Back to Journals » Clinical Ophthalmology » Volume 18
Fungal Keratitis: Diagnosis, Management, and Recent Advances
Authors Awad R , Ghaith AA , Awad K, Mamdouh Saad M, Elmassry AA
Received 31 October 2023
Accepted for publication 9 December 2023
Published 10 January 2024 Volume 2024:18 Pages 85—106
DOI https://doi.org/10.2147/OPTH.S447138
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Ramy Awad,1 Alaa Atef Ghaith,2 Khaled Awad,1 Marina Mamdouh Saad,1 Ahmed Ak Elmassry2
1Department of Ophthalmology, Alexandria General Ophthalmology Hospital, Alexandria, Egypt; 2Department of Ophthalmology, Faculty of Medicine, Alexandria University, Alexandria, Egypt
Correspondence: Ahmed Ak Elmassry, Ophthalmology Department, Faculty of Medicine, Alexandria University, Champollion Street, Al Attarin, Alexandria, Egypt, Tel +2 0122 215 2435, Email [email protected]
Abstract: Fungal keratitis is one of the major causes of microbial keratitis that may lead to corneal blindness. Many problems related to diagnosis and therapy are encountered in fungal keratitis, including difficulty in obtaining laboratory diagnoses and the availability and efficacy of antifungal medications. Intensive and prolonged use of antifungal topical preparations may not be enough. The use of antifungal medications is considered the main treatment for fungal keratitis. It is recommended to start antifungal therapy after confirmation of the clinical diagnosis with a smear or positive cultures. Topical application of antifungal medications is a mainstay for the treatment of fungal keratitis; however, systemic, intra-stromal, or intra-cameral routes may be used. Therapeutic keratoplasty is the main surgical procedure approved for the management of fungal keratitis with good success rate. Intrastromal corneal injection of antifungal medications may result in steady-state drug levels within the corneal tissue and prevent intervals of decreased antifungal drug concentration below its therapeutic level. In cases of severe fungal keratitis with deep stromal infiltration not responding to treatment, intracameral injection of antifungal agents may be effective. Collagen cross-linking has been proposed to be beneficial for cases of fungal keratitis as a stand-alone therapy or as an adjunct to antifungal medications. Although collagen cross-linking has been extensively studied in the past few years, its protocol still needs many modifications to optimize UV fluence levels, irradiation time, and concentration of riboflavin to achieve 100% microbial killing.
Keywords: fungal keratitis, keratomycosis, PACK-CXL, antifungal, voriconazole, intracorneal injection, targeted therapy
Introduction
Infectious corneal ulcers, or what we call microbial keratitis, are considered the leading cause of vision loss, especially in developing countries. It may be caused by a wide range of microorganisms (bacteria, viruses, fungi, or parasites). The proliferation of microorganisms within corneal tissues and the associated inflammatory response results in corneal tissue destruction, loss of transparency, and vision diminution.1,2
Fungi are considered opportunistic pathogens and can rarely invade an intact cornea; however, in a state of immunosuppression, after trauma; particularly when caused by vegetable matter, in cases of topical steroid use or ocular surface disease, they become pathogenic.3
Fungal keratitis is also known as mycotic keratitis (MK) and is one of the major causes of microbial keratitis that may lead to corneal blindness. It is more common in tropical and subtropical countries.4 It is estimated that more than a million cases of fungal keratitis are diagnosed every year. The risk of perforation following infection is about 10%. More than half of the patients with fungal keratitis will lose their vision and live with monocular blindness.5
Many problems related to diagnosis and therapy are encountered in fungal keratitis, including difficulty in obtaining laboratory diagnosis of fungal organisms and the availability and efficacy of antifungal medications. Intensive and prolonged use of antifungal topical preparations may not be enough, and other methods of drug delivery, such as systemic, intrastromal, and intracameral routes, should be considered. The development of new treatment modalities is also essential to achieve more rapid results and overcome drug resistance.6
Etiological Organisms
Fungi that can lead to corneal infection are classified broadly into filamentous fungi or molds (eg, Aspergillus, Fusarium, Paecilomyces, Curvularia, and other phaeohyphomycetes), yeast, yeast-like fungi (eg, Candida, Cryptococcus, and Geotrichum), or dimorphic fungi (eg, Coccidioides and Histoplasma).3,7
Epidemiology
Being the second most common cause of infectious keratitis after bacterial infection, fungal infection of the cornea is reported to be more serious and destructive. The course of infection in fungal keratitis is long-standing, with a high risk for complications and perforation. Thus, therapeutic keratoplasty is frequently needed in such cases.8
Geographic location highly affects the incidence of fungal keratitis. Even in the same county, incidence varies between different districts concerning humidity and other risk factors. The incidence of fungal keratitis varies in most reports between 17% and 36% of cases with corneal infection. In the United States, fungal keratitis is reported to account for 5–20% of corneal infections. The incidence is much higher in developing countries, and it was reported to be more than 50%.9–13 A recent study from the Egyptian delta reported fungal infections of the cornea to be as high as two-thirds of all microbial keratitis, of which mixed infection was culture-proven in 20%.14
Fungal or mycotic keratitis may affect any age but is found more in middle-aged individuals. It is more common in males than in females. It is reported to be more endemic during winter due to high humidity. Filamentous fungi are more common than yeast and yeast-like fungi. Aspergillus and Fusarium are the most frequently observed filamentous fungi, while Candida is the most common yeast.3
Clinical Features
History-taking is an important step in the evaluation of a case of suspected fungal keratitis. It is important to ask about the onset and course of the disease. Mycotic keratitis usually has an insidious onset and a gradual course. Asking the patient about a history of trauma, contact lens use, systemic diseases, and medications is essential.3
Symptoms of mycotic keratitis include blurring of vision, irritation, redness, photophobia, watering, and discharge. Pain is usually less than that of bacterial or Acanthamoeba keratitis and does not correlate with clinical signs.15
Clinical signs (Figure 1) include hyperemia in the form of circumcorneal ciliary flush, corneal infiltration, edema, loss of lusterness and ulcer borders are usually feathery. Ulcers are usually associated with grey or yellow stromal infiltration that extends beyond the edges of the ulcer. A grey ring of infiltration may be seen in the cornea as fluffy elevated lesions with dirty yellow or brown pigmentation. Dense infiltration at the level of endothelium may be presented by endothelial plaques. Satellites in the form of multifocal micro abscesses are seen in more than 10% of cases. The anterior chamber reaction in the form of a thick hypopyon is seen in more than half of the cases. Variation in clinical appearance is usually related to causative fungal species.10,16
It was found that clinical signs including serrated or feathery ulcer margins, raised borders, presence of satellite lesions, and color of infiltrates can be helpful in clinical differentiation between fungal and bacterial keratitis (Figure 2). 17
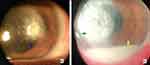 |
Figure 2 Clinical signs differentiating fungal corneal ulcer (a) from bacterial (b). The signs are infiltration (Green arrow), feathery borders (blue arrow), and hypopyon (Yellow arrow). |
In candida and other yeast-induced keratitis, the ulcer configuration shows a “collar button” appearance. The ulcer is usually small with expanding infiltration of the stroma. It resembles bacterial keratitis but has a slow progressive course, a more discrete stromal infiltration, and shows no response to topical antibiotics.6
Laboratory Diagnosis
Clinical signs of fungal keratitis are valuable in giving an initial diagnosis, but confirmation of the diagnosis and identification of the causative organism requires other diagnostic tests, including microbiological laboratory tests. The possibility of a combined infection should also be suspected. Microbiological tests must take place at presentation and before the initiation of medications.18
The first step for laboratory tests is corneal scraping to obtain tissues for examination and culture. Corneal scraping also assists in debulking of the fungi and debridement of the surface, which helps in medication penetration. It is performed using a spatula or surgical blade or, less preferably, using swabs or sponge-like materials. Scraping of the ulcer base along its edge is advisable. Anterior chamber tap may be needed if there is an endothelial plaque or deep keratitis. A corneal biopsy may also be performed. Contact lenses, their solution, topical medications used, and eye cosmetics should also be sent for culture if indicated.18,19 New laboratory diagnostic techniques were evaluated for their diagnostic performance for fungal keratitis. One of these techniques is the use of a lateral-flow device in the diagnosis of Aspergillus in corneal scrapes from patients with suspected fungal keratitis. It was found to have a high diagnostic accuracy in identifying Aspergillus species from corneal scrapes and swabs.20
Direct Microscope
Examination of corneal smears using direct microscopy is an important initial step in laboratory diagnosis. We may use stains such as Gram stain and Giemsa stain or potassium hydroxide (KOH) wet preparation. Recently, one report described the use of trypan blue stain in the diagnosis of fungal ulcers.21
KOH Wet Mount Preparation
KOH at a concentration of 10–20% can be used to identify fungal growth with a direct microscope (Figure 3). KOH’s role is to dissolve human tissues leaving alkali-resistant structures so that it allows visualization of fungi. It is a cheap, simple method of diagnosis. The sensitivity of that simple test ranges from 72% to 91%.22
 |
Figure 3 Microscopic examination of KOH wet mount corneal scrapping (X10) showing fungal filaments. |
Gram and Giemsa Stains
In clinical specimens, Gram and Giemsa stains are the most commonly used methods for the detection of microorganisms. Gram stain can be used to detect fungal hyphae and stain yeast. With Giemsa stain, many fungi, especially yeast and dimorphic types, stain blue. The sensitivity of those stains ranges from 27% to 90%.3,21,22
Trypan Blue Stain
Trypan blue dye is used in many ophthalmic procedures, such as staining of the anterior lens capsule to ease the capsulorhexis step in cataract surgery. It may also be used to stain the Descemet membrane in endothelial keratoplasty and in epiretinal membrane staining during retinal procedures. Trypan blue dye was recently identified to help in the staining of fungal filaments so that it helps in better visualization and improves diagnosis and photography for documentation. It is usually used in combination with KOH and applied after dryness of the solution on a glass slide.21
Other Stains Used
Lactophenol cotton blue, Grocott’s methenamine-silver stain, and Calcofluor white can also be helpful in the diagnosis of fungal keratitis with a sensitivity of 70% to 90%.23
Fungal Culture
For the diagnosis of fungal keratitis, the isolation of fungal elements by culture is considered the most sensitive method. Culture is important not only to identify the causative organism and detect mixed infections but also to test the organism’s in vitro susceptibility to antimicrobial agents. Fungal infection cannot be detected in a single specific culture medium, so two types of media: selective and nonselective should be used.18
In patients with suspected fungal keratitis, culture media used should include non-selective media originally used in the workup of general microbial keratitis, eg, blood agar. On the other hand, selective media such as Sabouraud dextrose agar (SDA) should be used (Figure 4). Gentamycin 50 µg/mL should be added to SDA. Cycloheximide should be excluded from SDA as it suppresses saprophytic fungal growth.24
 |
Figure 4 Culture positive for fusarium Solani on blood agar (a) and SDA (b). |
Fungal Keratitis Definitive Diagnosis is Achieved If
- Fungal elements are seen in corneal smears.
- Single medium showing fungal growth in the presence of fungus in smears.
- Growth of fungus in two or more media in the absence of fungus-positive smears.
- Fungal growth in a single solid medium at the site of inoculation in confluent form.3
Fungal growth in positive cultures occurs in more than 80% after 3 days and in more than 95% after 7 days. Microbiologists should wait for at least 7–14 days before reporting culture-negative fungi. Brain heart infusion may be useful as a transport medium but is not routinely used. Both yeast and filamentous fungi can grow readily on blood agar and SDA at room temperature. The use of plastic bags to place the agar inside is usually useful to increase humidity and enhance fungal growth. Fungal growth can be seen by the naked eye or using a dissecting microscope.25,26
Keratectomy and Biopsy
Corneal biopsy with diagnostic Keratectomy is more diagnostic than corneal scraping for fungal keratitis. It may be considered if corneal smears and cultures are negative. Partial-thickness trephination is performed using a 2–3 mm sterile disposable trephine to excise part of a clear cornea along with the infected cornea. Thereafter, to complete a partial-thickness keratectomy, the base is dissected and separated using a surgical blade. It is essential to avoid the visual axis. The procedure is usually performed under topical anaesthesia under an operating microscope or a slit lamp. The removed tissue is used for microbiological and histopathological examination. An excised corneal button during therapeutic keratoplasty may also be used for corneal biopsy. On histopathological examination, Hematoxylin and Eosin (H&E) and Periodic Acid-Schiff (PAS) are the most commonly used stains to detect fungal hyphae and inflammatory signs.27
Confocal Microscopy
In vivo confocal microscopy (IVCM) is an imaging technique that provides corneal tissue real-time imaging at the cornea’s microstructural level. It provides high-resolution optical sectioning of stromal fibers, cells, and any material within corneal tissues. Therefore, it can detect hyphae of filamentous fungi as well as yeasts. It may be helpful in the identification of organisms even in the early stages of the disease. It is considered a noninvasive technique to diagnose fungal corneal infection and detect hyphal density in vivo. There are three types of confocal microscopes available: tandem scanning, slit scanning, and laser scanning. Limitations of using a confocal microscope are that it cannot differentiate different types of filamentous fungi, difficulty in imaging the same area again (low reproducibility), and limited resolution for smaller organisms in mixed infections.28–31
Polymerase Chain Reaction
Polymerase chain reaction (PCR) is considered a rapid and sensitive technique in the diagnosis of mycotic keratitis. Positive results may be reported after a few hours in contrast to 2–14 days that may be taken by culture. Most publications reported high sensitivity, more than 90%. Limitations of PCR use are its cost, inability to perform drug sensitivity tests, and difficulty in monitoring drug efficacy in addition to the possibility of false-positive results due to contamination. Therefore, culture and sensitivity remain the most specific diagnostic tools.15,32
Artificial Intelligence and Its Role in Fungal Keratitis
Artificial intelligence (AI) is a promising technology that may play a great role in the diagnosis, follow-up, and management of all types of infectious keratitis. New AI algorithms were developed to diagnose fungal keratitis and to differentiate it from other types of infectious keratitis. It can also categorize fungal species based on slit-lamp photographs, confocal microscopy images, and clinical data.33 Saini et al34 developed a model based on AI to classify infectious keratitis into bacterial or fungal and compared it with experienced human observers. They reported 100% specificity for diagnosis of fungal and 77% specificity for bacterial infections. AI can also be used in follow-up of fungal keratitis cases: monitoring the progression and the response to treatment. Therefore, it may be helpful in the future to personalize the treatment for each patient.35
Misdiagnosis of Fungal Keratitis
Misdiagnosis is mainly due to some ophthalmologists’ lack of experience with differentiating clinical signs between different types of microbial keratitis. Other causes for misdiagnosis are deficiency of diagnostic equipment at many hospitals and primary care units and a lack of qualified personnel for that equipment. Corneal scraping for culture is not performed routinely, and noninvasive confocal microscopes are not available. As such, most of the cases with hypopyon ulcers are diagnosed primarily as bacterial infections and treated empirically with broad-spectrum antibiotics. Therefore, referral to a higher-level hospital is considered late after the failure of treatment and the progression of the ulcer with more complications.18 Dahlgren et al36 found that fungal keratitis was the most challenging to diagnose, with a sensitivity and specificity of 38% and 45%, respectively. Even cornea specialists cannot depend on clinical diagnosis alone. Dalmon et al37 reported that cornea specialists can differentiate between fungal and bacterial keratitis on a clinical basis in approximately two-thirds of cases.
Pythium keratitis is one of the most challenging infections that may be misdiagnosed as fungal keratitis. Most of the cases with Pythium keratitis were diagnosed as suspected fungi or unidentified fungi. Pythium keratitis resembles fungal keratitis in its clinical features and in its microscopic appearance. It can be diagnosed after culture using a difficult technique that is not routinely used.38
Management
The management of corneal fungal infections is primarily medical. Surgical intervention may be needed in progressive resistant cases or for the management of complications. Sharma et al39 proposed a topical, systemic, and targeted therapy (TST) protocol for management of fungal keratitis with an overall success rate approaching 80%. Recently, corneal collagen cross-linking is a rising new surgical treatment for active cases. Treatment algorithm for different cases of fungal keratitis is shown in Figure 5.
 |
Figure 5 Algorithm for management of different cases of fungal keratitis. |
Medical Therapy
The use of antifungal medications is considered the main treatment for fungal keratitis. It is recommended to start antifungal therapy after confirmation of the clinical diagnosis with a smear or positive cultures. Topical application of antifungal medications is a mainstay for the treatment of fungal keratitis; however, systemic, intra-stromal, or intra-cameral routes may be used.40 Topical broad-spectrum antibiotics should be added to guard against superadded bacterial infection. Additionally, cycloplegic eye drops may be given to decrease pain and relieve associated iridocyclitis. In case of elevated intraocular pressure, anti-glaucoma medications should be added.40,41 Topical steroids should be avoided in fungal keratitis. It decreases the efficacy of antifungal medication when given together and if alone they worsen the condition.41,42
Topical Antifungal Agents
Natamycin 5% is considered the first-line treatment for fungal keratitis and the first approved antifungal medication. Initially, it should be given hourly till improvement is noticed by partial resolution of infiltration, then the frequency is reduced to two-hourly. After the resolution of the infection, natamycin should be continued for 2 weeks.30,43
Voriconazole is an azole drug that is derived from fluconazole. It is proven to be a broad-spectrum antifungal for both filamentous and yeast fungi. Topical voriconazole 1% is not commercially available but may be prepared in the pharmacy. The minimal inhibitory concentration of voriconazole is 0.5 μg/mL, which is less than that of other imidazole drugs. It is considered a good alternative to natamycin in resistant cases, to be used alone or in combination.15,41,44
Amphotericin B 0.15% may be prepared for topical use in cases not responding to natamycin 5%. It is also considered a first-line treatment in cases of fungal keratitis caused by Candida. In Fusarium infection, amphotericin B is not effective.28
Econazole 1% is found to be as effective as natamycin 5% for the treatment of fungal keratitis caused by filamentous fungi. Fluconazole 2% is fungistatic that is usually used in combination with other antifungal drugs such as amphotericin B in the treatment of fungal keratitis caused by Candida or Aspergillus. Clotrimazole 1% is also available as a topical medication for the treatment of fungal keratitis.45–47
Posaconazole is a newly developed synthetic triazole. It is an analog to itraconazole. It has been reported in many studies that Posaconazole has a broad-spectrum activity against most pathogenic fungal species. Therefore, it can be used in natamycin and voriconazole-resistant cases.48–50
Echinocandins is a recently developed group of antifungal drugs that lead to fungal cell lysis through inhibition of (1,3)-D-glucan synthesis. Caspofungin, micafungin, and anidulafungin are commercially available echinocandins that were FDA-approved for the management of invasive systemic fungal infections. Many reports have proven the clinical efficacy of echinocandins in the treatment of refractory cases of fungal keratitis.51,52
Mycotic ulcer treatment trial I (MUTT I) compared topical natamycin to topical voriconazole in the treatment of fungal keratitis and concluded that topical natamycin treatment has significantly better outcomes concerning visual acuity and is associated with a decreased risk of complications. The difference was found more in cases with Fusarium infection.53 Therefore, in non-Fusarium cases, voriconazole may have similar or better results especially in the absence of epithelial defect, as the voriconazole molecule is much smaller than natamycin with better penetration power.54
Several topical antifungal drugs may be used in combination as they act synergistically. Amphotericin B may be used in combination with flucytosine for Candida. Natamycin may also be used with ketoconazole in filamentous fungal keratitis.55,56
Medical treatment for fungal keratitis should last longer than that for bacterial infection as the response for topical antifungal medications is slow over a period of weeks. The length of treatment varies according to the clinical response and ranges between 4 to 6 weeks. Poor response to treatment is considered if the size of the ulcer, the depth of stromal infiltration, and the level of hypopyon remain the same or become worse. In such cases, the medical treatment should be modified and surgical treatment may be initiated.57
Problems related to medical therapy include the difficulty in obtaining sensitivity testing and the lack of correlation between in-vitro and in-vivo responses to antifungal medications. This may also explain the high rate of treatment failure.58
Prolonged use of antifungal topical medications may be associated with toxic effects on the ocular surface. The signs of toxicity may mimic persistent inflammatory signs of fungal infection. In case of suspected toxicity especially after 6 weeks of treatment, medications should be stopped along with careful follow-up of the patient to exclude persistent active infection. Clinical improvement can be proved by decreased cellular infiltration, resolution of satellite lesions, and decrease in pain experienced by the patient.43,59
Systemic Antifungal Agents
Oral or parenteral antifungal medications may be indicated if large corneal ulcers are reaching the limbus, severe deep keratitis or if the ulcer is associated with scleritis or endophthalmitis. Systemic antifungal medication is also indicated in cases of penetrating keratoplasty for fungal keratitis as a prophylactic treatment.60 Systemic antifungal therapy must be continued for 6–8 weeks.
Mycotic ulcer treatment trial II (MUTT II) studied the outcomes of adding oral voriconazole to topical treatment in patients with filamentous fungal ulcers. They concluded that adding oral voriconazole exposes the patient to systemic side effects and does not improve the final outcome.61 However, other studies have claimed beneficial effects of oral voriconazole alone or in combination with topical antifungal treatment.62,63
Systemic Anti-Fungal Agents Include
- Oral voriconazole 200 mg twice daily exhibits excellent ocular penetration and can reach minimal inhibitory concentration and provide consistent drug levels.15
- Oral ketoconazole 600 mg/day. Liver functions must be monitored every 2 weeks after its use.3
- Oral itraconazole 200 mg/day.
- Oral fluconazole 200 mg/day.
- Intravenous miconazole.
Surgical Therapy
The main surgical procedure accepted for the management of fungal keratitis is therapeutic keratoplasty. Nevertheless, new surgical techniques are emerging and gaining popularity in managing fungal keratitis, such as Rose Bengal Photodynamic therapy and Corneal Collagen Cross-Linking.18
Therapeutic Keratoplasty (TKP)
Fungal corneal ulcer progression can occur even with proper medical treatment, and this may lead to corneal perforation, and the spread of infection to the limbus, sclera, and uveal tract. Late diagnosis and improper treatment may lead to an increase in the rate of complications. In such cases, therapeutic keratoplasty may be essential to control infection, prevent more severe complications, and restore the globe’s anatomic integrity.64–66 Indications for therapeutic keratoplasty are:
- Progression of corneal ulcer despite proper medical treatment.
- Impending perforation or actual perforation >2 mm.
In therapeutic keratoplasty, the size of trephination should leave a 1–1.5 mm clinically uninvolved, clear zone of the cornea. The cornea left peripheral to trephination should not have any residual fungal infection to decrease recurrence risk. The sutures should be interrupted, with slightly longer bites to avoid cheese wiring of the edge of the recipient. Anterior chamber irrigation is performed to eliminate any remaining exudates or organisms. It is better not to touch the lens to prevent the spread of infection to the posterior segment. Antifungal agents should be injected intracameral at the end of surgery. If endophthalmitis is suspected, intravitreal injection of antifungal agents should also be performed.15,64
Recurrence of infection after TKP can complicate 6–16% of grafts. Risk factors for recurrence of fungal keratitis after TKP include presence of corneal perforation, hypopyon, lens infection, or fungal infection reaching the limbus. Patients who were given topical steroid for a long time before corneal transplantation are also at high risk of recurrence.67
Deep anterior lamellar keratoplasty (DALK) can be used instead of penetrating keratoplasty with a lower risk for allograft rejection. In DALK, the risk for the spread of infection to intraocular structures is decreased. It also reduces the need for postoperative steroid treatment. Studies have reported that DALK with the big bubble technique is a safe therapeutic approach for infective keratitis cases with a high success rate. This eliminates infection from a central optical zone of the cornea and provides better physiological graft survival outcomes with less risk for recurrence of infection (7.5%). However, if the infection is reaching the level of corneal endothelium or in cases with corneal perforation, DALK cannot be performed.68,69
The corneal button excised and any other tissues removed must be sent to microbiology and pathology laboratories. Culture and sensitivity test is important to detect causative organisms if they are not known before surgery and determine sensitive antimicrobials. In 75% of patients, a histopathologic examination can reveal fungal elements. Fungal hyphae can be seen within the corneal stroma in the histopathologic examination. Hyphae usually run parallel to the corneal surface, but perpendicular orientation is usually seen in patients on steroids or in severe cases with virulent organisms.70,71
Postoperative treatment should primarily include antifungal agents to prevent the recurrence of fungal infection. Systemic and topical antifungal drugs might be used and continued for at least 2 weeks if the histopathology reported that the corneal button excised edges are not involved by infection. The patient must be followed carefully for the possibility of recurrence. In cases in which histopathology reveals an infection of the edges of excised cornea or an infection of the graft is clinically detected, topical and systemic antifungals should be continued for at least 6–8 weeks.72,73
The main goal of therapeutic keratoplasty in mycotic keratitis is to get rid of the infection and eliminate the organisms, but avoiding graft rejection is a crucial second goal. After therapeutic keratoplasty, topical corticosteroids should be avoided except if the infection is well controlled clinically. Immunosuppressive drugs such as Cyclosporin A can replace corticosteroids, but their effects on fungal growth are not well studied.72
New Modalities in the Treatment of Fungal Keratitis
Intrastromal Therapy
Intrastromal corneal injection of antifungal medications eg voriconazole and amphotericin B may result in steady-state drug levels within the corneal tissue and prevent intervals of decreased antifungal dosing below its therapeutic level. However, this method of targeted drug delivery ensures antifungal penetration in cases presented with the involvement of deep layers of the corneal stroma. Many studies have evaluated the use of intrastromal voriconazole and/or amphotericin B for keratomycosis (Table 1).
 |
Table 1 Summary of Studies Reporting Intrastromal Corneal Injection of Antifungal Medications |
Most studies42,74–90 use either amphotericin B 3–5 µg in 0.1 mL or voriconazole 50 μg/0.1 mL. Aydin et al85 injected both drugs in combination (Voriconazole 0.05 mg/0.1 mL + amphotericin B 0.01 mg/0.1 mL) and reported a success rate was 87.5%. Saluja et al87 compared the intrastromal injection of voriconazole 50 ug/0.1 mL versus amphotericin B 5 μg/0.1 mL and natamycin 10 μg/0.1 mL and reported that the overall success rate was 93.3%. They found intrastromal voriconazole to be superior to other drugs. They also noticed similar visual outcomes in cases treated with intrastromal natamycin and amphotericin B but faster healing in the former. Most of the studies performed single, two, or three repeated injections. Aydin et al85 repeated intrastromal injections up to 18 times and reported a success rate of 87.5% with nearly no complications.
The procedure is performed in the operation room under complete aseptic measurements using topical, with or without peribulbar anesthesia. The reconstituted solution is loaded in a disposable 1 mL insulin syringe with a 30-gauge needle. Using an operating microscope, the needle is inserted bevel-down obliquely in a clear unaffected cornea to mid-stromal level. Up to 5 divided doses are injected to form a barrage of intrastromal drugs around the ulcer till corneal hydration is achieved.
Three randomized control trials77,82,87 studied the use of intrastromal corneal voriconazole 50 μg/0.1 mL in fungal keratitis. Solaiman et al77 reported an increased healing rate and a decrease in the period of infiltration resolution for deep or resistant fungal keratitis. However, Narayana et al82 found no benefit in adding intrastromal voriconazole as the primary treatment for cases of fungal keratitis caused by filamentous fungi. They described many complications as injections may increase the level of hypopyon or increase the risk of glaucoma and perforation. They also reported decreased times of healing and an increased degree of scarring. Intrastromal bleeding and postoperative pain were also reported.
Intracameral Therapy
In cases of severe fungal keratitis with deep stromal infiltration not responding to treatment, intracameral injection of antifungal agents may be effective. It provides a high drug delivery level into the anterior chamber. Injection should be done in an operating room under strictly aseptic conditions. Anterior chamber wash may also be performed to remove exudates and hypopyon, but care must be taken if the infection involves the anterior lens capsule to avoid capsular injury and cataract formation.
Many studies80,82,91–101 evaluated the use of intracameral antifungal drugs in deep keratomycosis (Table 2). Most of them reported a high success rate with few complications. They believe that this method of drug delivery can achieve a high concentration of antifungal medication in the deep corneal layers, reducing the infiltration and leading to the resolution of the endothelial plaque. Most of the studies used Amphotericin B in a dose of 5–10 µg in 0.1 mL, some used Voriconazole 50–100 µg in 0.1 mL. The injection can be repeated up to 13 times if the response was not adequate.91 The reported complications of intracameral antifungal drugs include a transient increase in hypopyon and intraocular pressure, postoperative pain, and intrastromal bleeding.82
 |
Table 2 Summary of Studies Reporting Intracameral Injection of Antifungal Medications |
A single randomized controlled trial by Sharma et al97 found no difference in time of healing and final visual acuity between cases of intracameral injection and cases treated with topical medications. They reported an increased incidence of cataract after intracameral injection.
Rose Bengal Photo-Dynamic Antimicrobial Therapy (PDAT)
Bascom Palmer Eye Institute initiated research on the antimicrobial efficacy of PDAT using rose bengal stain as photosensitizers in vitro. Bascom Palmer Ocular Biophysics Laboratory reported in vitro antimicrobial efficacy of rose Bengal PDAT against fungal organisms such as Fusarium Solani, Aspergillus fumigatus, Candida albicans, and other fungal organisms. Clinical efficacy was then established in patients with Fusarium Keratoplasticum keratitis that was resistant to many antifungal agents. The procedure was performed using rose bengal 0.1% then green light exposure with a total energy of 2.7 J/cm2. The patient was treated with two rose Bengal PDAT sessions. Successful treatment of infections, with no complications or recurrences, was reported to occur within 10 months. Rose Bengal PDAT safety is therefore established in vivo, and no resistance was reported.102–104
Photo-Activated Chromophore for Keratitis-Corneal Collagen Cross-Linking (PACK-CXL)
Riboflavin photoactivation with Ultraviolet “UV” light will lead to a release of reactive oxygen species. Therefore, it will promote the formation of chemical covalent bonds between collagen fibers. Effects of CXL on cornel tissues include biomechanical stiffening, increased collagen fiber diameter, increased resistance of corneal fibers against enzymatic degradation, higher corneal shrinking temperature, and decreased corneal swelling, along with many others that were determined over the course of multiple studies.105–109 The studies’ results reported an equivalent effect, on the cornea’s biomechanical stability, with higher intensity CXL (30 mW/cm2 for 3 min) to standard treatment. However, for a power range of 45–90 mW/cm2, there was no statistically significant difference in the cornea’s biomechanical stability from corresponding lower-intensity treatment.110,111
The first clinical use of CXL for the treatment of patients with infective keratitis was described by Iseli et al in 2008. They used CXL for five cases of microbial keratitis with a successful outcome.112 Before that time, the antimicrobial mechanisms of CXL were not known, but they used it for two reasons: firstly, UV light has a well-known antimicrobial effect, and the second reason is that the increased corneal tensile strength induced by CXL can prevent deep stromal invasion of the microorganisms.
The term “Photo-Activated Chromophore for Keratitis – Corneal Collagen Cross-linking (PACK-CXL)” was first proposed during the 9th International Cross-Linking Congress that was in Dublin in 2013. This special terminology was proposed to distinguish between CXL for cases of infective keratitis from that used for cases of ectatic corneal disorders. This term can be used to describe any chromophore that can be activated for corneal stiffening and is not limited to a single type.113
PACK-CXL, when described, was using the same Dresden protocol with some modifications:111
- Manual epithelial removal from the surface and the edges of the ulcer along with debridement and removal of the discharge and necrotic tissues. This will help deeper penetration of riboflavin. Removed tissues can also be used for microbiological testing.
- Riboflavin is used without any viscosity agent.
- UV beam should be directed to the lesion, even in peripheral lesions approaching the limbus.
- Fluorescein should be avoided during the procedure, as it competes with riboflavin for UVA absorption, reducing the procedure’s efficacy.114
PACK-CXL was noted to be ineffective in the treatment of viral or acanthamoeba keratitis. It has even been reported to reactivate herpes simplex virus infection. Price et al reported the development of dendritic lesions after PACK-CXL for the culture-negative melting cornea. Therefore, it is better to avoid CXL in patients with active herpetic keratitis or a history of recurrent herpetic keratitis.115
Many clinical studies112,116–136 reported varied outcomes regarding the efficacy of PACK-CXL (Table 3). It has been proposed to be beneficial for cases of bacterial and fungal keratitis as a stand-alone therapy or as an adjunct to antimicrobial medications (Figure 6). It can also be used for mixed infections that are considered challenging in most cases with a greater risk of treatment failure and the development of complications.
 |
Table 3 Summary of Studies Reporting Collagen Cross-Linking for Fungal Keratitis |
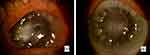 |
Figure 6 Fungal keratitis before PACK-CXL (a) and after PACK-CXL (b). |
In vitro, in vivo, and clinical studies have confirmed that PACK-CXL has beneficial antimicrobial efficacy, especially for moderate ulcers.120,128,131 It accelerates healing, speeds up epithelization, and decreases the risk of perforation. Other studies claimed no added benefit of PACK-CXL to the standard antimicrobial treatment.123,125,129 Most of the studies used the original Dresden protocol in CXL but Tabibian et al122 was the first to use accelerated protocol (UVA irradiation at 365 μm and 9 mW/cm2 for 10 min) and found it to be effective in reducing infiltration and increasing the process of healing. Hafezi et al136 recently used UVA of 9 mW/cm2 for either 10 min, 13 min, or 20 s with a total fluence of either 5.4 J/cm2 or 7.2 J/cm2. Higher fluences of UVA (up to 15 J/cm2) were tested by Awad et al137,138 and gave better clinical, microbiological, and pathological outcomes on rabbit eyes. Repeated high fluence PACK-CXL was reported by Hafezi et al134 and they noticed significant clinical improvement.
Although PACK-CXL has been extensively studied in the past few years, its protocol still needs many modifications to optimize UV fluence levels (J/cm2), irradiation time, and concentration of riboflavin to achieve 100% microbial killing.
Conclusion
It is recommended to start with antifungal therapy after confirmation of the clinical diagnosis with a smear or positive cultures. Intrastromal corneal injection of antifungal medications may result in steady-state drug levels within the corneal tissue and prevent intervals of decreased antifungal dosing below its therapeutic level. In cases of severe fungal keratitis with deep stromal infiltration not responding to treatment, intracameral injection of antifungal agents may be effective. Collagen cross-linking (CXL) is proposed to be beneficial for cases of fungal keratitis as a stand-alone therapy or as an adjunct to antifungal medications.
Abbreviations
AC, Anterior chamber; AI, Artificial intelligence; CXL, Corneal collagen cross-linking; DALK, Deep anterior lamellar keratoplasty; H&E, Hematoxylin and Eosin; IVCM, In vivo confocal microscopy; MK, Mycotic Keratitis; MUTT, Mycotic ulcer treatment trial; PAS, Periodic Acid-Schiff; PACK-CXL, Photo-Activated Chromophore for Keratitis – Collagen Cross-linking; PDAT, Photo-Dynamic Antimicrobial Therapy; PCR, Polymerase chain reaction; KOH, Potassium Hydroxide; ROS, Reactive oxygen species; SDA, Sabouraud dextrose agar; TST, Topical, systemic and targeted therapy; TKP, Therapeutic keratoplasty; UVA, Ultraviolet A.
Data Sharing Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ong HS, Corbett MC. Corneal infections in the 21st century. Postgrad Med J. 2015;91(1080):565–571. doi:10.1136/postgradmedj-2015-133323
2. Khalili MR, Jahadi HR, Karimi M, Yasemi M. Corneal collagen cross-linking for treatment of bacterial and herpetic keratitis. J Clin Diagn Res. 2017;11(7):Nc12–nc6. doi:10.7860/JCDR/2017/24863.10253
3. Garg P, Rao GN. Corneal ulcer: diagnosis and management. Community Eye Health. 1999;12(30):21–23.
4. Maharana PK, Sharma N, Nagpal R, Jhanji V, Das S, Vajpayee RB. Recent advances in diagnosis and management of mycotic keratitis. Indian J Ophthalmol. 2016;64(5):346–357. doi:10.4103/0301-4738.185592
5. Brown L, Leck AK, Gichangi M, Burton MJ, Denning DW. The global incidence and diagnosis of fungal keratitis. Lancet Infect Dis. 2021;21(3):e49–57. doi:10.1016/S1473-3099(20)30448-5
6. Liesegang TJ, Forster RK. Spectrum of microbial keratitis in South Florida. Am J Ophthalmol. 1980;90(1):38–47. doi:10.1016/S0002-9394(14)75075-5
7. Armstrong RA. The microbiology of the eye. Ophthalmic Physiol Opt. 2000;20(6):429–441. doi:10.1111/j.1475-1313.2000.tb01121.x
8. Colby K, Dana R. Foundations of Corneal Disease: Past, Present and Future. New York: Springer Nature; 2019.
9. Robin JB, Nielson S, Trousdale MD. Fluorescein-conjugated lectin identification of a case of human keratomycosis. Am J Ophthalmol. 1986;102(6):797–798. doi:10.1016/0002-9394(86)90413-7
10. Coster D, Lightman S. Cornea, Fundamentals of Clinical Ophthalmology Series. London: BMS Books; 2002.
11. Beebe DC. Maintaining transparency: a review of the developmental physiology and pathophysiology of two avascular tissues. Semin Cell Dev Biol. 2008;19(2):125–133. doi:10.1016/j.semcdb.2007.08.014
12. Dandona L, Ragu K, Janarthanan M, Naduvilath TJ, Shenoy R, Rao GN. Indications for penetrating keratoplasty in India. Indian J Ophthalmol. 1997;45(3):163–168.
13. McDonnell PJ, Nobe J, Gauderman WJ, Lee P, Aiello A, Trousdale M. Community care of corneal ulcers. Am J Ophthalmol. 1992;114(5):531–538. doi:10.1016/S0002-9394(14)74479-4
14. Sadik N, Elzeiny SM, Ali YE, Sobeih D. Fungal keratitis in the Egyptian delta: epidemiology, risk factors, and microbiological diagnosis. Ophthalmic Epidemiol. 2022;29(2):198–205. doi:10.1080/09286586.2021.1914667
15. Ansari Z, Miller D, Galor A. Current thoughts in fungal keratitis: diagnosis and treatment. Curr Fungal Infect Rep. 2013;7(3):209–218. doi:10.1007/s12281-013-0150-1
16. Xie L, Dong X, Shi W. Treatment of fungal keratitis by penetrating keratoplasty. Br J Ophthalmol. 2001;85(9):1070–1074. doi:10.1136/bjo.85.9.1070
17. Thomas PA, Leck AK, Myatt M. Characteristic clinical features as an aid to the diagnosis of suppurative keratitis caused by filamentous fungi. Br J Ophthalmol. 2005;89(12):1554–1558. doi:10.1136/bjo.2005.076315
18. Kalkanci A. Microbiological Diagnosis of Fungal Keratitis. In: Kalkanci A, editor. Mycotic Keratitis. Florida, United States: CRC Press; 2019:45–55.
19. Rai M, Occhiutto ML. Mycotic Keratitis. Florida, United States: CRC Press; 2019.
20. Gunasekaran R, Chandrasekaran A, Rajarathinam K, et al. Rapid point-of-care identification of aspergillus species in microbial keratitis. JAMA Ophthalmol. 2023;141(10):966–973. doi:10.1001/jamaophthalmol.2023.4214
21. Prasher P, Singh B, Singh K. Novel use of trypan blue dye to stain fungus in corneal scrapings in eye clinics. Cornea. 2020;39(11):e26. doi:10.1097/ICO.0000000000002446
22. Garg P, Gopinathan U, Choudhary K, Rao GN. Keratomycosis: clinical and microbiologic experience with dematiaceous fungi. Ophthalmology. 2000;107(3):574–580. doi:10.1016/S0161-6420(99)00079-2
23. Srinivasan M. Fungal keratitis. Curr Opin Ophthalmol. 2004;15(4):321–327. doi:10.1097/00055735-200408000-00008
24. Thomas PA, Kaliamurthy J. Mycotic keratitis: epidemiology, diagnosis and management. Clin Microbiol Infect. 2013;19(3):210–220. doi:10.1111/1469-0691.12126
25. Bharathi MJ, Ramakrishnan R, Vasu S, Meenakshi R, Palaniappan R. Epidemiological characteristics and laboratory diagnosis of fungal keratitis. A three-year study. Indian J Ophthalmol. 2003;51(4):315–321.
26. Ortega-Rosales A, Quizhpe-Ocampo Y, Montalvo-Flores M, Burneo-Rosales C, Romero-Ulloa G. A case of fungal keratitis due to Fusarium solani after an indigenous healing practice. IDCases. 2019;18:e00618.
27. Robaei D, Chan UT, Khoo P, et al. Corneal biopsy for diagnosis of recalcitrant microbial keratitis. Graefes Arch Clin Exp Ophthalmol. 2018;256(8):1527–1533. doi:10.1007/s00417-018-3981-1
28. Mahmoudi S, Masoomi A, Ahmadikia K, et al. Fungal keratitis: an overview of clinical and laboratory aspects. Mycoses. 2018;61(12):916–930. doi:10.1111/myc.12822
29. Kumar RL, Cruzat A, Hamrah P. Current state of in vivo confocal microscopy in management of microbial keratitis. Semin Ophthalmol. 2010;25(5–6):166–170. doi:10.3109/08820538.2010.518516
30. Brasnu E, Bourcier T, Dupas B, et al. In vivo confocal microscopy in fungal keratitis. Br J Ophthalmol. 2007;91(5):588–591. doi:10.1136/bjo.2006.107243
31. Chidambaram JD, Prajna NV, Larke N, et al. In vivo confocal microscopy appearance of Fusarium and Aspergillus species in fungal keratitis. Br J Ophthalmol. 2017;101(8):1119. doi:10.1136/bjophthalmol-2016-309656
32. Ferrer C, Alió JL. Evaluation of molecular diagnosis in fungal keratitis. Ten years of experience. J Ophthalmic Inflamm Infect. 2011;1(1):15–22. doi:10.1007/s12348-011-0019-9
33. Tahvildari M, Singh RB, Saeed HN. Application of artificial intelligence in the diagnosis and management of corneal diseases. Semin Ophthalmol. 2021;36(8):641–648. doi:10.1080/08820538.2021.1893763
34. Saini JS, Jain AK, Kumar S, Vikal S, Pankaj S, Singh S. Neural network approach to classify infective keratitis. Curr Eye Res. 2003;27(2):111–116. doi:10.1076/ceyr.27.2.111.15949
35. Soleimani M, Cheraqpour K, Sadeghi R, Pezeshgi S, Koganti R, Djalilian AR. Artificial intelligence and infectious keratitis: where are we now? Life. 2023;13(11):2117. doi:10.3390/life13112117
36. Dahlgren MA, Lingappan A, Wilhelmus KR. The clinical diagnosis of microbial keratitis. Am J Ophthalmol. 2007;143(6):940–944. doi:10.1016/j.ajo.2007.02.030
37. Dalmon C, Porco TC, Lietman TM, et al. The clinical differentiation of bacterial and fungal keratitis: a photographic survey. Invest Ophthalmol Vis Sci. 2012;53(4):1787–1791. doi:10.1167/iovs.11-8478
38. Chatterjee S, Agrawal D, Gomase SN. Clinical differentiation of Pythium keratitis from fungal keratitis and development of a scoring system. Indian J Ophthalmol. 2022;70(10):3515. doi:10.4103/ijo.IJO_870_22
39. Sharma N, Sahay P, Maharana PK, et al. Management algorithm for fungal keratitis: the TST (topical, systemic, and targeted therapy) protocol. Cornea. 2019;38(2):141–145. doi:10.1097/ICO.0000000000001781
40. Qiu S, Zhao GQ, Lin J, et al. Natamycin in the treatment of fungal keratitis: a systematic review and Meta-analysis. Int J Ophthalmol. 2015;8(3):597–602. doi:10.3980/j.issn.2222-3959.2015.03.29
41. FlorCruz NV, Peczon IV, Evans JR. Medical interventions for fungal keratitis. Cochrane Database Syst Rev. 2012;2012(2):Cd004241.
42. Garcia-Valenzuela E, Song CD. Intracorneal injection of amphotericin B for recurrent fungal keratitis and endophthalmitis. Arch Ophthalmol. 2005;123(12):1721–1723. doi:10.1001/archopht.123.12.1721
43. Yildiz EH, Abdalla YF, Elsahn AF, et al. Update on fungal keratitis from 1999 to 2008. Cornea. 2010;29(12):1406–1411. doi:10.1097/ICO.0b013e3181da571b
44. Arora R, Gupta D, Goyal J, Kaur R. Voriconazole versus natamycin as primary treatment in fungal corneal ulcers. Clin Exp Ophthalmol. 2011;39(5):434–440. doi:10.1111/j.1442-9071.2010.02473.x
45. Prajna NV, John RK, Nirmalan PK, Lalitha P, Srinivasan M. A randomised clinical trial comparing 2% econazole and 5% natamycin for the treatment of fungal keratitis. Br J Ophthalmol. 2003;87(10):1235–1237. doi:10.1136/bjo.87.10.1235
46. Rao SK, Madhavan HN, Rao G, Padmanabhan P. Fluconazole in filamentous fungal keratitis. Cornea. 1997;16(6):700. doi:10.1097/00003226-199711000-00019
47. Mselle J. Use of topical clotrimazole in human keratomycosis. Ophthalmologica. 2001;215(5):357–360. doi:10.1159/000050885
48. Torres HA, Hachem RY, Chemaly RF, Kontoyiannis DP, Raad II. Posaconazole: a broad-spectrum triazole antifungal. Lancet Infect Dis. 2005;5:775–785. doi:10.1016/S1473-3099(05)70297-8
49. Tu EY, McCartney DL, Beatty RF, Springer KL, Levy J, Edward D. Successful treatment of resistant ocular fusariosis with posaconazole (SCH-56592. Am J Ophthalmol. 2007;143:222–227. doi:10.1016/j.ajo.2006.10.048
50. Altun A, Kurna SA, Sengor T, et al. Effectiveness of posaconazole in recalcitrant fungal keratitis resistant to conventional antifungal drugs. Case Rep Ophthalmol Med. 2014;2014:701653. doi:10.1155/2014/701653
51. Neoh CF, Daniell M, Chen SC, Stewart K, Kong DC. Clinical utility of caspofungin eye drops in fungal keratitis. Int J Antimicrob Agents. 2014;44(2):96–104. doi:10.1016/j.ijantimicag.2014.04.008
52. Patil A, Majumdar S. Echinocandins in ocular therapeutics. J Ocul Pharmacol Ther. 2017;33(5):340–352. doi:10.1089/jop.2016.0186
53. Prajna NV, Krishnan T, Mascarenhas J, et al. The mycotic ulcer treatment trial: a randomized trial comparing natamycin vs voriconazole. JAMA Ophthalmol. 2013;131(4):422–429. doi:10.1001/jamaophthalmol.2013.1497
54. Mascarenhas M, Chaudhari P, Lewis SA. Natamycin ocular delivery: challenges and advancements in ocular therapeutics. Advan Therapy. 2023;8:1–28.
55. Stern GA, Okumoto M, Smolin G. Combined amphotericin B and rifampin treatment of experimental Candida albicans keratitis. Arch Ophthalmol. 1979;97(4):721–722. doi:10.1001/archopht.1979.01020010373020
56. Beggs WH. Mechanisms of synergistic interactions between amphotericin B and flucytosine. J Antimicrob Chemother. 1986;17(4):402–404. doi:10.1093/jac/17.4.402
57. Sha XY, Shi Q, Liu L, Zhong JX. Update on the management of fungal keratitis. Intl Ophthalmol. 2021;41(9):3249–3256. doi:10.1007/s10792-021-01873-3
58. Shapiro BL, Lalitha P, Loh AR, et al. Susceptibility testing and clinical outcome in fungal keratitis. Br J Ophthalmol. 2010;94(3):384–385. doi:10.1136/bjo.2009.158675
59. Shi W, Li S, Liu M, Jin H, Xie L. Antifungal chemotherapy for fungal keratitis guided by in vivo confocal microscopy. Graefes Arch Clin Exp Ophthalmol. 2008;246(4):581–586. doi:10.1007/s00417-007-0719-x
60. Tuli SS. Fungal keratitis. Clin Ophthalmol. 2011;5:275–279. doi:10.2147/OPTH.S10819
61. Prajna NV, Krishnan T, Rajaraman R, et al. Effect of oral voriconazole on fungal keratitis in the mycotic ulcer treatment trial II (MUTT II): a randomized clinical trial. JAMA Ophthalmol. 2016;134(12):1365–1372. doi:10.1001/jamaophthalmol.2016.4096
62. Cai Y, Song S, Chen Y, Xu X, Zou W. Oral voriconazole monotherapy for fungal keratitis: efficacy, safety, and factors associated with outcomes. Front Med. 2023;10:1174264. doi:10.3389/fmed.2023.1174264
63. Sharma N, Singhal D, Maharana PK, et al. Comparison of oral voriconazole versus oral ketoconazole as an adjunct to topical natamycin in severe fungal keratitis: a randomized controlled trial. Cornea. 2017;36(12):1521–1527. doi:10.1097/ICO.0000000000001365
64. Sharma N, Sachdev R, Jhanji V, Titiyal JS, Vajpayee RB. Therapeutic keratoplasty for microbial keratitis. Curr Opin Ophthalmol. 2010;21(4):293–300. doi:10.1097/ICU.0b013e32833a8e23
65. Ang M, Mehta JS, Sng CC, Htoon HM, Tan DT. Indications, outcomes, and risk factors for failure in tectonic keratoplasty. Ophthalmology. 2012;119(7):1311–1319. doi:10.1016/j.ophtha.2012.01.021
66. Benson MD, Kurji K, Tseng C, Bao B, Mah D. Analysis of penetrating keratoplasty in Northern Alberta, Canada, from 2000 to 2015. Can J Ophthalmol. 2018;53(6):568–573. doi:10.1016/j.jcjo.2018.01.024
67. Shi W, Wang T, Xie L, et al. Risk factors, clinical features, and outcomes of recurrent fungal keratitis after corneal transplantation. Ophthalmology. 2010;117(5):890–896. doi:10.1016/j.ophtha.2009.10.004
68. Gao H, Song P, Echegaray JJ, et al. Big bubble deep anterior lamellar keratoplasty for management of deep fungal keratitis. J Ophthalmol. 2014;2014:209759. doi:10.1155/2014/209759
69. Sabatino F, Sarnicola E, Sarnicola C, Tosi GM, Perri P, Sarnicola V. Early deep anterior lamellar keratoplasty for fungal keratitis poorly responsive to medical treatment. Eye (Lond). 2017;31(12):1639–1646. doi:10.1038/eye.2017.228
70. Cristol SM, Alfonso EC, Guildford JH, Roussel TJ, Culbertson WW. Results of large penetrating keratoplasty in microbial keratitis. Cornea. 1996;15(6):571–576. doi:10.1097/00003226-199611000-00006
71. Killingsworth DW, Stern GA, Driebe WT, Knapp A, Dragon DM. Results of therapeutic penetrating keratoplasty. Ophthalmology. 1993;100(4):534–541. doi:10.1016/S0161-6420(13)31631-5
72. Wang T, Li S, Gao H, Shi W. Therapeutic dilemma in fungal keratitis: administration of steroids for immune rejection early after keratoplasty. Graefes Arch Clin Exp Ophthalmol. 2016;254(8):1585–1589. doi:10.1007/s00417-016-3412-0
73. Gregory M, Macdonald E, Lockington D, Ramaesh K. Recurrent fungal keratitis following penetrating keratoplasty: an unusual source of infection. Arch Ophthalmol. 2010;128(11):1490–1491. doi:10.1001/archophthalmol.2010.264
74. Prakash G, Sharma N, Goel M, Titiyal JS, Vajpayee RB. Evaluation of intrastromal injection of voriconazole as a therapeutic adjunctive for the management of deep recalcitrant fungal keratitis. Am J Ophthalmol. 2008;146(1):56–59. doi:10.1016/j.ajo.2008.02.023
75. Sharma N, Agarwal P, Sinha R, Titiyal JS, Velpandian T, Vajpayee RB. Evaluation of intrastromal voriconazole injection in recalcitrant deep fungal keratitis: case series. Br J Ophthalmol. 2011;95(12):1735–1737. doi:10.1136/bjo.2010.192815
76. Sharma N, Chacko J, Velpandian T, et al. Comparative evaluation of topical versus intrastromal voriconazole as an adjunct to natamycin in recalcitrant fungal keratitis. Ophthalmology. 2013;120(4):677–681. doi:10.1016/j.ophtha.2012.09.023
77. Solaiman K, Alkawas A, Ibrahim B, Mahdy M, Shalaby M. Topical voriconazole drops with and without intrastromal voriconazole injection for treatment of deep or resistant fungal keratitis. J Clin Exp Ophthalmol. 2015;6(469):1000469. doi:10.4172/2155-9570.1000469
78. Killani S, Atti S, Gupta A, et al. Intracameral and intracorneal voriconazole in deep keratomycosis with endothelial plaque. J Evol Med Dent Sci. 2015;4:16476–16480. doi:10.14260/jemds/2015/2446
79. Kalaiselvi G, Narayana S, Krishnan T, Sengupta S. Intrastromal voriconazole for deep recalcitrant fungal keratitis: a case series. Br J Ophthalmol. 2015;99(2):195–198. doi:10.1136/bjophthalmol-2014-305412
80. Hu J, Zhang J, Li Y, et al. A combination of intrastromal and intracameral injections of amphotericin B in the treatment of severe fungal keratitis. J Ophthalmol. 2016;2016:3436415. doi:10.1155/2016/3436415
81. Nada WM, Al Aswad MA, El-Haig WM. Combined intrastromal injection of amphotericin B and topical fluconazole in the treatment of resistant cases of keratomycosis: a retrospective study. Clin Ophthalmol. 2017;11:871–874. doi:10.2147/OPTH.S135112
82. Narayana S, Krishnan T, Ramakrishnan S, et al. Mycotic antimicrobial localized injection: a randomized clinical trial evaluating intrastromal injection of voriconazole. Ophthalmology. 2019;126(8):1084–1089. doi:10.1016/j.ophtha.2019.03.020
83. Rathi A, Chauhan RS, Singh N. To compare therapeutic efficacy of topical voriconazole eye drops alone versus topical voriconazole eye drops combined with intrastromal injection of voriconazole in recalcitrant deep fungal keratitis. Saudi J Med Pharma Sci. 2019;5(2):137–146.
84. Konar P, Joshi S, Mandhare SJ, Thakur R, Deshpande M, Dayal A. Intrastromal voriconazole: an adjuvant approach for recalcitrant mycotic keratitis. Indian J Ophthalmol. 2020;68(1):35–38. doi:10.4103/ijo.IJO_378_19
85. Aydin B, Cubuk MO, Ucgul A, et al. Combined intrastromal voriconazole and amphotericin B treatment for persistent fungal keratitis. Eye Contact Lens. 2020;46(5):269–273. doi:10.1097/ICL.0000000000000723
86. Li C, Pang K, Du L, Wu X. Efficacy of voriconazole corneal intrastromal injection for the treatment of fungal keratitis. J Ophthalmol. 2021;2021:5597003. doi:10.1155/2021/5597003
87. Saluja G, Sharma N, Agarwal R, et al. Comparison of safety and efficacy of intrastromal injections of voriconazole, amphotericin b and natamycin in cases of recalcitrant fungal keratitis: a randomized controlled trial. Clin Ophthalmol. 2021;15:2437–2446. doi:10.2147/OPTH.S301878
88. Wannapanich T, Anutarapongpan O. Treatment outcomes of intrastromal voriconazole injection for fungal keratitis. Int J Biomed Res. 2022;13:6.
89. Bhirud A, Mishra A, Agrawal M, Sharma J. Intrastromal voriconazole as successful adjunctive approach for recalcitrant deep fungal keratitis. Roman J Ophthalmol. 2023;67(1):7. doi:10.22336/rjo.2023.3
90. Goudar S, Amulya P, Mridula AK, Seethalakshmi DK. Prospective interventional study of intrastromal voriconazole injection in nonhealing fungal keratitis. MRIMS J Health Sci. 2023;2023:1.
91. Kuriakose T, Kothari M, Paul P, Jacob P, Thomas R. Intracameral amphotericin B injection in the management of deep keratomycosis. Cornea. 2002;21(7):653–656. doi:10.1097/00003226-200210000-00004
92. Yoon KC, Jeong IY, Im SK, Chae HJ, Yang SY. Therapeutic effect of intracameral amphotericin B injection in the treatment of fungal keratitis. Cornea. 2007;26(7):814–818. doi:10.1097/ICO.0b013e31806c791e
93. Shen YC, Wang CY, Tsai HY, Lee HN. Intracameral voriconazole injection in the treatment of fungal endophthalmitis resulting from keratitis. Am J Ophthalmol. 2010;149(6):916–921. doi:10.1016/j.ajo.2010.01.024
94. Shao Y, Yu Y, Pei CG, et al. Therapeutic efficacy of intracameral amphotericin B injection for 60 patients with keratomycosis. Int J Ophthalmol. 2010;3(3):257–260. doi:10.3980/j.issn.2222-3959.2010.03.18
95. Mittal V, Mittal R. Intracameral and topical voriconazole for fungal corneal endoexudates. Cornea. 2012;31(4):366–370. doi:10.1097/ICO.0b013e318233f0a8
96. Sharma B, Kataria P, Anand R, et al. Efficacy profile of intracameral amphotericin B. Often Forgotten Step Asia Pac J Ophthalmol. 2015;4(6):360–366. doi:10.1097/APO.0000000000000107
97. Sharma N, Sankaran P, Agarwal T, et al. Evaluation of intracameral amphotericin B in the management of fungal keratitis: randomized controlled trial. Ocul Immunol Inflamm. 2016;24(5):493–497. doi:10.3109/09273948.2015.1057597
98. Maniam A, Min L, Phang L, Vendargon F, Othman O. Postoperative Fungal Keratitis managed by anterior chamber washout and intracameral amphotericin-B: a report of two cases. Cureus. 2021;13:12.
99. Dong LK, Krebs DB. An intracameral approach for recalcitrant fungal keratitis. Am J Ophthalmol Case Rep. 2022;25:101369. doi:10.1016/j.ajoc.2022.101369
100. Nasrin Y, Satya HR, Rath R. A hospital-based observational comparative study of efficacy of intracameral voriconazole and oral ketoconazole in deep keratomycosis. Ophthalmol J. 2022;7(3):6–11. doi:10.5603/OJ.2021.0045
101. Okonkwo A, Sethi K, Anand S. Repeated intracameral amphotericin B: a safe approach for management of fungal anterior chamber reactivations after therapeutic penetrating keratoplasty. Cornea. 2023;42(8):1041–1044. doi:10.1097/ICO.0000000000003268
102. Arboleda A, Miller D, Cabot F, et al. Assessment of rose bengal versus riboflavin photodynamic therapy for inhibition of fungal keratitis isolates. Am J Ophthalmol. 2014;158(1):64–70.e2. doi:10.1016/j.ajo.2014.04.007
103. Amescua G, Arboleda A, Nikpoor N, et al. Rose bengal photodynamic antimicrobial therapy: a novel treatment for resistant fusarium keratitis. Cornea. 2017;36(9):1141–1144. doi:10.1097/ICO.0000000000001265
104. Zhu H, Alt C, Webb RH, Melki S, Kochevar IE. Corneal crosslinking with rose bengal and green light: efficacy and safety evaluation. Cornea. 2016;35(9):1234–1241. doi:10.1097/ICO.0000000000000916
105. Spoerl E, Wollensak G, Seiler T. Increased resistance of crosslinked cornea against enzymatic digestion. Curr Eye Res. 2004;29(1):35–40. doi:10.1080/02713680490513182
106. Wollensak G, Spoerl E, Wilsch M, Seiler T. Endothelial cell damage after riboflavin-ultraviolet-A treatment in the rabbit. J Cataract Refract Surg. 2003;29(9):1786–1790. doi:10.1016/S0886-3350(03)00343-2
107. Wollensak G. Histological changes in human cornea after cross-linking with riboflavin and ultraviolet A. Acta Ophthalmol. 2010;88(2):e17–8. doi:10.1111/j.1755-3768.2008.01474.x
108. Wollensak G, Spörl E, Seiler T. Treatment of keratoconus by collagen cross linking. Ophthalmologe. 2003;100(1):44–49. doi:10.1007/s00347-002-0700-3
109. Wollensak G, Wilsch M, Spoerl E, Seiler T. Collagen fiber diameter in the rabbit cornea after collagen crosslinking by riboflavin/UVA. Cornea. 2004;23(5):503–507. doi:10.1097/01.ico.0000105827.85025.7f
110. Wernli J, Schumacher S, Spoerl E, Mrochen M. The efficacy of corneal cross-linking shows a sudden decrease with very high intensity UV light and short treatment time. Invest Ophthalmol Vis Sci. 2013;54(2):1176–1180. doi:10.1167/iovs.12-11409
111. Sinjab M, Cummings A. Corneal Collagen Cross Linking. London: Springer International Publishing; 2017.
112. Iseli HP, Thiel MA, Hafezi F, Kampmeier J, Seiler T. Ultraviolet A/riboflavin corneal cross-linking for infectious keratitis associated with corneal melts. Cornea. 2008;27(5):590–594. doi:10.1097/ICO.0b013e318169d698
113. Hafezi F, Randleman JB. PACK-CXL: defining CXL for infectious keratitis. J Refract Surg. 2014;30(7):438–439. doi:10.3928/1081597X-20140609-01
114. Richoz O, Gatzioufas Z, Francois P, Schrenzel J, Hafezi F. Impact of fluorescein on the antimicrobial efficacy of photoactivated riboflavin in corneal collagen cross-linking. J Refract Surg. 2013;29(12):842–845. doi:10.3928/1081597X-20131115-01
115. Ting DSJ, Henein C, Said DG, Dua HS. Photoactivated chromophore for infectious keratitis - Corneal cross-linking (PACK-CXL): a systematic review and meta-analysis. Ocul Surf. 2019;17(4):624–634. doi:10.1016/j.jtos.2019.08.006
116. Anwar HM, El-Danasoury AM, Hashem AN. Corneal collagen crosslinking in the treatment of infectious keratitis. Clin Ophthalmol. 2011;5:1277–1280. doi:10.2147/OPTH.S24532
117. Panda A, Krishna SN, Kumar S. Photo-activated riboflavin therapy of refractory corneal ulcers. Cornea. 2012;31(10):1210–1213. doi:10.1097/ICO.0b013e31823f8f48
118. Price MO, Tenkman LR, Schrier A, Fairchild KM, Trokel SL, Price FW. Photoactivated riboflavin treatment of infectious keratitis using collagen cross-linking technology. J Refract Surg. 2012;28(10):706–713. doi:10.3928/1081597X-20120921-06
119. Müller L, Thiel MA, Kipfer-Kauer AI, Kaufmann C. Corneal cross-linking as supplementary treatment option in melting keratitis: a case series. Klin Monbl Augenheilkd. 2012;229(4):411–415. doi:10.1055/s-0031-1299420
120. Li Z, Jhanji V, Tao X, Yu H, Chen W, Mu G. Riboflavin/ultraviolet light-mediated crosslinking for fungal keratitis. Br J Ophthalmol. 2013;97(5):669–671. doi:10.1136/bjophthalmol-2012-302518
121. Sorkhabi R, Sedgipoor M, Mahdavifard A. Collagen cross-linking for resistant corneal ulcer. Int Ophthalmol. 2013;33(1):61–66. doi:10.1007/s10792-012-9633-2
122. Tabibian D, Richoz O, Riat A, Schrenzel J, Hafezi F. Accelerated photoactivated chromophore for keratitis-corneal collagen cross-linking as a first-line and sole treatment in early fungal keratitis. J Refract Surg. 2014;30(12):855–857. doi:10.3928/1081597X-20141113-06
123. Said DG, Elalfy MS, Gatzioufas Z, et al. Collagen cross-linking with photoactivated riboflavin (PACK-CXL) for the treatment of advanced infectious keratitis with corneal melting. Ophthalmology. 2014;121(7):1377–1382. doi:10.1016/j.ophtha.2014.01.011
124. Shetty R, Nagaraja H, Jayadev C, Shivanna Y, Kugar T. Collagen crosslinking in the management of advanced non-resolving microbial keratitis. Br J Ophthalmol. 2014;98(8):1033–1035. doi:10.1136/bjophthalmol-2014-304944
125. Vajpayee RB, Shafi SN, Maharana PK, Sharma N, Jhanji V. Evaluation of corneal collagen cross-linking as an additional therapy in mycotic keratitis. Clin Exp Ophthalmol. 2015;43(2):103–107. doi:10.1111/ceo.12399
126. Uddaraju M, Mascarenhas J, Das MR, et al. Corneal cross-linking as an adjuvant therapy in the management of recalcitrant deep stromal fungal keratitis: a randomized trial. Am J Ophthalmol. 2015;160(1):131–4.e5. doi:10.1016/j.ajo.2015.03.024
127. Igal V, Pikkel Igal YS, Pikkel YY. Corneal cross-linking as a treatment for fungal keratitis associated with corneal melting. Case Rep Ophthalmol. 2017;8(1):148–151. doi:10.1159/000456537
128. Erdem E, Harbiyeli II, Boral H, Ilkit M, Yagmur M, Ersoz R. Corneal collagen cross-linking for the management of mycotic keratitis. Mycopathologia. 2018;183(3):521–527. doi:10.1007/s11046-018-0247-8
129. Prajna NV, Radhakrishnan N, Lalitha P, et al. Cross-linking-assisted infection reduction: a randomized clinical trial evaluating the effect of adjuvant cross-linking on outcomes in fungal keratitis. Ophthalmology. 2020;127(2):159–166. doi:10.1016/j.ophtha.2019.08.029
130. Mikropoulos DG, Kymionis GD, Voulgari N, et al. Intraoperative photoactivated chromophore for infectious keratitis-corneal cross-linking (PACK-CXL) during penetrating keratoplasty for the management of fungal keratitis in an immunocompromised patient. Ophthalmol Ther. 2019;8(3):491–495. doi:10.1007/s40123-019-0196-4
131. Wei A, Wang K, Wang Y, Gong L, Xu J, Shao T. Evaluation of corneal cross-linking as adjuvant therapy for the management of fungal keratitis. Graefes Arch Clin Exp Ophthalmol. 2019;257(7):1443–1452. doi:10.1007/s00417-019-04314-1
132. Bamdad S, Khalili MR, Khosravi A, Attarzade A, Movahedan H, Nejabat M. Corneal cross-linking as an adjunct for the management of refractory fungal keratitis. Middle East Afr J Ophthalmol. 2020;27(4):204–209. doi:10.4103/meajo.MEAJO_130_19
133. González Castellanos JC, Osaba M, Reviglio V, Canchi MT, Arrigone MC, Reviglio VE. Early treatment of bilateral fungal keratitis with corneal cross-linking as adjuvant therapy. Oxford Med Case Rep. 2020;2020(6):omaa032. doi:10.1093/omcr/omaa032
134. Hafezi F, Munzinger A, Goldblum D, Hillen M, Tandogan T. Repeated high-fluence accelerated slitlamp-based photoactivated chromophore for keratitis corneal cross-linking for treatment-resistant fungal keratitis. Cornea. 2022;41(8):1058–1061. doi:10.1097/ICO.0000000000002973
135. Khurana S, Gupta P, Gupta A, Sharma S, Ram J. Accelerated photoactivated chromophore for infectious keratitis-collagen cross-linking in deep mycotic keratitis. Indian J Ophthalmol Case Rep. 2022;2(1):257. doi:10.4103/ijo.IJO_998_21
136. Hafezi F, Hosny M, Shetty R, et al. PACK-CXL vs. antimicrobial therapy for bacterial, fungal, and mixed infectious keratitis: a prospective randomized phase 3 trial. Eye and Vision. 2022;9(1):2. doi:10.1186/s40662-021-00272-0
137. Awad R, Hafezi F, Ghaith AA, et al. Comparison between three different high fluence UVA levels in corneal collagen cross-linking for treatment of experimentally induced fungal keratitis in rabbits. Eur J Ophthalmol. 2022;32(4):1907–1914. doi:10.1177/11206721221092224
138. Elbassiouny R, Ghaith A, Farhad H, Baddour M, Eman S, Elmassry A. Evaluation of the efficacy of high-fluence corneal collagen cross-linking in fusarium corneal ulcer in rabbits. J Egypt Ophthalmol Soc. 2022;115(2):43–48. doi:10.4103/ejos.ejos_35_21
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

