclase-activating polypeptide 1 receptor type 1 (ADCYAP1R1) was associated with reduced bronchodilator response (BDR), significantly associated with high levels of chronic stress.88 Additionally, in a cohort of Puerto Rican children and adolescents, SNP rs34548976 was shown to be associated with reduced BDR, only in subjects with high levels of chronic stress. Of note, SNP rs34548976 was also associated with reduced expression of the gene for the β2-adrenergic receptor (ADRB2) in CD4+ lymphocytes from children and adults with asthma.89 Meta‐analysis of selected stress‐ or violence‐associated CpG sites in a cohort of childhood asthma in Puerto Ricans, 12 CpG sites were identified to associate with atopic asthma (in genes STARD3NL, TSR3, CDC42SE2, KLHL25, GALR1, TMEM196, ANAPC13, SLC35F4, PLCB1, BUD13, OR2B3, and TEAD4). These findings offer “proof of concept” and support the observed associations in longitudinal studies of chronic stress, DNA methylation markers in asthma in children.90
Except neuro-immunological interactions triggered by psychological stress that contribute to asthma symptoms and severity, stress can increase asthma morbidity by reducing response to inhaled corticosteroids and β2-agonists. Both short-term and long-term stress have been associated with reduced expression of genes encoding the glucocorticoid receptor (by 5.5-fold) and the β2 adrenergic receptors (by 9.5-fold) in leukocytes of children with asthma (P < 0.05 in both instances).91 In a well-established mouse model on stress and asthma, it was shown that social disruption stress induced decreased spleen cell responsiveness to corticosteroids and concurrently reduced glucocorticoid receptor expression in the lungs.92 Endogenous glucocorticoids released due to stressful stimuli are responsible for exacerbating inflammation but may also trigger airway hyperresponsiveness.93 Surprisingly enough, these results are contrary to the anti-inflammatory effects of glucocorticoids that are to be expected.
Experiments in a murine model of asthma have shown that allergic reactions in the lungs can increase the activity of specific hypothalamic and amygdaloid nuclei that are involved in emotional and behavioral alteration, and they can trigger stress responses via the HPA axis activation. These areas are involved directly or indirectly in adaptive behavioral changes and can regulate endogenous steroid secretion, among other endocrine pathways. These changes may build avoidance behavior as an adaptive homeostatic mechanism towards avoidance of further exposure to the culprit allergens to minimize future allergic reactions. The consequences of allergic asthma on brain stimulation and behavior alterations are mediated by at least peripheral mast cells and are IgE-dependent. Interestingly, it has been shown that brain activity and behavior alterations occur in the absence of any allergic inflammatory cell influx in the lungs at the early stages of the allergic responses. This suggests that organized pulmonary inflammation is not a prerequisite for brain activation that might be induced directly and immediat from the infiltrate of allergic effector cells in the CNS.94 These phenomena resemble the observed in food allergy central neuroinflammatory responses with behavioral consequences that will be described in the next section.95
Except for the well-known innervation of smooth muscle cells by sympathetic and parasympathetic nerves and their prominent role in asthmatic symptoms, the nervous system might also directly affect immune responses in asthma. In the sympathetic system, the mast cells that contribute to the cellular infiltrate through transforming growth factor-β (TGF-β) production induce the phosphorylation of β2 adrenergic receptor (β2AR), which in turn results in a reduced response to β2AR agonists. Besides, in mice models, it has been shown that β2AR-deficient mice have more intense T2 high inflammation as β2AR agonists dampen IL-33 and Alternaria-induced inflammation via suppressing innate lymphoid cells 2 (ILC2s).96 However, this needs to be confirmed in humans as well, although human lung ILC2s express β2AR. Accordingly, referring to the parasympathetic nerves, several mechanisms of neuro-immune crosstalk are implied. The eosinophil-derived major basic protein mediates the dysfunction of the muscarinic M2 receptor, which might contribute to this increased cholinergic neuron activity. Vice-versa, the vagal nerve suppresses the inflammatory response upon stimulation; this action is attributed to the action of acetylocholine to specific receptors as α7nAChR. Besides, recent data showed that α7nAChR agonists directly suppressed ILC2s, resulting in decreased T2 inflammation in IL-33– and Alternaria-induced airway inflammation.97 Thus, beyond the classical knowledge of the acetylocholine-induced bronchoconstriction via muscarinic M3 receptor, it seems that parasympathetic neurons may also have a beneficial effect on airway inflammation depending on the kind of their receptors.
Sensory nerves play a critical role in asthma pathophysiology. Various neuropeptides such as SP and CGRP have been shown to be increased in induced sputum and bronchoalveolar lavage of asthmatic patients and correlate with airway obstruction.98,99 Recently, experimental models have demonstrated that blocking sensory nerves decreases the severity of Th2 airway inflammation. According to these models, IL5 is produced mainly by ILC2s, and CD4+ cells stimulate pulmonary noniceptors to release VIP that activates ILC2s and CD4+ in a positive feedback loop with the final effect the enhancement of Th2 inflammation.100 Allergen sensitization triggers a feedforward inflammatory loop between IgE-producing plasma cells, FcεR1-expressing vagal sensory neurons, and Th2 cells, which helps to both initiate and amplify allergic airway inflammation. These data highlight a novel target for reducing allergy; namely, FcεR1γ expressed by nociceptors.
Moreover, the ion channels that are expressed in sensory nerve endings also contribute to antigen-induced neuronal activation. Transient receptor potential ankyrin 1 (TRPA1) induces the release of the neuropeptides CGRP, SP, and neurokinin A from sensory nerve terminals, which enhance T2 inflammation, although TRPA1 deficient mice do express the same asthma phenotype.101
Collectively, neurotransmitters and neuropeptides can directly affect immune cells, especially the ILC2s and Th2 cells that orchestrate T2 inflammation in asthma. Hence, their involvement in asthma pathophysiology might be much more important than considered until now.
Food Allergy
Food allergy is a hypersensitivity reaction due to an abnormal immune response to common food proteins. Food intake is inextricably linked to our nervous system. Food characteristics like taste, smell, and texture help determine food preferences and eating habits. Conditioned taste avoidance or aversion is a characteristic paradigm of classical conditioning that applies to food allergy.102 In the theory of taste aversion, the allergic subject evades specific foods that signal danger to avoid exposures that most of the time cause (at least) abdominal discomfort. In this sense, a subject sensitized to a particular food allergen can associate it with a specific taste or environment (exteroceptive stimulus).
Consequently, an adaptive response is initially elicited at a behavioral level, leading to avoidance of the culprit food (reactive behavior, eg, a fish-allergic individual avoids whatever smells or tastes like fish) or an associated environment (proactive behavior, eg, avoidance of a seafood restaurant). In the case of an intentional or unintentional exposure, the organism will try to minimize the time of interaction with the allergen by vomiting, coughing, or sneezing.103,104 This tactic is known as social or behavioral immune system.105
The typical such clinical paradigm in food allergy is the food protein-induced enterocolitis syndrome (FPIES), in which the gastrointestinal mucosa and nearby bystander immune cells, upon interaction with the culprit food allergens, struggle to eliminate them by increased retroperistalsis, resulting in emesis, and if not sufficient, increased peristalsis, resulting in diarrhea, in an effort to minimize exposure. This homeostatic reflex is coordinated by the vomiting center (located within the medulla) and the chemoreceptor trigger zone (located on the floor of the fourth ventricle). Key neurotransmitters like histamine, acetylcholine, SP, dopamine, and serotonin are involved in afferent feedback to these areas.106 It is self-explanatory that neuroimmune crosstalk and interactions between their components contribute to the clinical manifestations of this entity. At least one cell should either play the role of a direct chemo-sensor of the culprit food allergens,107 or produce appropriate immune byproducts that interact with the nerve endings of the ENS to communicate the deleterious effect of the food allergen. Overstimulation and dysregulation of this signaling may progress to profound gastrointestinal symptoms and exaggerated hemodynamic response. The reduction of nausea and vomiting caused by the selective serotonin or 5-hydroxytryptamine(3) receptor antagonist ondansetron in acute FPIES reactions further supports an underlying neuroimmune pathomechanism.108,109
Interestingly, more complex clinical manifestations, including symptoms from more than one system or organ, namely anaphylaxis, have been shown to be induced in rats sensitized to ovalbumin (OVA) by imposing Pavlovian conditioning even in the absence of exposure to OVA. These anaphylactic reactions were significantly related to stress or anxiety as they are measured by serum cortisol levels, suggesting that food anaphylaxis and anaphylaxis-related stress are prone to Pavlovian conditioning.110
Cognitive and behavioral changes may be explained by acquired neuroinflammation in the CNS. Experiments in mice sensitized to β-lactoglobulin showed that mast cells can accumulate to the CNS (brain and meninges) during the mice sensitization procedure and can be activated by circulating β-lactoglobulin during the repeated allergen exposure by IgE-FcεRI-mediated mechanisms, intracranially. Histamine levels and the expression of at least H3-receptors were significantly elevated in particular regions of the brain. Blood–brain barrier impairment only in sensitized mice was evident, providing an explanation for this activation. Remarkably, repeated allergen consumption resulted in no acute clinical objective allergic reactions but decreased mobility and depression-like behavior only in sensitized mice suggesting central neuroinflammatory responses with behavioral consequences.95 These observations seem to be genetically predisposed.111
The gastrointestinal tract has the privilege of possessing the ENS, its own nervous system that innervates the submucosal plexus and the myenteric plexus densely. The sensory neurons send signals to coordinate gut motility and gastric secretion. Lack of proper coordination could result in hypercontractility or hypersecretion that manifests with nausea, vomiting, diarrhea, or abdominal cramping symptoms encountered in the context of an allergic reaction. These symptoms can be attributed to degranulating mast cells that are lining the intestinal submucosa and interacting directly with the culprit allergens through the specific IgE antibodies bound on their surface FcεRI. Preformed mediators contained in their secretory granules are immediately released and interact not only with specific receptors on nearby T2 immune cells but also with the enteric sensory neurons of the ENS.
Indeed, immunoneural signaling has been demonstrated between human mast cells and enteric nerves. A mediator cocktail [including histamine, leukotrienes, platelet-activating factor (PAF), PGD2, and nitric oxide] released from mast cells stimulated by nonspecific IgE receptor-cross-linking (mAb 22E7 that can crosslink high-affinity FcεRI receptors by binding to a non-IgE binding epitope of the FcεRIα) excited human submucosal enteric neurons.112
In a microdissected preparation containing large intestine submucosal neurons, mast cells, and other immune cells from milk-sensitized guinea pigs, direct stimulation with β-lactoglobulin showed neural depolarization utilizing electrophysiological methods. This effect was mainly attributed to histamine. Pharmacological inhibition with antihistamines confirmed this finding, suggesting that histamine constitutes a major paracrine signal in this neuroimmune communication.113 There is evidence suggesting that H1114 and H2,115 histamine receptors mediate this histaminergic effect on the neuronal cell bodies, and H3 histamine receptors at the nicotinic synapses in enteric ganglia.116 These neuronal responses were additionally attributed, at least partially, to prostaglandins and leukotrienes. Combined pharmacological inhibition of prostaglandin and leukotrienes synthesis on top of histamine receptors suppressed these responses almost completely.117 This study of the role of leukotrienes in neuroimmune interactions and the previously mentioned experiment examining the role of PAF,112 are the only ones until now suggesting a potential role of bioactive lipids in neuroimmune interplay in allergy.
Neuroimmune crosstalk has been shown in enteric mast cells from guinea pigs and humans. Intestinal mast cells receive input from spinal afferent terminals in the digestive tract via CGRP receptors and neurokinin 1 receptors for SP. Sequentially, mast cells degranulate preformed and newly synthesized mediators that reach relevant receptors expressed on the same afferents and the ENS neurons by paracrine-like diffusion manner. These antidromic signals are mediated by the release of SP and CGRP at the level of mast cells-afferent junctions and ENS synapses. In term, mast cells express receptors for SP and CGRP, which evokes degranulation of several mediators, including histamine and mast cell protease II, which has been shown to play a critical role in this crosstalk. These interactions form a positive feedforward loop that autoregulates the excitability and sensitization of the afferent terminals to their preferred modality.118
Similar to histamine, mast-cell-degranulated tryptase can also induce acute and long-term hyperexcitability in guinea pig submucosal neurons and myenteric sensory neurons in the small intestine by interacting with protease-activated receptor 2 (PAR2) on these neurons’ bodies.119,120
Alterations in purinergic (related to binding and responding to purines), capsaicin-sensitive (eg, certain nociceptive sensory-efferent neurons that are sensitive to capsaicin), and cholinergic sensory neurotransmission have been observed to contribute to the disturbed intestinal motility in a food allergy mice model of intestinal anaphylaxis.121
A functional gastrointestinal motility regulator is also the VIP that primarily inhibits non-adrenergic non-cholinergic neurotransmitters to cause smooth muscle relaxation.122 The two known receptors for the VIP, VPAC1 and VPAC2 are expressed not only on neurons but also on several immune cells, including dendritic cells, macrophages, neutrophils, and ILC2.123 VIP dysregulation is involved in colitis, but there is no direct evidence that it is involved in food allergy, too.124
Evidence suggests that CGRP, which is a marker of both intrinsic and extrinsic primary afferent neurons in mice, may be involved in the pathophysiology of food allergy. The density of CGRP-immunoreactive nerve fibers was found to be increased in the intestinal mucosa of a murine model of experimental food allergy to OVA.125
There is much more to learn about the neuroimmune inflammation in food allergic individuals. More studies, especially on humans, are needed to understand the underlying neuro-immuno-pathomechanisms of food allergy and the potential interventions that could offer therapeutic alternatives in this difficult-to-treat entity.
Atopic Dermatitis
Atopic dermatitis (AD) is a common inflammatory skin disorder with a chronic or relapsing course, characterized by eczematous lesions and intense pruritus. Its complex pathophysiology integrates multifactorial elements, including strong genetic disposition, disordered microbiome, T2-skewed immune dysregulation, and skin barrier dysfunction. Cutaneous inflammation and barrier impairment, have been both hypothesized to precede each other as the initial steps of the disease, leading to what has been described as the “outside-in” and “inside-out” hypotheses, respectively. These two suggested mechanisms describe barrier defects, stemming, for example, from defects in the filaggrin gene that lead to disruption of barrier function. This disruption allows penetration of allergens and irritants and increased pathogens counts, hence triggering skin inflammation, allowing additional external stimuli to enhance this established inflammation. On the other hand, immunological aberrations may precede contributing to the development of skin barrier dysfunction. In the last years, the role of neural components in this complex and dynamic interplay has received much attention, and our understanding of this aspect of AD pathophysiology is constantly evolving.126
Skin is mostly innervated by a complex network of cutaneous somatosensory nerve fibers which originate from dorsal root ganglia (DRG) in the spinal cord or from the trigeminal ganglia for the skin of the head and face. These neurons depolarize when transient receptor potential (TRP) channels open and mediate physical sensations, along with pain and, most importantly, pruritus, the cardinal symptom of AD. Pruritus serves a protective role as a mechanism to remove noxious stimuli mechanically. The itch-scratch cycle is an important parameter of AD inflammation since the trauma caused to the skin enhances alarmin secretion by epithelial cells, Th2 activation, and further release of pruritogens. However, it is now recognized that the sensory neurons are involved in far more complex crosstalk with immune cells and keratinocytes, which is facilitated by close anatomical localization with resident immune cells.127
Pruritus consists of an important part of this neuro-immune interaction. In AD it is mainly mediated by histamine-independent signaling pathways, and its pathophysiology involves the stimulation of neurons by T2 cytokines produced in the inflammatory milieu. IL-31 is the first T2 immunity cytokine to be recognized to function as a pruritogen on TRPV1+/TRPA1+sensory neurons, besides its proinflammatory effect on keratinocytes and immune cells. IL-31 is produced by activated Th2 cells and other immune cells, including eosinophils and mast cells. In addition to directly causing pruritus and activating sensory neurons, IL-31 can also act as a neurotrophin. It can promote sensory nerve elongation and branching by inducing genes related to neuronal growth in DRG neurons, leading to increased cutaneous sensory nerve density.128,129 Branching may also be induced by other neurotrophins released locally, including NGF by keratinocytes, mast cells, basophils and other immune cells, and BDNF induced by eosinophils. By this means, it contributes to the development of chronic pruritus.130 Following IL-31, the T2 cytokines IL-4 and IL-13, which orchestrate an inflammatory response critical in AD pathogenesis, have been found to directly activate mouse and human TRPV1+ sensory neurons that express their common receptor subunit IL-4Rα, and evoke itch in mice.131,132 Furthermore, IL-4 seems to enhance the responsiveness of non-peptidergic neurons to multiple pruritogens, hence promoting neural hypersensitivity. It remains to be elucidated if the expression of IL31Rα and IL4Rα coexist on the same subsets of neurons, whether there are distinct subsets of TRPV1+ neurons, and what the required thresholds to confer responsiveness are.133
Sensory neurons do not only integrate signals coming from the immune system. Apart from immune cells, keratinocytes, the other important pillar of AD inflammation, could also activate a subset of TRPA1+ sensory neurons, which have receptors for the epithelial cell-derived cytokine thymic stromal lymphopoietin (TSLP), an alarmin involved in the initiation and maintenance of AD.3 The same has been demonstrated for another alarmin, IL-33, which activates DRG neurons expressing its receptor ST2 via both TRPV1 and TRPA1 ion channels.134 Hence, these alarmins not only play a central proinflammatory role in AD pathogenesis by recruiting Th2 cells, activating innate lymphoid cells and other cells of innate immunity, and leading to the production of Th2 cytokines, but they also directly activate sensory neurons.135 Evidence now suggests that all the cytokines mentioned above, along with TNF-α (a cytokine of Th1 inflammation, which complements Th2 inflammation at the chronic phase of AD) exert direct or indirect effects on transcription, synthesis and membrane insertion of TRP channels in sensory neurons, potentiating them to modulate the progression of pruritic inflammation from acute to chronic stages.136 Another possible circuit of the epithelial neuronal itch axis involves the TSLP-induced release of periostin by keratinocytes which activates TRPV1+ sensory neurons to induce itch, as well.137 At the same time, other epidermal proteases might also provoke pruritus, through distinct neuronal pathways, such as kallikreins, possibly through PAR2.138
In the other direction of this crosstalk, sensory neurons can regulate inflammation and barrier immunity via the release of neuropeptides, like CGRP. CGRP is an important pruritogenic mediator in spinal interneurons, transmitting the sensation of pruritus in the CNS, but it also interacts locally in the skin with other tissue and immune cells.139
In mice, IL-31 induces synthesis and release of BNP by neurons which then upregulate transcription and potentiate the activity of TRPV3 channels on keratinocytes. TRPV3 channel up-regulation in human keratinocytes is considered to increase TSLP levels, propagating Th2 inflammation in a feedforward loop and activating neurons.136 Furthermore, CGRP has been shown to influence the functional identity of immune cells. Langerhans cells exposed to nerve-derived CGRP in situ are polarized towards promoting Th2 inflammation.140
Another neuropeptide, SP, has been recently described as part of a proposed mechanism through which sensory neurons may be involved, not in the propagation but in the initiation of the inflammation in AD. In 2019 following the development of a clinically relevant mouse model of “AD-like” T2 skin inflammation, there was evidence that HDM cysteine protease activity may activate TRPV1+ neurons that are able to release SP. SP can subsequently activate mast cells, which reside in close opposition to nerve fibers, through the recently recognized Mas-related G protein-coupled receptor MRGPRB2 (the mouse equivalent of human MRGPRX2). This triggers mast cell degranulation releasing a multitude of mediators, which may constitute one of the initial events of the complex pathogenesis of T2-dominated inflammation.141
The released mast cell mediators also interact with their cognate receptors on sensory neurons, inducing itch, as in the case of mast cell proteases interacting with PAR+ sensory neurons. In another mouse model of allergic contact dermatitis, it was shown that the mediators released following MRGPRB2 activation (rich in tryptase) were different from those released by IgE-mediated mast cell activation (rich in histamine) and provoked non-histaminergic itch, which was accommodated by a different neuron population when compared to classic histaminergic pruritus.142 This bidirectional communication of mast cells with neurons, along with their close anatomical proximity to mast cells has started shaping the idea of the tissue-resident neuro-immune sensory unit related to T2 immunity.141,143
Further interactions can be identified and add to the complexity of this interplay and progression of AD. The neurotrophins not only lead to neuronal branching but participate in crosstalk among keratinocytes, immune cells, and neurons, which secrete and have receptors for these factors. Serum neurotrophin levels correlate with AD severity. Their contribution in AD cutaneous inflammation includes promoting endothelial barrier permeability, proinflammatory cytokine release, cellular recruitment and infiltration, and further release of neurotrophins. Typical receptors of immune cells and keratinocytes, such as Toll-like receptors, have also been recognized on sensory neurons and vice versa, classic neurotransmitter receptors on immune cells and keratinocytes, the biological importance of which remains to be elucidated.144
Other cell types may also present unique types of interactions, like basophils which participate in a distinct basophil-sensory neuronal itch axis, involved in acute itch flares, mediated by the production of leukotriene C4.135 Neutrophils may interact with sensory neurons by inducing CXCL-10 release, which binds to CXCR3 on neurons and promotes itch.145,146
Beyond the local neuro-immune interactions in the skin of AD patients, it is also well recognized that stress may be an aggravating or triggering factor for this inflammatory condition.147,148 Apart from psychosomatic considerations, the exact mechanisms through which stress-related central processes affect peripheral inflammation are still explored, with evidence pointing to possible neuro-immunoendocrine connections.149 Mast cells have been placed centrally in this process due to their ability to be stimulated by peripherally released corticotropin-releasing hormone (CRH) and related peptides, leading to inflammation.
Along with the HPA axis activation, peripheral sensory neural activation may consist of another stress axis, and the subsequent stress-induced release of SP and NGF could contribute to the local inflammation.150 Interestingly, the inflammation of AD has also been postulated to lead to inflammation of the CNS and associated developmental consequences starting in childhood and extending into adulthood.151 AD has been linked to autism spectrum disorders, attention-deficit/hyperactivity disorder, and, more recently, cognitive dysfunction, with possible underlying neuroinflammatory mechanisms, among other postulations. Mast cells may again play an important role due to their ability to release mediators that could affect the blood–brain barrier, allowing penetration of proinflammatory cytokines.151,152 However, further studies are needed to clarify these associations and further elucidate our understanding of these complex neuro-immune interplays in the skin.
Chronic Urticaria
There is rising interest and research into the pathophysiology of chronic urticaria (CU) as this disorder inevitably results in increasingly poor quality of the patients’ lives. In recent years, several studies have uncovered a conceivably vital role for the nervous system and neuro-immune interactions in CU’s development, potentially explaining the clinical responsiveness heterogeneity and the variable treatment responses.153,154
Extant knowledge indicates that the underlying mechanisms for CU onset include histamine and other inflammatory mediators released in the skin by mast cells and basophils.155 In a recently published systematic review, a complex neuro-immune-cutaneous model involving numerous neuropeptides and neurokinins, inflammatory mediators and cells, hormones, and the skin was discussed with an eye on the pathways involved in the onset and maintenance of CU. The authors also questioned whether the imbalance or irregularity of this neuro-immune-cutaneous circuit could also result in elevated stress levels that have been closely related to CU.153 In line with the latter, the prevalence of psychopathology among CU patients had been previously estimated to be 31.6%.154 However, it is unclear if stress causes or prompts the onset of CU when combined with a pre-existing dysregulation of the neuroimmune system.
In line with the other allergic disorders, the immune and nervous systems are not acting separately but work instead in tandem in CU. The neurons modulate the function of immune cells by releasing neuropeptides and neurotransmitters. Consequently, the activated immune cells produce and release proinflammatory mediators such as chemokines, cytokines, and neuropeptides that trigger inflammation in the skin.153 In addition to the intense biochemical crosstalk between immune cells and neurons, the interactions within the neuro-immune system also derive from sharing many properties, such as receptor and ligand expression, that enable efficient communication between the two systems.7,156
The homeostatic role of mast cells seems crucial for retaining CU pathophysiology through a complex neuro-immune cascade as they exist in every single organ, produce and secrete multiple molecules, express a considerable variety of receptors, and interact peripherally and centrally with the nervous system.157 The mast cells respond to neuropeptides in the inflammation process by releasing various proinflammatory, vasoactive, and nociceptive chemicals. Cytokines such as interferon-γ, tumor necrosis factor-a, IL-1, IL-2, IL-6, IL-9, IL-10, IL-16, the vascular endothelial growth factor, nitric oxide, the basic fibroblast growth factor, the TGF-β, NGF, neuropeptides such as endorphins, CRF, SP, somatostatin, kinins (bradykinin), and VIP are also produced by the mast cells implicated in CU.157–159 Receptors for neuropeptides, such as pituitary adenylate cyclase-activating polypeptide, CRF, neurotensin, VIP, and neuropeptide Y, are also expressed by the CU-implicated mast cells.157,160,161 Thus, the evidence of a systemic inflammatory process in patients with CU that has been suggested in several studies, and the correlation with disease severity could be potentially explained by the aforementioned inflammatory cascade that involves the mast cells in a leading role.
SP has an essential role in the cutaneous neuroimmune network and is recognized as a significant mediator responsible for neurogenic inflammation in the skin. It modulates many pathophysiological events, such as immunomodulation, antimicrobial host defense, inflammation, itch, and pain.150,162 Recent studies in CU patients have demonstrated significantly elevated circulating levels, in correlation with disease severity, and that SP-positive basophils are upregulated.155 SP has been shown to trigger degranulation in basophils derived from CU patients. The data suggest that the biological activity of SP can be exerted through the conventional NK-1 receptor and the Mas-related G protein-coupled receptors. MRGPRX2 can cause mast cell activation and has been shown to be upregulated in the skin of patients with severe CU.155
The skin not only serves as a physical barrier to the external factors but can also express the effects of internal processes. It interacts closely with the CNS through mechanical and chemical receptors, nerves, muscle, and vasculature. As either the primary detector or the secondary receiver of the central stress response, the skin is highly susceptible to the effects of stress. Through the peripheral nerve terminals, the local HPA axis, and the immune system, skin-homing cells, such as keratinocytes, T cells, and mast cells, play an active role in the stress response. Between the brain and the skin, there are feedback and crosstalk systems.163 Inflammatory neurogenic pathways and pre-inflammatory and proinflammatory cytokines play essential roles in the modulation of such responses.164 Several studies have examined the serum concentration of neuro-immune factors in CU patients (ie, SP, IL-6, CGRP, IL-18, NGF).153 Among other evidence, an increased release of proinflammatory cytokines, including IL-6 and IL-18 has been correlated with CU severity and autoimmunity165,166 and can explain the enhanced neuroimmune inflammation.
Chronic urticaria patients have also been found to have secondary sleeplessness due to stress, disrupting the circadian cycle of cortisol secretion and aggravating CU.167 Cortisol deficiency leads to inflammatory dysregulation, which flares up and intensifies the vicious cycle.168 Further evidence suggests that the peripheral HPA axis in the skin also plays a vital role in the etiopathogenesis of CU via inflammatory and neurotransmission disturbances.153,164,169 Mast cells and epidermal and hair follicle keratinocytes are effector cells in this axis expressing CRH receptor 1 (R1) receptors and produce CRH and IL-18 in response to stress.170 The neuroendocrine, behavioral, and autonomic responses to stress are all orchestrated by CRH-R1, whereas IL-18 contributes to severe cutaneous inflammation. As a result, the cutaneous HPA axis maintains the local inflammatory cytokine equilibrium, resulting in increased CRH production due to the imbalanced secretion. By stimulating mast cell degranulation and enhancing in vivo vascular permeability in rat skin, CRH has been demonstrated to have proinflammatory properties.171 CRH can also produce an increase in IL-6 secretion. In patients with CU involving the CRH receptors, elevated levels of the mast cell-related gene histidine decarboxylase and CRH-R1 were discovered.170 Vasodilation and increased vascular permeability, which are pathogenetic features of urticaria, are caused by CRH-induced activation of cutaneous mast cells via a CRH-R1-dependent pathway. These conditions can be exacerbated or precipitated by stress, as well.170,171
Conclusion
The complicated interaction between the CNS and the ANS, on the one hand, and the immune system and its components, on the other hand, seems to substantially contribute to allergy pathophysiology, uncovering a clearly under-recognized association that could have diagnostic and therapeutic potentials. Neurons connect directly with and regulate the function of many immune cells, including mast cells, the cells that have a leading role in allergic disorders. Proinflammatory mediators such as cytokines, neurotrophins, chemokines, and neuropeptides are released by these immune cells, which stimulate sensory neurons. The release of neurotransmitters and neuropeptides caused by the activation of these neurons directly impacts the functional activity of immune cells and vice versa. Predominantly T2 cytokines modulate different features of neuroimmune pathomechanisms involved in the recurrent and debilitating symptoms of different allergic disorders. Similarities in sensory neurons that innervate the involved organs and conservation of cytokine receptors on such neurons highlight the role of at least T2 cytokines as key mediators of neuroimmune responses to deleterious stimuli from the external environment. Bidirectional crosstalk between immune cells implicated in allergic inflammation and neurons preserves or deteriorates the ongoing vicious cycle of immune dysregulation.
It is essential to understand the unknown neurological networks that not only physiologically cooperate with the immune system to regulate homeostasis but also pathogenetically interact with more or less known immunological pathways, contribute to what is known as neuroimmunological inflammation and shift homeostasis to instability and disease clinical expression. This understanding will provide recognition of new allergic phenotypes or endotypes and the potential to study and focus on specialized, personalized treatments.
Disclosure
CK is an employee of Pfizer. Dr Michael Makris reports grants, personal fees from ASTRA ZENECA, GSK, CHIESI, MENARINI, SANOFI, NOVARTIS, and PFIZER, outside the submitted work. The rest of the authors do not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
1. Waxenbaum JA, Reddy V, Varacallo M. Anatomy, Autonomic Nervous System. Available from. Treasure Island (FL): StatPearls Publishing;2022. Available from: https://www.ncbi.nlm.nih.gov/books/NBK539845/.
2. Maier SF, Goehler LE, Fleshner M, Watkins LR. The role of the vagus nerve in cytokine-to-brain communication. Ann N Y Acad Sci. 1998;840:289–300. doi:10.1111/j.1749-6632.1998.tb09569.x
3. Wilson SR, Thé L, Batia LM, et al. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell. 2013;155(2):285–295. doi:10.1016/j.cell.2013.08.057
4. Kashem SW, Riedl MS, Yao C, Honda CN, Vulchanova L, Kaplan DH. Nociceptive Sensory Fibers Drive Interleukin-23 Production from CD301b+ Dermal Dendritic Cells and Drive Protective Cutaneous Immunity. Immunity. 2015;43(3):515–526. doi:10.1016/j.immuni.2015.08.016
5. Lee S, Jo S, Talbot S, et al. Novel charged sodium and calcium channel inhibitor active against neurogenic inflammation. eLife. 2019:8. doi:10.7554/eLife.48118
6. Nockher WA, Renz H. Neurotrophins in allergic diseases: from neuronal growth factors to intercellular signaling molecules. J Allergy Clin Immunol. 2006;117(3):583–589. doi:10.1016/j.jaci.2005.11.049
7. Oetjen LK, Kim BS. Interactions of the immune and sensory nervous systems in atopy. FEBS J. 2018;285(17):3138–3151. doi:10.1111/febs.14465
8. Frieri M. Neuroimmunology and inflammation: implications for therapy of allergic and autoimmune diseases. Ann Allergy Asthma Immunol. 2003;90(6 Suppl 3):34–40. doi:10.1016/s1081-1206(10)61658-4
9. Bienenstock J, Perdue M, Blennerhassett M, et al. Inflammatory cells and the epithelium. Mast cell/nerve interactions in the lung in vitro and in vivo. Am Rev Respir Dis. 1988;138(6 Pt 2):S31–4. doi:10.1164/ajrccm/138.6_Pt_2.S31
10. Kiernan JA. Degranulation of mast cells in the trachea and bronchi of the rat following stimulation of the vagus nerve. Int Arch Allergy Appl Immunol. 1990;91(4):398–402. doi:10.1159/000235149
11. Greene R, Fowler J, MacGlashan DJ, Weinreich D. IgE-challenged human lung mast cells excite vagal sensory neurons in vitro. J Appl Physiol. 1988;64(5):2249–2253. doi:10.1152/jappl.1988.64.5.2249
12. Stead RH, Dixon MF, Bramwell NH, Riddell RH, Bienenstock J. Mast cells are closely apposed to nerves in the human gastrointestinal mucosa. Gastroenterology. 1989;97(3):575–585. doi:10.1016/0016-5085(89)90627-6
13. Williams RM, Berthoud HR, Stead RH. Vagal afferent nerve fibres contact mast cells in rat small intestinal mucosa. Neuroimmunomodulation. 1997;4(5–6):266–270. doi:10.1159/000097346
14. Crosson T, Wang JC, Doyle B, et al. FcεR1-expressing nociceptors trigger allergic airway inflammation. J Allergy Clin Immunol. 2021;147(6):2330–2342. doi:10.1016/j.jaci.2020.12.644
15. van der Kleij H, Charles N, Karimi K, et al. Evidence for neuronal expression of functional Fc (epsilon and gamma) receptors. J Allergy Clin Immunol. 2010;125(3):757–760. doi:10.1016/j.jaci.2009.10.054
16. Undem BJ, Clark TT. Mechanisms underlying the neuronal-based symptoms of allergy. J Allergy Clin Immunol. 2014;133(6):1521–1534. doi:10.1016/j.jaci.2013.11.027
17. Hadamitzky M, Lückemann L, Pacheco-López G, Schedlowski M. Pavlovian Conditioning of Immunological and Neuroendocrine Functions. Physiol Rev. 2020;100(1):357–405. doi:10.1152/physrev.00033.2018
18. Dekker E, Pelser HE, Groen J. Conditioning as a cause of asthmatic attacks; a laboratory study. J Psychosom Res. 1957;2(2):97–108. doi:10.1016/0022-3999(57)90015-6
19. Dark K, Peeke HV, Ellman G, Salfi M. Behaviorally conditioned histamine release. Prior stress and conditionability and extinction of the response. Ann N Y Acad Sci. 1987;496:578–582. doi:10.1111/j.1749-6632.1987.tb35816.x
20. Gauci M, Husband AJ, Saxarra H, King MG. Pavlovian conditioning of nasal tryptase release in human subjects with allergic rhinitis. Physiol Behav. 1994;55(5):823–825. doi:10.1016/0031-9384(94)90066-3
21. Goebel MU, Meykadeh N, Kou W, Schedlowski M, Hengge UR. Behavioral conditioning of antihistamine effects in patients with allergic rhinitis. Psychother Psychosom. 2008;77(4):227–234. doi:10.1159/000126074
22. Vits S, Cesko E, Benson S, et al. Cognitive factors mediate placebo responses in patients with house dust mite allergy. PLoS One. 2013;8(11):e79576. doi:10.1371/journal.pone.0079576
23. Wechsler ME, Kelley JM, Boyd IOE, et al. Active albuterol or placebo, sham acupuncture, or no intervention in asthma. N Engl J Med. 2011;365(2):119–126. doi:10.1056/NEJMoa1103319
24. Exton MS, Elfers A, Jeong WY, Bull DF, Westermann J, Schedlowski M. Conditioned suppression of contact sensitivity is independent of sympathetic splenic innervation. Am J Physiol Regul Integr Comp Physiol. 2000;279(4):R1310–5. doi:10.1152/ajpregu.2000.279.4.R1310
25. Konno A, Togawa K. Role of the vidian nerve in nasal allergy. Ann Otol Rhinol Laryngol. 1979;88(2 Pt 1):258–266. doi:10.1177/000348947908800219
26. Baroody FM, Wagenmann M, Naclerio RM. Comparison of the secretory response of the nasal mucosa to methacholine and histamine. J Appl Physiol. 1993;74(6):2661–2671. doi:10.1152/jappl.1993.74.6.2661
27. Philip G, Jankowski R, Baroody FM, Naclerio RM, Togias AG. Reflex activation of nasal secretion by unilateral inhalation of cold dry air. Am Rev Respir Dis. 1993;148(6 Pt 1):1616–1622. doi:10.1164/ajrccm/148.6_Pt_1.1616
28. Sanico AM, Philip G, Lai GK, Togias A. Hyperosmolar saline induces reflex nasal secretions, evincing neural hyperresponsiveness in allergic rhinitis. J Appl Physiol. 1999;86(4):1202–1210. doi:10.1152/jappl.1999.86.4.1202
29. Sanico AM, Koliatsos VE, Stanisz AM, Bienenstock J, Togias A. Neural hyperresponsiveness and nerve growth factor in allergic rhinitis. Int Arch Allergy Immunol. 1999;118(2–4):154–158. doi:10.1159/000024054
30. Riccio MM, Proud D. Evidence that enhanced nasal reactivity to bradykinin in patients with symptomatic allergy is mediated by neural reflexes. J Allergy Clin Immunol. 1996;97(6):1252–1263. doi:10.1016/s0091-6749(96)70193-8
31. Anton F, Peppel P. Central projections of trigeminal primary afferents innervating the nasal mucosa: a horseradish peroxidase study in the rat. Neuroscience. 1991;41(2–3):617–628. doi:10.1016/0306-4522(91)90354-q
32. Baraniuk JN. Sensory, parasympathetic, and sympathetic neural influences in the nasal mucosa. J Allergy Clin Immunol. 1992;90(6 Pt 2):1045–1050. doi:10.1016/0091-6749(92)90121-h
33. Wallois F, Macron JM, Jounieaux V, Duron B. Trigeminal afferences implied in the triggering or inhibition of sneezing in cats. Neurosci Lett. 1991;122(2):145–147. doi:10.1016/0304-3940(91)90843-i
34. Jackson RT, Rooker DW. Stimulation and section of the vidian nerve in relation to autonomic control of the nasal vasculature. Laryngoscope. 1971;81(4):565–569. doi:10.1288/00005537-197104000-00007
35. Lisney SJW, Bharali LAM. The Axon Reflex: an Outdated Idea or a Valid Hypothesis? Physiology. 1989;4(2):45–48. doi:10.1152/physiologyonline.1989.4.2.45
36. McDonald DM, Mitchell RA, Gabella G, Haskell A. Neurogenic inflammation in the rat trachea. II. Identity and distribution of nerves mediating the increase in vascular permeability. J Neurocytol. 1988;17(5):605–628. doi:10.1007/BF01260989
37. McDonald DM. Neurogenic inflammation in the rat trachea. I. Changes in venules, leucocytes and epithelial cells. J Neurocytol. 1988;17(5):583–603. doi:10.1007/BF01260988
38. Baraniuk JN, Kaliner M. Neuropeptides and nasal secretion. Am J Physiol. 1991;261(4 Pt 1):L223–35. doi:10.1152/ajplung.1991.261.4.L223
39. Baluk P, Bertrand C, Geppetti P, McDonald DM, Nadel JA. NK1 receptors mediate leukocyte adhesion in neurogenic inflammation in the rat trachea. Am J Physiol. 1995;268(2 Pt 1):L263–9. doi:10.1152/ajplung.1995.268.2.L263
40. Revington M, Lacroix JS, Potter EK. Sympathetic and parasympathetic interaction in vascular and secretory control of the nasal mucosa in anaesthetized dogs. J Physiol. 1997;505(Pt 3):823–831. doi:10.1111/j.1469-7793.1997.823ba.x
41. Yao W, Sheikh SP, Ottesen B, Jørgensen JC. The effect of neuropeptides on vessel tone and cAMP production. Ann N Y Acad Sci. 1996;805:784–788. doi:10.1111/j.1749-6632.1996.tb17557.x
42. El-Shazly AE, Begon DY, Kustermans G, et al. Novel association between vasoactive intestinal peptide and CRTH2 receptor in recruiting eosinophils: a possible biochemical mechanism for allergic eosinophilic inflammation of the airways. J Biol Chem. 2013;288(2):1374–1384. doi:10.1074/jbc.M112.422675
43. Shiraishi Y, Asano K, Nakajima T, et al. Prostaglandin D2-induced eosinophilic airway inflammation is mediated by CRTH2 receptor. J Pharmacol Exp Ther. 2005;312(3):954–960. doi:10.1124/jpet.104.078212
44. Royer JF, Schratl P, Lorenz S, et al. A novel antagonist of CRTH2 blocks eosinophil release from bone marrow, chemotaxis and respiratory burst. Allergy. 2007;62(12):1401–1409. doi:10.1111/j.1398-9995.2007.01452.x
45. Uller L, Mathiesen JM, Alenmyr L, et al. Antagonism of the prostaglandin D2 receptor CRTH2 attenuates asthma pathology in mouse eosinophilic airway inflammation. Respir Res. 2007;8(1):16. doi:10.1186/1465-9921-8-16
46. Nomiya R, Okano M, Fujiwara T, et al. CRTH2 plays an essential role in the pathophysiology of Cry j 1-induced pollinosis in mice. J Immunol. 2008;180(8):5680–5688. doi:10.4049/jimmunol.180.8.5680
47. Downey GP. Mechanisms of leukocyte motility and chemotaxis. Curr Opin Immunol. 1994;6(1):113–124. doi:10.1016/0952-7915(94)90042-6
48. Bokoch GM. Chemoattractant signaling and leukocyte activation. Blood. 1995;86(5):1649–1660.
49. Bacon KB, Szabo MC, Yssel H, Bolen JB, Schall TJ. RANTES induces tyrosine kinase activity of stably complexed p125FAK and ZAP-70 in human T cells. J Exp Med. 1996;184(3):873–882. doi:10.1084/jem.184.3.873
50. Knall C, Worthen GS, Johnson GL. Interleukin 8-stimulated phosphatidylinositol-3-kinase activity regulates the migration of human neutrophils independent of extracellular signal-regulated kinase and p38 mitogen-activated protein kinases. Proc Natl Acad Sci U S A. 1997;94(7):3052–3057. doi:10.1073/pnas.94.7.3052
51. Okita W, Ichimura K. Contribution of nitric oxide and sensory transmitters to non-adrenergic, non-cholinergic innervation of nasal blood vessels. Acta Otolaryngologica Supplementum. 1998;539:76–78. doi:10.1080/00016489850182189
52. Lacroix JS. Adrenergic and non-adrenergic mechanisms in sympathetic vascular control of the nasal mucosa. Acta Physiol Scand Suppl. 1989;581:1–63.
53. Kawarai M, Koss MC. Sympathetic control of nasal blood flow in the rat mediated by alpha(1)-adrenoceptors. Eur J Pharmacol. 2001;413(2–3):255–262. doi:10.1016/s0014-2999(01)00759-2
54. Wang M, Lung MA. Adrenergic mechanisms in canine nasal venous systems. Br J Pharmacol. 2003;138(1):145–155. doi:10.1038/sj.bjp.0705020
55. Lewin GR, Barde YA. Physiology of the neurotrophins. Annu Rev Neurosci. 1996;19:289–317. doi:10.1146/annurev.ne.19.030196.001445
56. Raap U, Goltz C, Deneka N, et al. Brain-derived neurotrophic factor is increased in atopic dermatitis and modulates eosinophil functions compared with that seen in nonatopic subjects. J Allergy Clin Immunol. 2005;115(6):1268–1275. doi:10.1016/j.jaci.2005.02.007
57. Bresciani M, Lalibertè F, Lalibertè MF, Gramiccioni C, Bonini S. Nerve growth factor localization in the nasal mucosa of patients with persistent allergic rhinitis. Allergy. 2009;64(1):112–117. doi:10.1111/j.1398-9995.2008.01831.x
58. Wu X, Myers AC, Goldstone AC, Togias A, Sanico AM. Localization of nerve growth factor and its receptors in the human nasal mucosa. J Allergy Clin Immunol. 2006;118(2):428–433. doi:10.1016/j.jaci.2006.04.037
59. Raap U, Fokkens W, Bruder M, Hoogsteden H, Kapp A, Braunstahl GJ. Modulation of neurotrophin and neurotrophin receptor expression in nasal mucosa after nasal allergen provocation in allergic rhinitis. Allergy. 2008;63(4):468–475. doi:10.1111/j.1398-9995.2008.01626.x
60. Noga O, Englmann C, Hanf G, Grützkau A, Guhl S, Kunkel G. Activation of the specific neurotrophin receptors TrkA, TrkB and TrkC influences the function of eosinophils. Clin Exp Allergy. 2002;32(9):1348–1354. doi:10.1046/j.1365-2745.2002.01442.x
61. Hamada A, Watanabe N, Ohtomo H, Matsuda H. Nerve growth factor enhances survival and cytotoxic activity of human eosinophils. Br J Haematol. 1996;93(2):299–302. doi:10.1046/j.1365-2141.1996.5151055.x
62. Costello RW, Schofield BH, Kephart GM, Gleich GJ, Jacoby DB, Fryer AD. Localization of eosinophils to airway nerves and effect on neuronal M2 muscarinic receptor function. Am J Physiol. 1997;273(1 Pt 1):L93–103. doi:10.1152/ajplung.1997.273.1.L93
63. Banerji A, Piccirillo JF, Thawley SE, et al. Chronic rhinosinusitis patients with polyps or polypoid mucosa have a greater burden of illness. Am J Rhinol. 2007;21(1):19–26. doi:10.2500/ajr.2007.21.2979
64. Khan A, Vandeplas G, Huynh TMT, et al. The Global Allergy and Asthma European Network (GALEN) rhinosinusitis cohort: a large European cross-sectional study of chronic rhinosinusitis patients with and without nasal polyps. Rhinology. 2019;57(1):32–42. doi:10.4193/Rhin17.255
65. Hastan D, Fokkens WJ, Bachert C, et al. Chronic rhinosinusitis in Europe–an underestimated disease. A GA2LEN study. Allergy. 2011;66(9):1216–1223. doi:10.1111/j.1398-9995.2011.02646.x
66. Thilsing T, Rasmussen J, Lange B, Kjeldsen AD, Al-Kalemji A, Baelum J. Chronic rhinosinusitis and occupational risk factors among 20- to 75-year-old Danes-A GA(2) LEN-based study. Am J Ind Med. 2012;55(11):1037–1043. doi:10.1002/ajim.22074
67. LeMessurier KS, Tiwary M, Morin NP, Samarasinghe AE. Respiratory Barrier as a Safeguard and Regulator of Defense Against Influenza A Virus and Streptococcus pneumoniae. Front Immunol. 2020;11:3. doi:10.3389/fimmu.2020.00003
68. Schleimer RP. Immunopathogenesis of Chronic Rhinosinusitis and Nasal Polyposis. Annu Rev Pathol. 2017;12:331–357. doi:10.1146/annurev-pathol-052016-100401
69. Takabayashi T, Kato A, Peters AT, et al. Excessive fibrin deposition in nasal polyps caused by fibrinolytic impairment through reduction of tissue plasminogen activator expression. Am J Respir Crit Care Med. 2013;187(1):49–57. doi:10.1164/rccm.201207-1292OC
70. Takabayashi T, Kato A, Peters AT, et al. Increased expression of factor XIII-A in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2013;132(3):584–592.e4. doi:10.1016/j.jaci.2013.02.003
71. Simon HU, Yousefi S, Schranz C, Schapowal A, Bachert C, Blaser K. Direct demonstration of delayed eosinophil apoptosis as a mechanism causing tissue eosinophilia. J Immunol. 1997;158(8):3902–3908.
72. Coffey CS, Mulligan RM, Schlosser RJ. Mucosal expression of nerve growth factor and brain-derived neurotrophic factor in chronic rhinosinusitis. Am J Rhinol Allergy. 2009;23(6):571–574. doi:10.2500/ajra.2009.23.3412
73. Deng YK, Ma J, Wang ZC, et al. Hemokinin-1 stimulates C-C motif chemokine ligand 24 production in macrophages to enhance eosinophilic inflammation in nasal polyps. Int Forum Allergy Rhinol. 2019;9(11):1334–1345. doi:10.1002/alr.22430
74. Cserháti E. The history of bronchial asthma from the Renaissance till the beginning of the twentieth century. Acta Physiol Hung. 2005;92(2):181–192. doi:10.1556/APhysiol.92.2005.2.9
75. Oren E, Gerald L, Stern DA, Martinez FD, Wright AL. Self-Reported Stressful Life Events During Adolescence and Subsequent Asthma: a Longitudinal Study. J Allergy Clin Immunol Pract. 2017;5(2):427–434.e2. doi:10.1016/j.jaip.2016.09.019
76. Sandberg S, Järvenpää S, Penttinen A, Paton JY, McCann DC. Asthma exacerbations in children immediately following stressful life events: a Cox’s hierarchical regression. Thorax. 2004;59(12):1046–1051. doi:10.1136/thx.2004.024604
77. Landeo-Gutierrez J, Forno E, Miller GE, Celedón JC. Exposure to Violence, Psychosocial Stress, and Asthma. Am J Respir Crit Care Med. 2020;201(8):917–922. doi:10.1164/rccm.201905-1073PP
78. Merrill A, Paracha A, Hemming E, et al. A structural model of high crime neighborhoods as a driver of toxic stress leading to asthma diagnoses among children of a large medical practice. Health Place. 2021;71:102665. doi:10.1016/j.healthplace.2021.102665
79. Gupta RS, Zhang X, Springston EE, et al. The association between community crime and childhood asthma prevalence in Chicago. Ann Allergy Asthma Immunol. 2010;104(4):299–306. doi:10.1016/j.anai.2009.11.047
80. Smyth JM, Soefer MH, Hurewitz A, Kliment A, Stone AA. Daily psychosocial factors predict levels and diurnal cycles of asthma symptomatology and peak flow. J Behav Med. 1999;22(2):179–193. doi:10.1023/a:1018787500151
81. Ciprandi G, Schiavetti I, Rindone E, Ricciardolo FLM. The impact of anxiety and depression on outpatients with asthma. Ann Allergy Asthma Immunol. 2015;115(5):408–414. doi:10.1016/j.anai.2015.08.007
82. Chen E, Hanson MD, Paterson LQ, Griffin MJ, Walker HA, Miller GE. Socioeconomic status and inflammatory processes in childhood asthma: the role of psychological stress. J Allergy Clin Immunol. 2006;117(5):1014–1020. doi:10.1016/j.jaci.2006.01.036
83. Liu LY, Coe CL, Swenson CA, Kelly EA, Kita H, Busse WW. School examinations enhance airway inflammation to antigen challenge. Am J Respir Crit Care Med. 2002;165(8):1062–1067. doi:10.1164/ajrccm.165.8.2109065
84. Heffner KL, Kiecolt-Glaser JK, Glaser R, Malarkey WB, Marshall GD. Stress and anxiety effects on positive skin test responses in young adults with allergic rhinitis. Ann Allergy Asthma Immunol. 2014;113(1):13–18. doi:10.1016/j.anai.2014.03.008
85. Lu Y, Ho R, Lim TK, et al. Neuropeptide Y may mediate psychological stress and enhance TH2 inflammatory response in asthma. J Allergy Clin Immunol. 2015;135(4):1061–1063.e4. doi:10.1016/j.jaci.2014.10.036
86. Kawano T, Ouchi R, Ishigaki T, et al. Increased Susceptibility to Allergic Asthma with the Impairment of Respiratory Tolerance Caused by Psychological Stress. Int Arch Allergy Immunol. 2018;177(1):1–15. doi:10.1159/000488289
87. Janssens T, Steele AM, Rosenfield D, Ritz T. Airway reactivity in response to repeated emotional film clip presentation in asthma. Biol Psychol. 2017;123:1–7. doi:10.1016/j.biopsycho.2016.11.006
88. Brehm JM, Ramratnam SK, Tse SM, et al. Stress and Bronchodilator Response in Children with Asthma. Am J Respir Crit Care Med. 2015;192(1):47–56. doi:10.1164/rccm.201501-0037OC
89. Chen W, Boutaoui N, Brehm JM, et al. ADCYAP1R1 and asthma in Puerto Rican children. Am J Respir Crit Care Med. 2013;187(6):584–588. doi:10.1164/rccm.201210-1789OC
90. Yan Q, Forno E, Cardenas A, et al. Exposure to violence, chronic stress, nasal DNA methylation, and atopic asthma in children. Pediatr Pulmonol. 2021;56(7):1896–1905. doi:10.1002/ppul.25372
91. Miller GE, Chen E. Life stress and diminished expression of genes encoding glucocorticoid receptor and beta2-adrenergic receptor in children with asthma. Proc Natl Acad Sci U S A. 2006;103(14):5496–5501. doi:10.1073/pnas.0506312103
92. Bailey MT, Kierstein S, Sharma S, et al. Social stress enhances allergen-induced airway inflammation in mice and inhibits corticosteroid responsiveness of cytokine production. J Immunol. 2009;182(12):7888–7896. doi:10.4049/jimmunol.0800891
93. Forsythe P, Ebeling C, Gordon JR, Befus AD, Vliagoftis H. Opposing effects of short- and long-term stress on airway inflammation. Am J Respir Crit Care Med. 2004;169(2):220–226. doi:10.1164/rccm.200307-979OC
94. Costa-Pinto FA, Basso AS, Russo M. Role of mast cell degranulation in the neural correlates of the immediate allergic reaction in a murine model of asthma. Brain Behav Immun. 2007;21(6):783–790. doi:10.1016/j.bbi.2007.01.002
95. Germundson DL, Nagamoto-Combs K. Potential Role of Intracranial Mast Cells in Neuroinflammation and Neuropathology Associated with Food Allergy. Cells. 2022;11(4):738. doi:10.3390/cells11040738
96. Moriyama S, Brestoff JR, Flamar AL, et al. β(2)-adrenergic receptor-mediated negative regulation of group 2 innate lymphoid cell responses. Science. 2018;359(6379):1056–1061. doi:10.1126/science.aan4829
97. Galle-Treger L, Suzuki Y, Patel N, et al. Nicotinic acetylcholine receptor agonist attenuates ILC2-dependent airway hyperreactivity. Nat Commun. 2016;7:13202. doi:10.1038/ncomms13202
98. Tomaki M, Ichinose M, Miura M, et al. Elevated substance P content in induced sputum from patients with asthma and patients with chronic bronchitis. Am J Respir Crit Care Med. 1995;151(3 Pt 1):613–617. doi:10.1164/ajrccm.151.3.7533601
99. Kay AB, Ali FR, Heaney LG, et al. Airway expression of calcitonin gene-related peptide in T-cell peptide-induced late asthmatic reactions in atopics. Allergy. 2007;62(5):495–503. doi:10.1111/j.1398-9995.2007.01342.x
100. Talbot S, Abdulnour REE, Burkett PR, et al. Silencing Nociceptor Neurons Reduces Allergic Airway Inflammation. Neuron. 2015;87(2):341–354. doi:10.1016/j.neuron.2015.06.007
101. Caceres AI, Brackmann M, Elia MD, et al. A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma. Proc Natl Acad Sci U S A. 2009;106(22):9099–9104. doi:10.1073/pnas.0900591106
102. Garcia J, Lasiter PS, Bermudez-Rattoni F, Deems DA. A general theory of aversion learning. Ann N Y Acad Sci. 1985;443:8–21. doi:10.1111/j.1749-6632.1985.tb27060.x
103. Parker LA. The role of nausea in taste avoidance learning in rats and shrews. Auton Neurosci. 2006;125(1–2):34–41. doi:10.1016/j.autneu.2006.01.010
104. Pinto A, Yanai M, Sekizawa K, Aikawa T, Sasaki H. Conditioned enhancement of cough response in awake Guinea pigs. Int Arch Allergy Immunol. 1995;108(1):95–98. doi:10.1159/000237124
105. Schaller M, Murray DR, Bangerter A. Implications of the behavioural immune system for social behaviour and human health in the modern world. Philos Trans R Soc Lond B Biol Sci. 2015;370:1669. doi:10.1098/rstb.2014.0105
106. Denholm L, Gallagher G. Physiology and pharmacology of nausea and vomiting. Anaesthesia Intensive Care Med. 2018;19(9):513–516. doi:10.1016/J.MPAIC.2018.06.010
107. Berin MC. Immunopathophysiology of food protein–induced enterocolitis syndrome. J Allergy Clin Immunol. 2015;135(5):1108–1113. doi:10.1016/J.JACI.2014.12.1948
108. Holbrook T, Keet CA, Frischmeyer-Guerrerio PA, Wood RA. Use of ondansetron for food protein-induced enterocolitis syndrome. J Allergy Clin Immunol. 2013;132(5):1219–1220. doi:10.1016/j.jaci.2013.06.021
109. Miceli Sopo S, Battista A, Greco M, Monaco S. Ondansetron for food protein-induced enterocolitis syndrome. Int Arch Allergy Immunol. 2014;164(2):137–139. doi:10.1159/000363384
110. Palermo-Neto J, Guimarães RK. Pavlovian conditioning of lung anaphylactic response in rats. Life Sci. 2000;68(6):611–623. doi:10.1016/s0024-3205(00)00966-8
111. Germundson DL, Nookala S, Smith NA, Warda Y. HLA-II Alleles Influence Physical and Behavioral Responses to a Whey Allergen in a Transgenic Mouse Model of Cow’s Milk Allergy. Front Allergy. 2022;3:870513. doi:10.3389/falgy.2022.870513
112. Schemann M, Michel K, Ceregrzyn M, Zeller F, Seidl S, Bischoff SC. Human mast cell mediator cocktail excites neurons in human and Guinea-pig enteric nervous system. Neurogastroenterol Motil. 2005;17(2):281–289. doi:10.1111/j.1365-2982.2004.00591.x
113. Frieling T, Cooke HJ, Wood JD. Neuroimmune communication in the submucous plexus of Guinea pig colon after sensitization to milk antigen. Am J Physiol. 1994;267(6 Pt 1):G1087–93. doi:10.1152/ajpgi.1994.267.6.G1087
114. Bell A, Althaus M, Diener M. Communication between mast cells and rat submucosal neurons. Pflugers Arch. 2015;467(8):1809–1823. doi:10.1007/s00424-014-1609-9
115. Tamura K, Wood JD. Effects of prolonged exposure to histamine on Guinea pig intestinal neurons. Dig Dis Sci. 1992;37(7):1084–1088. doi:10.1007/BF01300291
116. Tamura K, Palmer JM, Wood JD. Presynaptic inhibition produced by histamine at nicotinic synapses in enteric ganglia. Neuroscience. 1988;25(1):171–179. doi:10.1016/0306-4522(88)90016-4
117. Liu S, Hu HZ, Gao N, et al. Neuroimmune interactions in Guinea pig stomach and small intestine. Am J Physiol Gastrointest Liver Physiol. 2003;284(1):G154–64. doi:10.1152/ajpgi.00241.2002
118. Wang GD, Wang XY, Liu S, et al. Innervation of enteric mast cells by primary spinal afferents in Guinea pig and human small intestine. Am J Physiol Gastrointest Liver Physiol. 2014;307(7):G719–31. doi:10.1152/ajpgi.00125.2014
119. Gao C, Liu S, Hu HZ, et al. Serine proteases excite myenteric neurons through protease-activated receptors in Guinea pig small intestine. Gastroenterology. 2002;123(5):1554–1564. doi:10.1053/gast.2002.36581
120. Reed DE, Barajas-Lopez C, Cottrell G, et al. Mast cell tryptase and proteinase-activated receptor 2 induce hyperexcitability of Guinea-pig submucosal neurons. J Physiol. 2003;547(Pt 2):531–542. doi:10.1113/jphysiol.2002.032011
121. Leng Y, Yamamoto T, Kadowaki M. Alteration of cholinergic, purinergic and sensory neurotransmission in the mouse colon of food allergy model. Neurosci Lett. 2008;445(3):195–198. doi:10.1016/j.neulet.2008.09.014
122. Van Geldre LA, Lefebvre RA. Interaction of NO and VIP in gastrointestinal smooth muscle relaxation. Curr Pharm Des. 2004;10(20):2483–2497. doi:10.2174/1381612043383890
123. Lai NY, Mills K, Chiu IM. Sensory neuron regulation of gastrointestinal inflammation and bacterial host defence. J Intern Med. 2017;282(1):5–23. doi:10.1111/joim.12591
124. Lothe L, Ivarsson SA, Lindberg T. Motilin, vasoactive intestinal peptide and gastrin in infantile colic. Acta Paediatr Scand. 1987;76(2):316–320. doi:10.1111/j.1651-2227.1987.tb10467.x
125. Lee J, Yamamoto T, Hayashi S, Kuramoto H, Kadowaki M. Enhancement of CGRP sensory afferent innervation in the gut during the development of food allergy in an experimental murine model. Biochem Biophys Res Commun. 2013;430(3):895–900. doi:10.1016/j.bbrc.2012.12.058
126. Leung DYM, Berdyshev E, Goleva E. Cutaneous barrier dysfunction in allergic diseases. J Allergy Clin Immunol. 2020;145(6):1485–1497. doi:10.1016/j.jaci.2020.02.021
127. Yosipovitch G, Berger T, Fassett MS. Neuroimmune interactions in chronic itch of atopic dermatitis. J Eur Acad Dermatol Venereol. 2020;34(2):239–250. doi:10.1111/jdv.15973
128. Cevikbas F, Wang X, Akiyama T, et al. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: involvement of TRPV1 and TRPA1. J Allergy Clin Immunol. 2014;133(2):448–460. doi:10.1016/j.jaci.2013.10.048
129. Feld M, Garcia R, Buddenkotte J, et al. The pruritus- and TH2-associated cytokine IL-31 promotes growth of sensory nerves. J Allergy Clin Immunol. 2016;138(2):500–508.e24. doi:10.1016/j.jaci.2016.02.020
130. Guseva D, Rüdrich U, Kotnik N, et al. Neuronal branching of sensory neurons is associated with BDNF-positive eosinophils in atopic dermatitis. Clin Exp Allergy. 2020;50(5):577–584. doi:10.1111/cea.13560
131. Campion M, Smith L, Gatault S, Métais C, Buddenkotte J, Steinhoff M. Interleukin-4 and interleukin-13 evoke scratching behaviour in mice. Exp Dermatol. 2019;28(12):1501–1504. doi:10.1111/exd.14034
132. Oetjen LK, Mack MR, Feng J, et al. Sensory Neurons Co-opt Classical Immune Signaling Pathways to Mediate Chronic Itch. Cell. 2017;171(1):217–228.e13. doi:10.1016/j.cell.2017.08.006
133. Braz JM, Dembo T, Charruyer A, Ghadially R, Fassett MS, Basbaum AI. Genetic priming of sensory neurons in mice that overexpress PAR2 enhances allergen responsiveness. Proc Natl Acad Sci U S A. 2021;118(8):548. doi:10.1073/pnas.2021386118
134. Liu B, Tai Y, Achanta S, et al. IL-33/ST2 signaling excites sensory neurons and mediates itch response in a mouse model of poison ivy contact allergy. Proc Natl Acad Sci U S A. 2016;113(47):E7572–E7579. doi:10.1073/pnas.1606608113
135. Wang F, Trier AM, Li F, et al. A basophil-neuronal axis promotes itch. Cell. 2021;184(2):422–440.e17. doi:10.1016/j.cell.2020.12.033
136. Meng J, Wang J, Steinhoff M, Dolly JO. TNFα induces co-trafficking of TRPV1/TRPA1 in VAMP1-containing vesicles to the plasmalemma via Munc18-1/syntaxin1/SNAP-25 mediated fusion. Sci Rep. 2016;6:21226. doi:10.1038/srep21226
137. Mishra SK, Wheeler JJ, Pitake S, et al. Periostin Activation of Integrin Receptors on Sensory Neurons Induces Allergic Itch. Cell Rep. 2020;31(1):107472. doi:10.1016/j.celrep.2020.03.036
138. Guo CJ, Mack MR, Oetjen LK, et al. Kallikrein 7 Promotes Atopic Dermatitis-Associated Itch Independently of Skin Inflammation. J Invest Dermatol. 2020;140(6):1244–1252.e4. doi:10.1016/j.jid.2019.10.022
139. Barry DM, Munanairi A, Chen ZF. Spinal Mechanisms of Itch Transmission. Neurosci Bull. 2018;34(1):156–164. doi:10.1007/s12264-017-0125-2
140. Ding W, Stohl LL, Wagner JA, Granstein RD. Calcitonin gene-related peptide biases Langerhans cells toward Th2-type immunity. J Immunol. 2008;181(9):6020–6026. doi:10.4049/jimmunol.181.9.6020
141. Serhan N, Basso L, Sibilano R, et al. House dust mites activate nociceptor-mast cell clusters to drive type 2 skin inflammation. Nat Immunol. 2019;20(11):1435–1443. doi:10.1038/s41590-019-0493-z
142. Meixiong J, Anderson M, Limjunyawong N, et al. Activation of Mast-Cell-Expressed Mas-Related G-Protein-Coupled Receptors Drives Non-histaminergic Itch. Immunity. 2019;50(5):1163–1171.e5. doi:10.1016/j.immuni.2019.03.013
143. Thapaliya M, Chompunud Na Ayudhya C, Amponnawarat A, Roy S, Ali H. Mast Cell-Specific MRGPRX2: a Key Modulator of Neuro-Immune Interaction in Allergic Diseases. Curr Allergy Asthma Rep. 2021;21(1):3. doi:10.1007/s11882-020-00979-5
144. Pavlov VA, Chavan SS, Tracey KJ. Molecular and Functional Neuroscience in Immunity. Annu Rev Immunol. 2018;36:783–812. doi:10.1146/annurev-immunol-042617-053158
145. Qu L, Fu K, Yang J, Shimada SG, LaMotte RH. CXCR3 chemokine receptor signaling mediates itch in experimental allergic contact dermatitis. Pain. 2015;156(9):1737–1746. doi:10.1097/j.pain.0000000000000208
146. Walsh CM, Hill RZ, Schwendinger-Schreck J, et al. Neutrophils promote CXCR3-dependent itch in the development of atopic dermatitis. eLife. 2019:8. doi:10.7554/eLife.48448
147. Yosipovitch G, Goon ATJ, Wee J, Chan YH, Zucker I, Goh CL. Itch characteristics in Chinese patients with atopic dermatitis using a new questionnaire for the assessment of pruritus. Int J Dermatol. 2002;41(4):212–216. doi:10.1046/j.1365-4362.2002.01460.x
148. Chrostowska-Plak D, Reich A, Szepietowski JC. Relationship between itch and psychological status of patients with atopic dermatitis. J Eur Acad Dermatol Venereol. 2013;27(2):e239–42. doi:10.1111/j.1468-3083.2012.04578.x
149. Theoharides TC. Effect of Stress on Neuroimmune Processes. Clin Ther. 2020;42(6):1007–1014. doi:10.1016/j.clinthera.2020.05.002
150. Peters EMJ, Liezmann C, Klapp BF, Kruse J. The neuroimmune connection interferes with tissue regeneration and chronic inflammatory disease in the skin. Ann N Y Acad Sci. 2012;1262:118–126. doi:10.1111/j.1749-6632.2012.06647.x
151. Jackson-Cowan L, Cole EF, Silverberg JI, Lawley LP. Childhood atopic dermatitis is associated with cognitive dysfunction: a National Health Interview Survey study from 2008 to 2018. Ann Allergy Asthma Immunol. 2021;126(6):661–665. doi:10.1016/j.anai.2020.11.008
152. Billeci L, Tonacci A, Tartarisco G, Ruta L, Pioggia G, Gangemi S. Association Between Atopic Dermatitis and Autism Spectrum Disorders: a Systematic Review. Am J Clin Dermatol. 2015;16(5):371–388. doi:10.1007/s40257-015-0145-5
153. Konstantinou GN, Konstantinou GN. Psychological Stress and Chronic Urticaria: a Neuro-immuno-cutaneous Crosstalk. A Systematic Review of the Existing Evidence. Clin Ther. 2020;42(5):771–782. doi:10.1016/j.clinthera.2020.03.010
154. Konstantinou GN, Konstantinou GN. Psychiatric comorbidity in chronic urticaria patients: a systematic review and meta-analysis. Clin Transl Allergy. 2019;9:42. doi:10.1186/s13601-019-0278-3
155. Vena GA, Cassano N, Di Leo E, Calogiuri GF, Nettis E. Focus on the role of substance P in chronic urticaria. Clin Mol Allergy. 2018;16:24. doi:10.1186/s12948-018-0101-z
156. Ruppenstein A, Limberg MM, Loser K, Kremer AE, Homey B, Raap U. Involvement of Neuro-Immune Interactions in Pruritus With Special Focus on Receptor Expressions. Front Med. 2021;8:139. doi:10.3389/fmed.2021.627985
157. Theoharides TC, Kalogeromitros D. The critical role of mast cells in allergy and inflammation. Ann N Y Acad Sci. 2006;1088:78–99. doi:10.1196/annals.1366.025
158. Dvorak AM. Ultrastructural studies of human basophils and mast cells. J Histochem Cytochem. 2005;53(9):1043–1070. doi:10.1369/jhc.5R6647.2005
159. Conti P, Pregliasco FE, Bellomo RG, et al. Mast Cell Cytokines IL-1, IL-33, and IL-36 Mediate Skin Inflammation in Psoriasis: a Novel Therapeutic Approach with the Anti-Inflammatory Cytokines IL-37, IL-38, and IL-1Ra. Int J Mol Sci. 2021;22:15. doi:10.3390/ijms22158076
160. Kulka M, Sheen CH, Tancowny BP, Grammer LC, Schleimer RP. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology. 2008;123(3):398–410. doi:10.1111/j.1365-2567.2007.02705.x
161. Bulut K, Felderbauer P, Deters S, et al. Sensory neuropeptides and epithelial cell restitution: the relevance of SP- and CGRP-stimulated mast cells. Int J Colorectal Dis. 2008;23(5):535–541. doi:10.1007/s00384-008-0447-7
162. Zheng W, Wang J, Zhu W, Xu C, He S. Upregulated expression of substance P in basophils of the patients with chronic spontaneous urticaria: induction of histamine release and basophil accumulation by substance P. Cell Biol Toxicol. 2016;32(3):217–228. doi:10.1007/s10565-016-9330-4
163. Arck P, Paus R. From the brain-skin connection: the neuroendocrine-immune misalliance of stress and itch. Neuroimmunomodulation. 2006;13(5–6):347–356. doi:10.1159/000104863
164. Kim JE, Cho BK, Cho DH, Park HJ. Expression of hypothalamic-pituitary-adrenal axis in common skin diseases: evidence of its association with stress-related disease activity. Acta Derm Venereol. 2013;93(4):387–393. doi:10.2340/00015555-1557
165. Rasool R, Ashiq I, Shera IA, Yousuf Q, Shah ZA. Study of serum interleukin (IL) 18 and IL-6 levels in relation with the clinical disease severity in chronic idiopathic urticaria patients of Kashmir (North India). Asia Pac Allergy. 2014;4(4):206–211. doi:10.5415/apallergy.2014.4.4.206
166. Puxeddu I, Italiani P, Giungato P, et al. Free IL-18 and IL-33 cytokines in chronic spontaneous urticaria. Cytokine. 2013;61(3):741–743. doi:10.1016/j.cyto.2013.01.015
167. Yang HY, Sun CC, Wu YC, Wang JD. Stress, insomnia, and chronic idiopathic urticaria–a case-control study. J Formosan Med Assoc. 2005;104(4):254–263.
168. Varghese R, Rajappa M, Chandrashekar L, et al. Association among stress, hypocortisolism, systemic inflammation, and disease severity in chronic urticaria. Ann Allergy Asthma Immunol. 2016;116(4):344–348.e1. doi:10.1016/j.anai.2016.01.016
169. Graubard R, Perez-Sanchez A, Katta R. Stress and Skin: an Overview of Mind Body Therapies as a Treatment Strategy in Dermatology. Dermatol Practical Conceptual. 2021;11(4):e2021091. doi:10.5826/dpc.1104a91
170. Papadopoulou N, Kalogeromitros D, Staurianeas NG, Tiblalexi D, Theoharides TC. Corticotropin-releasing hormone receptor-1 and histidine decarboxylase expression in chronic urticaria. J Invest Dermatol. 2005;125(5):952–955. doi:10.1111/j.0022-202X.2005.23913.x
171. Theoharides TC, Singh LK, Boucher W, et al. Corticotropin-releasing hormone induces skin mast cell degranulation and increased vascular permeability, a possible explanation for its proinflammatory effects. Endocrinology. 1998;139(1):403–413. doi:10.1210/endo.139.1.5660
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.