Back to Journals » International Journal of Nanomedicine » Volume 10 » Issue 1
Functional consequences for primary human alveolar macrophages following treatment with long, but not short, multiwalled carbon nanotubes
Authors Sweeney S, Grandolfo D, Ruenraroengsak P, Tetley T
Received 21 November 2014
Accepted for publication 5 February 2015
Published 23 April 2015 Volume 2015:10(1) Pages 3115—3129
DOI https://doi.org/10.2147/IJN.S77867
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Thomas Webster
Sinbad Sweeney, Davide Grandolfo, Pakatip Ruenraroengsak, Teresa D Tetley
Lung Cell Biology, Section of Pharmacology and Toxicology, National Heart and Lung Institute, Imperial College London, London, UK
Purpose: Multiwalled carbon nanotubes (MWCNTs) are a potential human health hazard, primarily via inhalation. In the lung, alveolar macrophages (AMs) provide the first line of immune cellular defense against inhaled materials. We hypothesized that, 1 and 5 days after treating AMs with short (0.6 µm in length; MWCNT-0.6 µm) and long (20 µm in length; MWCNT-20 µm) MWCNTs for 24 hours, AMs would exhibit increased markers of adverse bioreactivity (cytokine release and reactive oxygen species generation) while also having a modified functional ability (phagocytosis and migration).
Methods: Primary human AMs were treated with short and long MWCNTs for 24 hours, 1 and 5 days after which toxicity end points, including cell death, reactive oxygen species generation, and inflammatory mediator release, were measured. AM functional end points involving phagocytic ability and migratory capacity were also measured.
Results: AM viability was significantly decreased at 1 and 5 days after treatment with MWCNT-20 µm, while superoxide levels and inflammatory mediator release were significantly increased. At the same time, there was reduced phagocytosis and migratory capacity alongside increased expression of MARCO; this coincided with frustrated phagocytosis observed by scanning electron microscopy. In contrast, the adverse bioreactivity of the shorter MWCNT-0.6 µm with AMs (and any resulting reduction in AM functional ability) was substantially less marked or absent altogether.
Conclusion: This study shows that after 24-hour treatment with long, but not short, MWCNTs, AM function is severely affected up to 5 days after the initial exposure. This has potentially significant pathophysiological consequences for individuals who may be intentionally (via therapeutic applications) or unintentionally exposed to these nanomaterials.
Keywords: nanotechnology, MWCNTs, alveolar macrophages, cytokines, phagocytosis, bioreactivity
Introduction
The nanotechnology sector has become an important and rapidly growing global industry, developing and producing a vast array of nanomaterials (materials with at least one external dimension <100 nm, [ISO/TS: 27687:2008]). Engineered multiwalled carbon nanotubes (MWCNTs) are one such nanomaterial whose production capacity is estimated at several thousand tons yearly.1 MWCNTs offer high strength-to-weight ratios, tensile strength, conductivity, and thermal stability. Typically occurring with diameters in the low-nanometer range and lengths in the high-micrometer range, MWCNTs are being applied in industries such as electronics, automobile, aerospace, and nanomedicine (eg, drug/gene delivery and scaffolds for tissue engineering).2 Products already incorporating MWCNTs include computer components, aircraft structural components, and sporting equipment such as tennis rackets, golf clubs, bicycles, golf irons, and baseball bats. Human exposure to MWCNTs can vary considerably, depending on the production method, application, and use.3 Inhalation of MWCNTs is a primary concern, not least because of lessons learned from exposure to long, thin, needle-like, and biopersistent amphibole asbestos fibers leading to the development of pulmonary mesothelioma.4 The fiber structure–activity paradigm is of particular concern, as described by Donaldson, as the structural characteristics of fibers that impart pathogenic activity are thinness (allowing for respirability), length (promoting retention), and biopersistence, resulting in increased toxicity, inflammation, and carcinogenicity.5 Indeed, MWCNTs can also fall under the World Health Organization’s definition of a fiber, ie, a particle longer than 5 μm, less than 3 μm in width, and having an aspect ratio of >3:1.6 It is generally accepted that nanosized particles have a high deposition rate in the alveolar region of the lung.7–9 As with other nanoparticles, aerodynamic diameter governs MWCNT deposition in the lung. However, for MWCNTs that may have lengths well into the micrometer range, their actual diameters have been proposed to determine their alveolar deposition,10 where the longitudinal axis of the MWCNTs aligns with the airstream.
Alveolar macrophages (AMs) provide the first line of immune cellular defense by removing foreign matter (microbes, particles, etc) from the alveolar space. AMs identify and subsequently phagocytose foreign matter using pattern recognition receptors such as toll-like receptors (TLR4), scavenger receptors, and receptors of endogenous opsonins.11–14 AMs also have important functional roles in removal of endogenous cellular debris and degradation of pulmonary surfactant (lipids and proteins).15 AMs that become laden with phagocytosed material are thought to migrate out of the alveolar space for eventual clearance via the mucociliary escalator system and the lymphatic system, thus critically maintaining a sterile and particle-free alveolar unit. While AMs are efficient operators in foreign matter removal from the peripheral lung, exposure to excessive burden or complex particle morphology can compromise their efficacy or result in their injury and eventual death. For example, AMs can suffer from a phenomenon termed particle “overload”, which results in compromised phagocytic and migratory clearance, while the attempted clearance of long thin fibers (eg, amphibole asbestos) can induce “frustrated” phagocytosis in exposed AMs.16–18 Such events result in protracted AM release of inflammatory cytokines, chemokines, interferons, and other soluble mediators; further recruitment of immune effector cells; increased production/release of free radicals (eg, reactive oxygen species [ROS]); and possible damage to the delicate underlying alveolar epithelium. These events may be a precursor to known MWCNT-induced pathologies such as pulmonary fibrosis19 and granulomatous inflammation20 in rodents following inhalation.
Previous work in our laboratory showed that the length of MWCNTs is a critical determinant of their adverse bioreactivity with primary human AMs (in terms of proinflammatory mediator release and cell death); following an acute (24 hours) treatment, AMs displayed greatest reactivity toward long MWCNTs (~20 μm in length) than toward short MWCNTs (~0.6 μm in length).21 Such acute injury to AMs may result in a more prolonged proinflammatory bioactivation and may impede their short- and long-term functional and migratory abilities. Thus, in the present study we hypothesized that, 1 and 5 days after treating AMs with long MWCNTs (with an acute 24-hour exposure), AMs would exhibit increased markers of adverse bioreactivity (cell death, ROS generation, and inflammatory mediator release) while also having a modified ability to phagocytose bacteria and a modified migratory capacity.
Materials and methods
Ethics statement
The human lung tissue used in this study was surplus tissue obtained following resection for lung carcinoma. Written informed consent was obtained for all samples, and the study was carried out with the approval of the Royal Brompton and Harefield Ethical Committee (Ref: 08/H0708/73).
AM cell model
Primary human AMs were isolated from human lung having grossly normal appearance obtained following resection for lung carcinoma, as described by Witherden and Tetley.22 AMs were isolated from a minimum of five subjects per experiment. Briefly, lung tissue sections were perfused by injection of 0.15 M sterile sodium chloride saline solution until the draining lavage ran clear and the cell count was <1×104 cells/mL. The saline perfusate was collected and centrifuged at 240× g for 10 minutes at 20°C. The cell pellet was resuspended in RPMI cell culture medium containing 1% penicillin/streptomycin/l-glutamine (PSG) and 1% fetal bovine serum (FBS) and seeded for respective experiments. After AM adherence during incubation at 37°C with 5% CO2 (~3 hours), the culture medium was removed and nonadherent cells (including contaminating blood) were removed by gentle phosphate-buffered saline (PBS) washing. For experiments, AM cultures were maintained in RPMI cell culture medium containing 1% PSG and FBS incubated at 37°C with 5% CO2. We have shown previously that >95% of these cells are CD68+,23 are phagocytic, and show macrophage morphology under scanning electron microscope (SEM).24
AM treatment with MWCNTs
The MWCNTs used in this study were purchased from Nanothinx, Rio Patras, Greece. We have previously characterized and reported on the physicochemical properties of these materials, both in distilled water and in cell culture medium.21 Briefly, MWCNT-0.6 μm and MWCNT-20 μm (as labeled by the manufacturer) were determined to have median lengths of 1.1 μm (range, 0.23–9.7 μm) and 19.3 μm (range, 0.6–30.8 μm), respectively. Both MWCNT-0.6 μm and MWCNT-20 μm had comparable median diameters of 30.6 nm (range, 19.5–47.6 nm) and 27.8 nm (range, 15.6–41.9 nm), respectively. Brunauer, Emmett, and Teller (BET) measurements indicated that MWCNT-0.6 μm and MWCNT-20 μm had surface areas of 255.24 and 204.8 m2/g, respectively. Both MWCNT-0.6 μm and MWCNT-20 μm also have comparable carbon purities (ie, the % atomic carbon) of 87% and 95%. As characterized by us previously, the remaining elemental % is predominantly oxygen while we also see trace amounts of calcium, chlorine, phosphorus, and sodium (likely residual from the raw materials or catalysts or both). While the metal catalysts for these MWCNTs were not disclosed by the manufacturer, our previous analysis suggests that these are likely to be aluminum (accounting for 0.6% and 0.3% of total atomic content for MWCNT-0.6 μm and MWCNT-20 μm, respectively) and iron (<0.1% for both of the MWCNT formats). The surface charge of both MWCNT-0.6 μm and MWCNT-20 μm was also comparable at −13.2 and −12 mV, respectively. Both MWCNT materials were assayed for endotoxin contamination, using a Pierce LAL Chromogenic Endotoxin Quantitation Kit (Thermo Scientific, Waltham, MA, USA) and both were below the 0.1 EU/mL limit of detection. AMs were treated with 10 μg/mL (prepared in RPMI 1640 culture medium) of MWCNT-0.6 μm or MWCNT-20 μm for 24 hours. This dose of MWCNT was chosen as we previously found it had maximal bioreactivity (interleukin [IL]-6 and IL-8 release) with sufficient viability, in AMs following a 24-hour treatment (in a dose range of 0.1–50 μg/mL), which we believed would allow us to examine whether AMs could maintain their immune functionality (phagocytosis, cytokine release, migration, etc) following treatment with these nanomaterials.21 Furthermore, this dose is comparable to those used in published in vivo rodent studies on MWCNT inhalation. For example, Ryman-Rasmussen et al25 exposed mice to 4 and 0.2 mg/kg CNTs (a calculated dose was based on a 10% estimated deposition fraction) in their study, where the estimated total AMs were 0.95×105,26 the low dose of which compares favorably with this study, where we exposed 1×105 AMs to 10 μg MWCNT. At the end of a 24-hour treatment period, AMs were washed three times with PBS, fresh RPMI 1640 culture medium was added, and AMs were incubated for a further 1 or 5 days posttreatment.
Scanning electron microscopy
AMs were seeded at 0.5×106 cells/well on glass coverslips in 24-well culture plates. Following treatment with 10 μg/mL of MWCNT-0.6 μm or MWCNT-20 μm for 24 hours, AMs were fixed with 2% glutaraldehyde in 0.1 M cacodylate buffer for 30 minutes, followed by three rinses (5 minutes each) with 0.1 M cacodylate buffer. The AMs were then dehydrated with ethanol and sealed onto a SEM stub with Araldite (Huntsman Advanced Materials, Duxford, UK). The samples were then sputter-coated with a thin film of gold and examined under SEM with a Hitachi s4000 (Hitachi, Tokyo, Japan).
Cell viability
MTS is a tetrazolium compound, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, that can be reduced by dehydrogenase enzymes within living cells demonstrating cellular metabolic activity and cell viability. Such reduction in the presence of an electron coupling reagent, phenazine ethosulfate, results in the production of formazan product that is soluble in tissue culture medium, and the absorbance of this formazan product can be measured using a spectrophotometer. AMs were seeded on 96-well plates at a density of 100,000 cells/well in RPMI 1640 culture medium (containing 5% PSG) at 37°C with 5% CO2. One and 5 days after treatment with MWCNTs, AMs were rinsed with PBS and incubated with RPMI 1640 culture medium containing the MTS reagent according to the manufacture’s protocol for 1.5 hours (CellTiter 96® AQueous One Solution Assay, Promega, Madison, WI, USA). The absorbance of formazan product in the culture medium was then read using a spectrophotometer at a wavelength of 490 nm. We have established that the MWCNTs used in this study do not interfere with the formation of formazan and therefore do not interfere with the MTS cell viability assay (data not shown).
Reactive oxygen species generation
One and 5 days posttreatment with MWCNTs, intracellular AM ROS (key contributors to cellular oxidative stress) were detected by measuring the oxidation of fluorescent dihydroethidium (DHE). DHE is readily permeable to cells and is oxidized by ROS, primarily superoxide, to yield ethidium. Ethidium subsequently binds to DNA, which produces a detectable red fluorescence. In the present study, ROS production was measured with the DHE probe using a variation of the protocol derived from Budd et al.27 AMs were seeded on 96-well plates (black, with a clear bottom) at a density of 100,000 cells/well in RPMI 1640 culture medium (containing 5% PSG) at 37°C with 5% CO2. One and 5 days posttreatment with MWCNTs, AMs were rinsed with PBS and incubated with RPMI 1640 culture medium containing 100 μL of 10 μM DHE (Invitrogen, Paisley, UK) in RPMI 1640 culture medium for 30 minutes. At the end of DHE incubation, AMs were washed twice to remove extracellular probe and read in a fluorescence plate reader at an excitation/emission (Ex/Em) of 485/595 nm. We assessed potential MWCNT interference in the DHE assay by adding the DHE probe to each of the MWCNT materials (10 μg/mL) in a cell-free setup. We did not observe any spontaneous ethidium production with detectable red fluorescence, and therefore concluded that the MWCNTs used in this study did not interfere with the DHE assay. In addition, the fluorescence of treated and nontreated AMs was measured in the absence of the DHE probe, to allow any interference by AM autofluorescence to be removed from the measurement.
Measurement of inflammatory mediator release
One and 5 days posttreatment with MWCNTs, AM mediator-conditioned culture medium was assayed for concentrations of the inflammatory cytokines (human IL-6, IL-1β, and tumor necrosis factor alpha [TNF-α]) and the inflammatory chemokine (human IL-8) using sandwich enzyme-linked immunosorbent assays (ELISA). The assays were performed using DuoSet® antibody kits and according to the manufacturer’s directions (R&D Systems Inc., Minneapolis, MN, USA). In the ELISA, we assessed potential MWCNT interference by spiking a 1,000 pg/mL standard solution of IL-6 or IL-8 with increasing doses of both MWCNT materials (10 μg/mL). ELISA was performed on the standard alone and the MWCNT spiked standard. We did not observe any significant differences in the measured concentration of the nonspiked and MWCNT spiked standard, and therefore concluded that the MWCNTs used in this study did not interfere with the ELISA.
Phagocytosis of Escherichia coli
The phagocytic capacity of AMs, 1 and 5 days after 24-hour treatment with 10 μg/mL MWCNT-0.6 μm or MWCNT-20 μm, was determined by measuring the uptake of fluorescein-labeled Escherichia coli (E. coli), using a commercially available kit, according to the manufacturer’s instructions (Vybrant Phagocytosis Assay Kit, Molecular Probes, Paisley, UK). Briefly, AMs were seeded on 96-well plates (black, with a clear bottom) at a density of 100,000 cells/well in RPMI 1640 culture medium (containing 5% PSG) at 37°C with 5% CO2. One and 5 days posttreatment with MWCNTs, AMs were rinsed with PBS and incubated with RPMI 1640 culture medium containing fluorescein-labeled E. coli for 3 hours. At the end of the incubation period, AMs were washed twice to remove extracellular, nonphagocytosed E. coli. Interfering fluorescence, from any residual extracellular nonphagocytosed E. coli, was quenched by addition of trypan blue according to the manufacturer’s instructions. AMs were then imaged using a fluorescent microscope and green fluorescence intensity was quantified using Simple PCI software (Hamamatsu, Shizuoka, Japan). The comparison between treated and nontreated AMs was made by quantifying total green fluorescence, which includes differences in both the number of AMs that contain bacteria and the amount of fluorescence per E-coli-positive AM.
Phagocytic receptor expression
The AM extracellular expression of the scavenger receptors (MARCO, MSR-1, and CD36) and TLR4, 1 and 5 days after 24-hour treatment with MWCNT-0.6 μm or MWCNT-20 μm, was measured using flow cytometry. Briefly, treated and nontreated AMs were detached using nonenzymatic cell dissociation solution (Sigma-Aldrich Co., St Louis, MO, USA) and washed in PBS. AMs were then incubated for 1 hour on ice with a mouse monoclonal antibody against human MARCO (Hycult Biotech, Uden, the Netherlands), human MSR-1 (R&D Systems), human CD36 (Hycult Biotech), and human TLR4 (BioLegend, San Diego, CA, USA) or a mouse isotype control antibody (New England Biolabs, MA, USA). AMs were then washed twice with PBS and incubated with phycoerythrin-labeled goat anti-mouse immunoglobulin G for 30 minutes on ice. AMs were again washed with PBS and resuspended for analysis in FACSFlow™ (Becton Dickinson and Company, NJ, USA) prior to analysis on a BD FACSCanto II™ flow cytometer (Becton Dickinson). A total of 10,000 gated events were measured for each sample.
Migratory capacity
The migratory capacity of AMs was determined by measuring their ability to migrate (via chemotaxis) toward zymosan-activated serum (Z-AS), 1 and 5 days after 24-hour treatment with MWCNT-0.6 μm or MWCNT-20 μm. Briefly, using a protocol adapted from Warheit et al28 zymosan (Sigma-Aldrich Ltd.) was prepared in sterile PBS and was added to FBS to yield a final concentration of 1 mg/mL; this was incubated in a shaking water bath at 37°C for 2 hours, followed by 56°C for 30 minutes to cease complement activation. The Z-AS was centrifuged at 12,000× g for 15 minutes and the supernatants were removed and diluted to 10% in RPMI 1640 culture medium. 1×105 AMs, in 0.5 mL of RPMI 1640 culture medium, were added to 8-μm-pore BD FluoroBlok™ (Becton Dickinson) inserts. These inserts were then placed in a 24-well plate containing 1.5 mL RPMI 1640 culture medium. The AMs were then treated as before with 10 μg/mL of MWCNT-0.6 μm or MWCNT-20 μm for 24 hours. At the end of this treatment period, AMs were washed three times with PBS, fresh RPMI 1640 culture medium was added to the insert chamber and the lower chamber, and AMs were incubated for a further 1 or 5 days posttreatment. After 1 or 5 days posttreatment, AMs were washed with PBS, fresh RPMI 1640 culture medium with 1% FBS was added to the insert chamber, and the 10% Z-AS in RPMI 1640 culture medium was added to the lower chamber. The AMs were incubated at 37°C with 5% CO2 for 8 hours to allow migration to take place. AMs that migrated toward the Z-AS were stained with fluorescent calcein, imaged by fluorescent microscope fluorescence intensity, and quantified using the Simple PCI software. A chemokinesis control (placing RPMI 1640 culture medium in both the insert and the lower chamber) was also performed. We were also wary of the possibility of MWCNTs physically obstructing the insert pores and falsely indicating reduced migration. To test for this phenomenon, we added 10 μg/mL of MWCNT-0.6 μm or MWCNT-20 μm to the culture insert for 24 hours; 1.5 mL of PBS was added to the lower chamber. We then added dyed microsphere 6.0 μm polystyrene Polybeads® (blue; Polysciences Inc., Eppelheim, Germany) to the insert (at 0.1% v/v), and the Polybeads® were left to pass through the pores, into the lower PBS-containing chamber, for 8 hours. The absorbance of the Polybeads® solution was measured at 450 nm, before and after passing through the insert, to determine if pore obstruction by MWCNT was a problem. We did not find any reduction in the concentration of Polybeads® before and after passage through the insert, thus MWCNTs did not obstruct the insert pores.
Statistical analyses
Data from cell viability, ROS generation, IL-8 release, phagocytosis, phagocytosis receptor expression, and migratory capacity experiments are presented as the mean ± standard error, where five independent experiments were performed using five separate donor tissues for AM harvest. Significant differences between nontreated and MWCNT-treated AMs were determined using nonparametric Mann–Whitney test. In all analyses, a P-value <0.05 was considered significant.
Results
Scanning electron microscopy
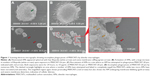
AMs were treated with 10 μg/mL of MWCNT-0.6 μm or MWCNT-20 μm for 24 hours to assess respective cellular morphological changes. Under SEM, untreated AMs appeared spherical with few filopodia and some membrane ruffling (Figure 1A). Following the 24-hour treatment with MWCNT-0.6 μm, AMs became activated and flattened out, exhibiting numerous filopodia (Figure 1B). Following the 24-hour treatment with MWCNT-20 μm, AM activation was also incited. However, recruitment of numerous AMs to sites of attempted phagocytosis was also observed (Figure 1C). Failure to completely engulf MWCNT-20 μm was found. Protruding MWCNTs were accompanied by membrane blebbing, severe membrane distortion, and multiple AM recruitment, suggesting MWCNT-induced frustrated phagocytosis (Figure 1D).
Cell viability
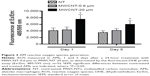
AM viability, 1 and 5 days after the 24-hour treatment with 10 μg/mL MWCNT-0.6 μm or MWCNT-20 μm, was measured using the MTS assay (Figure 2). AM viability was not significantly changed at 1 or 5 days after treatment with MWCNT-0.6 μm. In contrast, AM viability significantly decreased by 28% (P<0.05) and 51% (P<0.01) at 1 and 5 days, respectively, after treatment with MWCNT-20 μm.
Reactive oxygen species generation
The fluorescent probe, DHE, was used to measure the generation of ROS (primarily, the superoxide anion) in AMs, 1 and 5 days after 24-hour treatment with 10 μg/mL MWCNT-0.6 μm or MWCNT-20 μm (Figure 3). AM superoxide levels were not significantly changed at 1 or 5 days after treatment with MWCNT-0.6 μm. Conversely, AM superoxide levels significantly increased 1.9-fold (P<0.05) and 1.7-fold (P<0.05) at 1 and 5 days, respectively, after treatment with MWCNT-20 μm.
Inflammatory mediator release
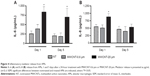
We observed that IL-6 release from AMs did not significantly change at 1 or 5 days after the 24-hour treatment with 10 μg/mL MWCNT-0.6 μm (Figure 4A). IL-6 release by AMs significantly increased 3.3-fold (P<0.05) and 3.5-fold (P<0.05) at 1 and 5 days, respectively, after treatment with MWCNT-20 μm. We observed that IL-8 release from AMs did not significantly change at 1 or 5 days after the 24-hour treatment with 10 μg/mL MWCNT-0.6 μm (Figure 4B). IL-8 release by AMs significantly increased 1.6-fold (P<0.05) 1 day after treatment with MWCNT-20 μm; however, no significant changes were observed at the 5 days posttreatment time point. In addition, we did not observe any differences in the release of IL-1β or TNF-α following treatment of AMs with MWCNTs (data not shown).
Phagocytosis of E. coli
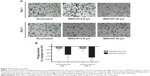
The phagocytic capacity of AMs, 1 or 5 days after the 24-hour treatment with 10 μg/mL MWCNT-0.6 μm or MWCNT-20 μm, was determined by measuring the uptake of fluorescein-labeled E. coli. We observed that AM phagocytic capacity was significantly reduced, by 15% (P<0.05), 1 day after the 24-hour treatment with 10 μg/mL MWCNT-0.6 μm (Figure 5); however, no significant changes were observed at the 5 days posttreatment time point. We observed that AM phagocytic capacity was significantly reduced, by 63% (P<0.01) and 85% (P<0.001), at 1 and 5 days, respectively, after treatment with 10 μg/mL MWCNT-20 μm.
Phagocytic receptor expression
The extracellular expression of the scavenger receptors (MARCO, MSR-1, and CD36) and TLR4, 1 and 5 days after the 24-hour treatment with MWCNT-0.6 μm or MWCNT-20 μm, was measured using flow cytometry. We observed a slight, but nonsignificant, increase in TLR4 expression 1 day after the 24-hour treatment with 10 μg/mL MWCNT-0.6 μm (Figure 6); in contrast, MARCO, MSR-1, and CD36 were significantly increased, by 18%, 15%, and 14%, respectively (P<0.05), at the same time point. Five days after the 24-hour treatment with MWCNT-0.6 μm, there were no significant changes in the expression of any of the four receptors analyzed. At 1 and 5 days after treatment with MWCNT-20 μm, MARCO expression was significantly increased 33% (P<0.05) and 23% (P<0.05), respectively; there were no significant changes for any of the other receptors at either time point.
Migratory capacity
The migratory capacity of AMs was determined by measuring their ability to migrate toward zymosan-activated FBS, 1 and 5 days after the 24-hour treatment with MWCNT-0.6 μm or MWCNT-20 μm. We observed that the migratory capacity of AMs did not significantly change at 1 or 5 days after the 24-hour treatment with 10 μg/mL MWCNT-0.6 μm (Figure 7). At 1 and 5 days after treatment with MWCNT-20 μm, AM migratory capacity significantly decreased by 49% (P<0.05) and 76% (P<0.01), respectively.
Discussion
We have previously shown that MWCNT length is a critical determinant of their adverse bioreactivity with primary human AMs (in terms of proinflammatory mediator release and cell death); following a 24-hour treatment, AMs displayed great reactivity toward long MWCNTs (~20 μm in length) when compared to short MWCNTs (~0.6 μm in length).21 In the present study, we determined if there were functional adverse consequences for these AMs, 1 and 5 days after the exposure to the MWCNTs. These time points allowed us to assess the longer term significance of acute human AM exposure to MWCNT and how this may translate to real-life exposure scenarios.
Immediately following the 24-hour treatment with MWCNT-0.6 μm or MWCNT-20 μm, AMs were imaged under SEM to assess their interaction with the MWCNTs and any cellular morphological changes. We observed a failure of AMs to completely engulf MWCNT-20 μm (and associated protruding MWCNTs, accompanied by membrane blebbing and severe membrane distortion), strongly indicating that AMs are not comfortable dealing with such high aspect ratio materials. This is further evidenced by the absence of such responses with AMs exposed to the shorter MWCNT-0.6 μm. Furthermore, 1 and 5 days posttreatment with MWCNT-20 μm, AM viability was significantly reduced, while the viability of AMs treated with MWCNT-0.6 μm was unchanged at these time points.
Phagocytosis of particles has been shown to involve increased superoxide and associated increased superoxide anion release by AMs. Even though macrophages fail to internalize high aspect ratio fibers, there is still increased release of cellular superoxide,17 possibly due to membrane damage or loss of cellular integrity allowing release of the elevated intracellular superoxide anion. This could in turn lead to oxidative damage of neighboring AMs or alveolar epithelial cells. In the present study, we observed significantly increased intracellular AM superoxide levels, 1 and 5 days after treatment with MWCNT-20 μm but not MWCNT-0.6 μm. The inflammatory mediators IL-1β and TNF-α are putatively involved in the adverse bioreactivity of carbon nanotubes with macrophages.29,30 However, following treatment of AMs with MWCNTs used in the present study, we did not observe any differences in the release of these mediators compared to the nontreated control AMs (data not shown). This may be due to the fact that we did not treat these AMs with priming agents, such as lipopolysaccharide, which in unpublished studies we have found to be necessary; thus the associated inflammasome pathway may not have been activated.
Previous research by us on the interactions between AM and MWCNT showed marked increases in IL-6 and IL-8 release, following 24-hour treatment with MWCNT-20 μm and, to a lesser extent, MWCNT-0.6 μm.21 Importantly, in the present study, we observed that IL-6 release by AMs was still significantly increased, 1 and 5 days after treatment with MWCNT-20 μm. IL-8 release by AMs was also significantly increased 1 day after treatment with MWCNT-20 μm; interestingly however, this increase had resolved at 5 days posttreatment time point. IL-6 is a pleiotropic cytokine associated with the pathogenesis of numerous lung diseases such as asthma, chronic obstructive pulmonary disease (COPD), and idiopathic pulmonary fibrosis,31,32 while IL-8 is a potent chemoattractant for neutrophils and is generally associated with acute inflammation33 but which is also elevated in chronic inflammatory lung diseases, such as COPD, where the elevated circulating levels of IL-6 and IL-8 are proposed to be predominantly AM derived,34,35 therefore there are important pathological consequences for increases in these mediators from MWCNT-treated AMs.
For those AMs that remained viable 1 and 5 days after the 24-hour treatment with MWCNT-0.6 μm or MWCNT-20 μm, we were interested in determining their functional capabilities in terms of bacterial phagocytosis (important for maintaining alveolar sterility) and migration (important for removal of phagocytosed material from the alveolar unit). Using fluorescein-labeled E. coli to measure phagocytosis, we discovered that there was no significant difference between phagocytosis by macrophages exposed to MWCNT-0.6 μm and untreated macrophages 5 days following exposure; the short MWCNTs inhibited phagocytosis at day 1 only by ~15%, indicating that treatment with the short MWCNT had no major, long-lasting effect on phagocytosis, correlating with the lack of induction of cell death by these MWCNT. In contrast, parallel studies of the effects of MWCNT-20 μm on macrophage phagocytosis resulted in markedly reduced phagocytosis compared to untreated control, which fell to only 27% and 15% that of untreated control cells on days 1 and 5, respectively. By comparison, the viability of these cells was 72% and 49% of control at these times, proportionally significantly higher than the phagocytic activity (Figure S1), indicating that the ability to phagocytose microbes was markedly inhibited by the long MWCNTs and the effect was chronic. The process of macrophage phagocytosis is still in discovery.36 However, there are a number of extracellular receptors known to be important in initiating the process, including TLR4 and scavenger receptors.37
Consequently, we were interested to see if the marked, chronic reduction in AM phagocytic capacity in the present study could be explained by differential expression of some of these extracellular receptors. There was a slight, nonsignificant, increase in TLR4 expression (important in recognition of lipopolysaccharide from Gram-negative bacteria) at 1 day following the 24-hour treatment with MWCNT-0.6 μm; however, in contrast, the scavenger receptors MARCO, MSR-1, and CD36 (important in recognition of nonopsonized material) were all significantly increased at the same time point. Five days after treatment with MWCNT-0.6 μm, there were no significant changes in the expression of any of the four receptors analyzed. In striking contrast, at 1 and 5 days after treatment with MWCNT-20 μm, MARCO expression was markedly increased while there were no significant changes for any of the other receptors at either time point. Given the significant reduction in E. coli phagocytosis, we expected to see a corresponding decrease in extracellular TLR4 expression; however, this was not the case. The upregulation of MARCO was particularly interesting, however, and this response may partly explain the decreased E. coli phagocytosis, as AMs may concentrate on phagocytosis of the long MWCNT rather than the bacteria. MARCO has been shown to be a critical AM receptor for nonopsonized particles, in both rodent and human AM studies.38,39 Using J774.1 murine macrophages, Hirano et al40 reported that MWCNTs (of unspecified length or functionalization; 67 nm in diameter) may elicit cytotoxic effects by reacting with MARCO on the plasma membrane and rupturing the plasma membrane. Wang et al41 showed that apoptosis in RAW 264.7 murine macrophages, induced by MWCNTs (~0.5 μm in length, 10 nm diameter; acid-treated and taurine-functionalized), could be abated by pre-treating the cells with a scavenger receptor Class A (which includes MARCO) specific neutralizing antibody. In agreement with these studies, the marked and sustained (at day 5 postexposure) increase in MARCO expression for AMs treated with MWCNT-20 μm in the present study indicates a key role for this receptor in AM–MWCNT interactions. An important study by Gao et al demonstrated that THP-1 macrophage recognition of MWCNTs can be changed by modification of their surface chemistry; the authors showed that increased binding of modified MWCNTs to scavenger receptors caused a reduction in MWCNT binding to mannose receptors, thus reducing inflammatory nuclear factor-κB activation and immunotoxicity in the THP-1 macrophages and in vivo in mice.42 Critically however, in the present study, we have shown that AMs that modify their receptor expression in favor of MARCO–MWCNT interactions may consequently have reduced microbial phagocytic capacity. Defective AM phagocytosis is seen in a number of chronic lung diseases (including COPD, asthma, and cystic fibrosis) and this potentially leads to increased bacterial colonization and increased frequency of bacterial-induced exacerbations of the diseases.14,43 The fact that we observed remarkably reduced E. coli phagocytosis, 5 days after extracellular MWCNT-20 μm were removed, indicates potential for development of similar pathological complications in individuals exposed to such materials.
This problem likely would be exacerbated by the persistence of MWCNTs in the alveolar space, because of reduced clearance of long, high aspect ratio, MWCNT. It is generally accepted that AMs laden with phagocytosed material must migrate out of the alveolar space to begin eventual clearance through proximal lymph nodes or via the mucociliary escalator.44 AMs have previously been shown to migrate toward complement component C5a.28,45,46 We therefore chose the zymosan-activated FBS (a rich source of C5a) method to measure migratory capacity. We observed that the migratory capacity of AMs did not significantly change at 1 or 5 days after the 24-hour treatment with MWCNT-0.6 μm; however, at 1 and 5 days after treatment with MWCNT-20 μm, AM migratory capacity was markedly decreased.
As outlined earlier in the observations of E. coli phagocytosis, any changes in migration should be considered in the context of the degree of cell death that occurred at the same time interval. Again, there was little effect of the short MWCNTs on macrophage migration. In contrast, exposure to long MWCNTs resulted in only 51% and 24% cell migration at days 1 and 5, in comparison to untreated control. This compares with 72% and 51% cell viability. Thus, the fall in cell migration is significantly more than the fall in cell death at the same time interval (Figure S1), indicating that, in addition to cell death, long MWCNTs cause a reduction in macrophage migration. Combined with the observed increased ROS generation and inflammatory mediator release and reduced microbial phagocytosis, the observed reduction in AM migratory capacity depicts an unfavorable situation for the alveolar unit and protection of its delicate epithelium. Such a high aspect ratio MWCNT exposure scenario could lead to cyclical episodes of attempted AM phagocytosis of the material, followed by AMs becoming stressed, their function being compromised, recruitment of more AMs, and an inability to migrate out of the alveolar space for clearance.
Conclusion
Our study reveals that treatment with long, but not short, MWCNTs can affect AM function. Our results clearly demonstrate that 1 and 5 days after treating AMs with long MWCNTs for 24 hours, AMs continue to exhibit increased markers of adverse bioreactivity (cell death, ROS generation, and inflammatory mediator release), while they also have a reduced ability to phagocytose bacteria and a limited migratory capacity. This is in contrast to shorter MWCNTs, where these effects are substantially less marked or absent altogether. Given the complexity and diversity of MWCNT exposure scenarios, it is difficult to extrapolate the significance of the MWCNT concentration examined in this in vitro study. Nevertheless, these findings, using primary human AMs, have important implications for the design and application of MWCNTs, and future studies are needed to clearly elucidate the in vivo relevance of the differential interactions presented here.
Acknowledgments
Financial support for SS from Unilever, UK, is gratefully acknowledged. PR is supported by the Leverhulme Trust, UK. We thank Dr Katerina Kouravelou at Nanothinx, Greece, for her generous advice throughout the study. We also thank Dr Andrew V Rogers (Royal Brompton Hospital, London) for assistance and expert advice on cell imaging using scanning electron microscopy. This research was supported by the Medical Research Council and Public Health England, Center for Environment and Health. The project was also supported by the NIHR Respiratory Disease Biomedical Research Unit at the Royal Brompton and Harefield NHS Foundation Trust and Imperial College London. The views expressed in this publication are those of the authors(s) and not necessarily those of the NHS, the National Institute for Health Research, or the Department of Health.
Disclosure
The authors report no conflicts of interest in this work.
References
De Volder MF, Tawfick SH, Baughman RH, Hart AJ. Carbon nanotubes: present and future commercial applications. Science. 2013;339(6119):535–539. doi:10.1126/science.1222453. | ||
Schnorr JM, Swager TM. Emerging applications of carbon nanotubes. Chem Mater. 2011;23(3):646–657. doi:10.1021/cm102406h. | ||
Wohlleben W, Brill S, Meier MW, et al. On the lifecycle of nanocomposites: comparing released fragments and their in vivo hazards from three release mechanisms and four nanocomposites. Small. 2011;7(16):2384–2395. doi:10.1002/smll.201002054. | ||
Donaldson K, Tran CL. An introduction to the short-term toxicology of respirable industrial fibres. Mutat Res. 2004;553(1–2):5–9. doi:10.1016/j.mrfmmm.2004.06.011. | ||
Donaldson K. The inhalation toxicology of p-aramid fibrils. Crit Rev Toxicol. 2009;39(6):487–500. doi:10.1080/10408440902911861. | ||
World Health Organization. Determination of Airborne Fibre Number Concentrations: A Recommended Method, by Phase-contrast Optical Microscopy (Membrane Filter Method). Geneva, Switzerland: WHO; 1997. | ||
Bailey M. The new ICRP model for the respiratory tract. Radiat Prot Dosimetry. 1994;53:107–114. | ||
Jaques PA, Kim CS. Measurement of total lung deposition of inhaled ultrafine particles in healthy men and women. Inhal Toxicol. 2000;12(8):715–731. doi:10.1080/08958370050085156. | ||
Daigle CC, Chalupa DC, Gibb FR, et al. Ultrafine particle deposition in humans during rest and exercise. Inhal Toxicol. 2003;15(6):539–552. doi:10.1080/08958370304468. | ||
Kennedy GL, Kelly DP. Introduction to fiber toxicology. In: Warheit D, editor. Fiber Toxicology. San Diego, CA: Academic Press; 1993:15–42. | ||
Miyata R, van Eeden SF. The innate and adaptive immune response induced by alveolar macrophages exposed to ambient particulate matter. Toxicol Appl Pharmacol. 2011;257(2):209–226. doi:10.1016/j.taap.2011.09.007. | ||
Thorley AJ, Grandolfo D, Lim E, Goldstraw P, Young A, Tetley TD. Innate immune responses to bacterial ligands in the peripheral human lung – role of alveolar epithelial TLR expression and signalling. PLoS One. 2011;6(7):e21827. doi:10.1371/journal.pone.0021827. | ||
Chavez-Santoscoy AV, Roychoudhury RR, Pohl NL, Wannemuehler MJ, Narasimhan B, Ramer-Tait AE. Tailoring the immune response by targeting C-type lectin receptors on alveolar macrophages using “pathogen-like” amphiphilic polyanhydride nanoparticles. Biomaterials. 2012;33(18):4762–4772. doi:10.1016/j.biomaterials.2012.03.027. | ||
Donnelly LE, Barnes PJ. Defective phagocytosis in airways disease. Chest. 2012;141(4):1055–1062. doi:10.1378/chest.11-2348. | ||
Baritussio A, Alberti A, Armanini D, Meloni F, Bruttomesso D. Different pathways of degradation of SP-A and saturated phosphatidylcholine by alveolar macrophages. Am J Physiol Lung Cell Mol Physiol. 2000;279(1):L91–L99. | ||
Oberdörster G, Ferin J, Gelein R, Soderholm S, Finkelstein J. Role of the alveolar macrophage in lung injury: studies with ultrafine particles. Environ Health Perspect. 1992;97:193. | ||
Hill IM, Beswick PH, Donaldson K. Differential release of superoxide anions by macrophages treated with long and short fibre amosite asbestos is a consequence of differential affinity for opsonin. Occup Environ Med. 1995;52(2):92–96. | ||
Renwick LC, Brown D, Clouter A, Donaldson K. Increased inflammation and altered macrophage chemotactic responses caused by two ultrafine particle types. Occup Environ Med. 2004;61(5):442–447. | ||
Ryman-Rasmussen JP, Tewksbury EW, Moss OR, Cesta MF, Wong BA, Bonner JC. Inhaled multiwalled carbon nanotubes potentiate airway fibrosis in murine allergic asthma. Am J Respir Cell Mol Biol. 2008;40(3):349–358. doi:10.1165/rcmb.2008-0276OC. | ||
Ma-Hock L, Treumann S, Strauss V, et al. Inhalation toxicity of multi-wall carbon nanotubes in rats exposed for 3 months. Toxicol Sci. 2009;112(2):468–481. doi:10.1093/toxsci/kfp146. | ||
Sweeney S, Berhanu D, Misra SK, Thorley AJ, Valsami-Jones E, Tetley TD. Multi-walled carbon nanotube length as a critical determinant of bioreactivity with primary human pulmonary alveolar cells. Carbon N Y. 2014;78(C):26–37. doi:10.1016/j.carbon.2014.06.033. | ||
Witherden IR, Tetley TD. Isolation and culture of human alveolar type II pneumocytes. Methods Mol Med. 2001;56:137–146. doi:10.1385/1-59259-151-5:137. | ||
Thorley AJ, Goldstraw P, Young A, Tetley TD. Primary human alveolar type II epithelial cell CCL20 (macrophage inflammatory protein-3alpha)-induced dendritic cell migration. Am J Respir Cell Mol Biol. 2005;32(4):262–267. doi:10.1165/rcmb.2004-0196OC. | ||
Payne JP, Kemp SJ, Dewar A, et al. Effects of airborne World Trade Center dust on cytokine release by primary human lung cells in vitro. J Occup Environ Med. 2004;46(5):420–427. doi:10.1097/01.jom.0000126021.25149.64. | ||
Ryman-Rasmussen JP, Cesta MF, Brody AR, et al. Inhaled carbon nanotubes reach the subpleural tissue in mice. Nat Nanotechnol. 2009; 4(11):747–751. doi:10.1038/nnano.2009.305. | ||
Snelgrove RJ, Goulding J, Didierlaurent AM, et al. A critical function for CD200 in lung immune homeostasis and the severity of influenza infection. Nat Immunol. 2008;9(9):1074–1083. doi:10.1038/ni.1637. | ||
Budd SL, Castilho RF, Nicholls DG. Mitochondrial membrane potential and hydroethidine-monitored superoxide generation in cultured cerebellar granule cells. FEBS Lett. 1997;415(1):21–24. | ||
Warheit DB, George G, Hill LH, Snyderman R, Brody AR. Inhaled asbestos activates a complement-dependent chemoattractant for macrophages. Lab Invest. 1985;52(5):505–514. | ||
Palomäki J, Välimäki E, Sund J, et al. Long, needle-like carbon nanotubes and asbestos activate the NLRP3 inflammasome through a similar mechanism. ACS Nano. 2011;5(9):6861–6870. doi:10.1021/nn200595c. | ||
Hamilton RF, Buford M, Xiang C, Wu N, Holian A. NLRP3 inflammasome activation in murine alveolar macrophages and related lung pathology is associated with MWCNT nickel contamination. Inhal Toxicol. 2012;24(14):995–1008. doi:10.3109/08958378.2012.745633. | ||
Pantelidis P, Fanning GC, Wells AU, Welsh KI, Bois Du RM. Analysis of tumor necrosis factor-α, lymphotoxin-α, tumor necrosis factor receptor II, and interleukin-6 polymorphisms in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2001;163(6):1432–1436. | ||
Celli BR, Locantore N, Yates J, et al. Inflammatory biomarkers improve clinical prediction of mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;185(10):1065–1072. doi:10.1164/rccm.201110-1792OC. | ||
Harada A, Sekido N, Akahoshi T, Wada T, Mukaida N, Matsushima K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J Leukoc Biol. 1994;56(5):559–564. | ||
Tetley TD. Macrophages and the pathogenesis of COPD. Chest. 2002; 121(5 Suppl):156S–159S. | ||
Barnes PJ. Alveolar macrophages as orchestrators of COPD. COPD. 2004;1(1):59–70. doi:10.1081/COPD-120028701. | ||
Karavitis J, Kovacs EJ. Macrophage phagocytosis: effects of environmental pollutants, alcohol, cigarette smoke, and other external factors. J Leukoc Biol. 2011;90(6):1065–1078. doi:10.1189/jlb.0311114. | ||
Taylor PR, Martinez-Pomares L, Stacey M, Lin H-H, Brown GD, Gordon S. Macrophage receptors and immune recognition. Annu Rev Immunol. 2005;23:901–944. doi:10.1146/annurev.immunol.23.021704.115816. | ||
Palecanda A, Paulauskis J, Al-Mutairi E, et al. Role of the scavenger receptor MARCO in alveolar macrophage binding of unopsonized environmental particles. J Exp Med. 1999;189(9):1497–1506. | ||
Arredouani MS, Palecanda A, Koziel H, et al. MARCO is the major binding receptor for unopsonized particles and bacteria on human alveolar macrophages. J Immunol. 2005;175(9):6058–6064. doi:10.4049/jimmunol.175.9.6058. | ||
Hirano S, Kanno S, Furuyama A. Multi-walled carbon nanotubes injure the plasma membrane of macrophages. Toxicol Appl Pharmacol. 2008;232(2):244–251. doi:10.1016/j.taap.2008.06.016. | ||
Wang X, Guo J, Chen T, et al. Multi-walled carbon nanotubes induce apoptosis via mitochondrial pathway and scavenger receptor. Toxicol In Vitro. 2012;26(6):799–806. doi:10.1016/j.tiv.2012.05.010. | ||
Gao N, Zhang Q, Mu Q, et al. Steering carbon nanotubes to scavenger receptor recognition by nanotube surface chemistry modification partially alleviates NFκB activation and reduces its immunotoxicity. ACS Nano. 2011;5(6):4581–4591. doi:10.1021/nn200283g. | ||
Berenson CS, Kruzel RL, Eberhardt E, Sethi S. Phagocytic dysfunction of human alveolar macrophages and severity of chronic obstructive pulmonary disease. J Infect Dis. 2013;208(12):2036–2045. doi:10.1093/infdis/jit400. | ||
Gehr P, Mühlfeld C, Rothen-Rutishauser B, Blank F. Particle-Lung Interactions. 2nd ed. New York, NY: Informa Healthcare USA, Inc.; 2010:1–339. | ||
Warheit DB, Overby LH, George G, Brody AR. Pulmonary macrophages are attracted to inhaled particles through complement activation. Exp Lung Res. 1988;14(1):51–66. | ||
Barlow PG, Brown DM, Donaldson K, Maccallum J, Stone V. Reduced alveolar macrophage migration induced by acute ambient particle (PM10) exposure. Cell Biol Toxicol. 2007;24(3):243–252. doi:10.1007/s10565-007-9033-y. |
Supplementary material
 © 2015 The Author(s). This work is published by Dove Medical Press Limited, and licensed under a
Creative Commons Attribution License.
The full terms of the License are available at http://creativecommons.org/licenses/by/4.0/.
The license permits unrestricted use, distribution, and reproduction in any medium, provided the
original author and source are credited.
© 2015 The Author(s). This work is published by Dove Medical Press Limited, and licensed under a
Creative Commons Attribution License.
The full terms of the License are available at http://creativecommons.org/licenses/by/4.0/.
The license permits unrestricted use, distribution, and reproduction in any medium, provided the
original author and source are credited.