Back to Journals » Drug Design, Development and Therapy » Volume 11
Functional and morphological effects of diazepam and midazolam on tumor vasculature in the 9L gliosarcoma brain tumor model using dynamic susceptibility contrast MRI: a comparative study
Authors Yan N, Zheng Y, Yang C
Received 13 June 2017
Accepted for publication 15 August 2017
Published 4 October 2017 Volume 2017:11 Pages 2931—2936
DOI https://doi.org/10.2147/DDDT.S143838
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sukesh Voruganti
Nuo Yan,1 Yuzhen Zheng,2 Cheng Yang1
1Second Department of Anesthesiology, The Affiliated Hospital to Logistics University of PAP, Tianjin, 2Department of Anesthesiology, Tianjin Huanhu Hospital, Tianjin, China
Abstract: Antiangiogenic therapy attenuates tumor growth by reducing vascularization. Diazepam (DZP) and midazolam (MZL) have antiangiogenic properties in human umbilical vein endothelial cells. Thus, we investigated the antiangiogenic activity of DZP and MZL in the rat 9L gliosarcoma brain tumor model. The effect on tumor vasculature was evaluated using dynamic susceptibility contrast magnetic resonance imaging with gradient-echo (GE) and spin-echo (SE) to assess perfusion parameters, including cerebral blood volume (CBV), cerebral blood flow (CBF), mean transit time (MTT), and mean vessel diameter. The GE-normalized CBF (nCBF) in the tumors of untreated controls was significantly lower than that in normal brain tissue, whereas the CBV and MTT were higher. DZP- and MZL-treated rats had higher CBF and lower CBV and MTT values than did untreated controls. The tumor size decreased significantly to 33.5% in DZP-treated rats (P<0.001) and 22.5% in MZL-treated rats (P<0.01) relative to controls. The SE-normalized CBV was lower in DZP-treated (32.9%) and MZL-treated (10.6%) rats compared with controls. The mean vessel diameter decreased significantly by 32.5% in DPZ-treated and by 24.9% in MZL-treated rats compared with controls (P<0.01). The GE and SE nCBF values were higher in DZP-treated (49.9% and 40.1%, respectively) and MZL-treated (41.2% and 32.1%, respectively) rats than in controls. The GE- and SE-normalized MTTs were lower in DZP-treated (48.2% and 59.8%, respectively) and MZL-treated (40.5% and 51.2%, respectively) rats than in controls. Both DZP and MZL had antiangiogenic effects on tumor perfusion and vasculature; however, the antiangiogenic activity of DZP is more promising than that of MZL.
Keywords: diazepam, midazolam, 9L gliosarcoma, antiangiogenic, DSC-MRI
Introduction
Antiangiogenic therapy has been shown to reduce the density and growth of tumor vessels and increase the overall effectiveness of conventional therapies in several animal tumor models.1,2 The therapy reduces tumor vascularization and perfusion prior to the administration of chemotherapy.3,4 A previous study found that tumor perfusion improved in murine fibrosarcoma-bearing mice after 2 days of thalidomide treatment.5 Findings from animal studies indicate that the sprouting of new vessels from surrounding tumor vessels is a major contributor to tumor growth,6 suggesting that inhibition of the sprouting process, referred to as angiogenesis, would suppress tumor growth.
Diazepam (DZP) and midazolam (MZL) are benzodiazepine drugs that are used as anesthetics clinically and in intensive care units.7,8 Benzodiazepines induce anesthesia/sedation by potentiating the inhibitory neurotransmitter gamma amino-butyric acid.9 A recent study found that DZP and MZL had antiangiogenic effects in human umbilical vein endothelial cells.10
Magnetic resonance imaging (MRI) has emerged as a potential technique for measuring several characteristics of tissue vasculature because of its sensitivity to endogenous and exogenous contrast mechanisms. Tissue blood flow can be measured using arterial spin labeling, which relies on endogenous contrast.11,12 Susceptibility-based MRI techniques such as dynamic susceptibility contrast MRI (DSC-MRI) are used to measure mean transit time (MTT), cerebral blood flow (CBF), mean vessel diameter (mVD), and cerebral blood volume (CBV).13 MRI-derived CBV values are useful for differentiating between grades of glial tumors and for predicting survival.14–16 mVD values have been shown to be correlated with brain tumor grades.15 MRI measurements of CBF and CBV provide information about vascular morphology and are useful for designing the optimal treatment for tumors.17,18
To date, no studies have investigated the antiangiogenic effects of DZP and MZL on tumor vasculature and hemodynamics. We hypothesized that DZP and MZL administration would alter the hemodynamic parameters of blood vessels in the rat 9L gliosarcoma brain tumor model. Thus, we used DSC-MRI to investigate DZP- and MZL-induced changes in the morphology and function of 9L brain tumor vessels. Specifically, we used DSC-MRI-guided CBV mapping with mVD studies to investigate the morphological dynamics of tumor angiogenesis with a focus on developing novel strategies for tumor treatment.
Materials and methods
9L gliosarcoma cell lines and inoculation in rats
We used 24 male Sprague–Dawley rats (Harlan, Indianapolis, IN, USA) in this study. All animal protocols were developed in accordance with the guidelines of the Institutional Ethics Committee of the Affiliated Hospital of Logistics University of Chinese People’s Armed Police Force. The Institutional Ethics Committee approved the experiments and the use of 9L gliosarcoma cells obtained from Massachusetts General Hospital (Boston, MA, USA). The rats were anesthetized with sodium pentobarbital (50 mg/kg, intraperitoneal [ip]) and immobilized. A 1-mm burr hole was drilled in the skull 2 mm lateral and 1 mm anterior to bregma, and then the animals were inoculated with 1×105 9L cells with a 10-μL gas-tight syringe. The cells were implanted 3 mm below the dural surface. The total duration of the injection was 5 min, and the syringe needle was gently retracted over a 5-min period. A surgical staple was used to close the skin, and the pins were removed before MRI.
The 9L cells were used to induce brain tumors in the rats. The cells were subjected to seeding in Dulbecco’s medium (Gibco, Grand Island, NY, USA) and 10% fetal calf serum. The cell lines were expanded and maintained with Protoprobe (ProtoPROBE Inc., Milwaukee, WI, USA) prior to inoculation. The cells were shaken manually before each inoculation.
DZP and MZL treatment
The rats were divided into DZP-treated (n=8), MZL-treated (n=8), and control vehicle-treated (n=8) groups. Ten days after inoculation with 9L gliosarcoma cells, the rats received ip injections of DZP (2 mg/kg) or MZL (5 mg/kg) over 4 days (ie, from days 10 to 14). On day 14, the rats underwent DSC-MRI.
MRI experiments
After 14 days of 9L gliosarcoma cell inoculation, the rats were subjected to MRI using a Bruker Medspec 3T MRI system (Ettlingen, Baden-Württemberg, Germany) as described previously.18 The rats were administered an intravenous (iv) loading dose of gadolinium-chelated contrast agent (0.05 mM/kg, Gadoteridol, ProHance; Bracco Diagnostics, Princeton, NJ, USA) prior to obtaining images localizing the tumors. Gadolinium was administered to reduce T1 effects caused by contrast agent extravasation. Then, coronal images were acquired using a simultaneous gradient-echo/spin-echo (GE/SE) echo-planar imaging pulse sequence (matrix size: 64×64, repetition time [TR] =1 s, GE, echo time [TE] =20 ms; SE [TE] =96 ms, field of view [FOV] =3.5 cm, slice thickness =2 mm, three slices) for 2 min. A bolus of contrast media (0.2 mmol/kg, iv) was injected for 60 s. The GE and SE signals were recorded simultaneously because the images showed a varied and complementary sensitivity profile corresponding to the diameter of the vessels: the SE images were highly sensitive to capillary blood volume, whereas the GE images were sensitive to vascular blood volume.19 The SE and GE images were used to evaluate the hemodynamic parameters for DSC-MRI as follows: T1-weighted SE images, TR =450 ms, matrix size =256×256, FOV =3.5 cm, TE =15 ms, slice thickness =2 mm, three slices.
Image analysis
The concentration of contrast agent in each voxel was estimated by the correlation between the change in GE and SE transverse relaxation rates (ΔR2* and ΔR2) after contrast injection and the concentration of the contrast media. The curves for ΔR2* and ΔR2 were calculated as the logarithm of the GE and SE MRI signal intensities, respectively. The vascular and microvascular hemodynamic parameters were calculated using a tracer kinetic analysis of the concentration–time curves. Briefly, the arterial input functions (AIF) of GE and SE were estimated in the arterial branches of normal contralateral tissue. The tissue residue function was obtained by deconvolving the tissue concentration time curves with the AIF using singular value decomposition.20 CBF was calculated as the residue function peak, and MTT was derived from the central volume principle as the ratio of CBV to CBF. CBF and MTT maps were normalized for comparison. Finally, intravoxel transit-time distributions (TTDs) were estimated from the residue function in each voxel.21,22 TTD differences between the tumor and the corresponding normal tissue were determined by estimating the cumulative TTD in a given voxel, TTDs as a function of transit time, and the normal cumulative TTD values.
Region of interest and statistical data analyses
Mean values for the selected parameters were obtained from a region of interest (ROI) placed over the enhancing tumor region as visualized on the T1-weighted SE image after the injection of gadolinium. The ROI values were normalized to a region of the same size in a normal brain. One-way analyses of variance were used to compare parameters among the DZP, MZL, and control groups.
Results
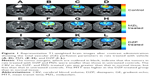
T1-weighted brain images of rats treated with DZP (2 mg/kg), MZL (5 mg/kg), or neither (untreated controls) were obtained after contrast administration for the assessment of total vascular (GE) CBF, CBV, mVD, and MTT (Figure 1). The tumor size, as determined by the contrast-enhancing region, was significantly smaller in the DZP and MZL treatment groups than in the untreated control group. The GE-normalized CBF (nCBF) in the untreated control tumors was significantly lower than that in normal brain tissue (29.03% and 33.33% lower compared with the MZL- and DZP-treated rats, respectively), whereas CBV and MTT were higher in the untreated controls than in the MZL- (40%) and DZP-treated (48%) rats (P<0.001). CBF was higher in the tumors of rats treated with DZP than in those of rats treated with MZL, and in some areas the CBF did not differ from that of normal tissue. CBV and MTT were lower in DZP-treated than in MZL-treated rats.
Tumor size, measured as the percent enhancement (PE) area, in the DZP-treated, MZL-treated, and untreated control rats is shown in Figure 2. The mean PE area decreased by 33% in DZP-treated rats (P<0.001) and by 22.5% in MZL-treated rats (P<0.01) relative to the untreated controls. These findings suggest that treatment with DZP and MZL had a highly significant effect on tumor size.
The mean GE-normalized CBV (nCBV) was reduced to 28.3% in DZP-treated rats (P<0.01) and to 15.8% in MZL-treated rats relative to control values (Figure 3A). The SE nCBV values were lower in the DZP (32.9%) and MZL (10.6%) groups relative to control values (Figure 3A). While our findings clearly indicate that DZP and MZL interfered with the formation of new microvessels, the fact that the effect of DZP was statistically significant suggests that the drug was more effective than MZL. The mVD decreased by 32.5% in DZP-treated and by 24.9% in MZL-treated rats compared with controls (P<0.001 for both; Figure 3A).
The mean GE and SE nCBF values of rats treated with DZP (49.9% and 40.1%, respectively) and MZL (41.2% and 32.1%, respectively) were higher relative to those of control rats (Figure 3B).
The normalized MTT (nMTT) in the control group was higher than that in normal rats (Figure 3C). The GE and SE nMTT values in rats treated with DZP (48.2% and 59.8%, respectively) and MZL (40.5% and 51.2%, respectively) were lower than those of untreated control rats.
Discussion
We used the simultaneous GE/SE DSC-MRI method to investigate the effects of DZP and MZL on tumor angiogenesis. DZP (2 mg/kg) and MZL (5 mg/kg) inhibited growth, decreased blood flow and MTT, and reduced blood volume in response to tumor angiogenesis; however, the attenuating effects of DZP were greater than those of MZL.
Previous studies have shown that antiangiogenic therapy decreases blood flow in tumors. Bevacizumab, an antiangiogenic agent, has been reported to reduce blood flow and volume in gliomas,23 and DC101 decreased tumor perfusion at low and high doses in pancreatic tumor xenografts.24 Furthermore, DC101 decreased interstitial fluid pressure in solid tumors by producing a morphologically and functionally “normalized” vascular network.4 Our findings are consistent with those of previous studies; in addition, we found that DZP inhibited the growth of vascular networks, attenuated vascular function, and improved blood perfusion in the tumor resulting in an overall decrease in transit time.
DSC-MRI revealed the antiangiogenic potential of DZP and MZL. Moreover, we found that DZP was more effective than MZL in decreasing the mVD and increasing tumor blood flow. The low CBV values in rats treated with DZP (2 mg/kg) and MZL (5 mg/kg) suggest a role of nutrient deficiency in the inhibition of tumor growth; however, the effect of DZP was more promising than that of MZL. Our finding that both DZP and MZL increased blood flow in the tumor suggests a novel strategy to enhance the effectiveness of available chemotherapeutic agents.
The CBV and MTT values in normal brain tissue may serve as standards for the amount of blood required to ensure adequate tissue oxygenation.25,26 The metabolic demands of tumors are thought to be greater than those of normal tissue, and, as such, would require an increased blood volume and MTT. Therefore, we speculate that the elevated MTT in the untreated tumor-induced control rats is indicative of longer erythrocyte residue times in the tumor vessels. The decreases in CBV and MTT in tumor-induced rats treated with DZP and MZL suggest that immature and unwanted blood vessels may have been trimmed, resulting in improved blood flow. The exchange of nutrients and oxygen occurs in the microvasculature; thus, decreases in SE nMTT and GE and SE nCBV may be an alternative indicator of vascular normalization following antiangiogenic therapy.
In conclusion, both DZP and MZL increased perfusion in the tumor vasculature. Our findings suggest that the antiangiogenic activity of DZP exceeds that of MZL. Our findings provide direction for future research into improving the delivery of cytotoxic agents to tumor tissue. In future studies, we plan to use DSC-MRI to evaluate the vascular hemodynamics of other cytotoxic agents as well as DZP and MZL to confirm our findings and further investigate the mechanisms underlying this antiangiogenic activity.
Disclosure
The authors report no conflicts of interest in this work.
References
Abrams TJ, Murray LJ, Pesenti E, et al. Preclinical evaluation of the tyrosine kinase inhibitor SU11248 as a single agent and in combination with “standard of care” therapeutic agents for the treatment of breast cancer. Mol Cancer Ther. 2003;2(10):1011–1021. | ||
Laird AD, Vajkoczy P, Shawver LK, et al. SU6668 is a potent antiangiogenic and antitumor agent that induces regression of established tumors. Cancer Res. 2000;60(15):4152–4160. | ||
Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9(6):685–693. | ||
Tong RT, Boucher Y, Kozin SV, Winkler F, Hicklin DJ, Jain RK. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004;64(11):3731–3736. | ||
Ansiaux R, Baudelet C, Jordan B, et al. Changes in the tumor microenvironment early after treatment with the anti-angiogenic agent thalidomide. In: Proceedings of the 11th Annual Meeting of the ISMRM; Kyoto, Japan; 2004. | ||
Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182–1186. | ||
Young CC, Prielipp RC. Benzodiazepines in the intensive care unit. Crit Care Clin. 2001;17(4):843–862. | ||
Payen JF, Chanques G, Mantz J, et al. Current practices in sedation and analgesia for mechanically ventilated critically ill patients: A prospective multicenter patient-based study. Anesthesiology. 2007;106(4):687–695; quiz 891–892. | ||
Stevanovic P. [Midazolam-clinical practice guidelines.] Med Pregl. 2006;59(1–2):89–94. Serbian. | ||
Nan YS, Li SY, Kang JL, Suzuki S, Ema Y, Nishiwaki K. Effects of midazolam, diazepam, propofol and dexmedetomidine on endothelial cell proliferation and angiogenesis induced by VEGF. Afr J Microbiol Res. 2010;4(23):2549–2555. | ||
Weber MA, Zoubaa S, Schlieter M, et al. Diagnostic performance of spectroscopic and perfusion MRI for distinction of brain tumors. Neurology. 2006;66(12):1899–1906. | ||
Wolf RL, Wang J, Wang S, et al. Grading of CNS neoplasms using continuous arterial spin labeled perfusion MR imaging at 3 Tesla. J Magn Reson Imaging. 2005;22(4):475–482. | ||
Morakkabati N, Leutner CC, Schmiedel A, Schild HH, Kuhl CK. Breast MR imaging during or soon after radiation therapy. Radiology. 2003;229(3):893–901. | ||
Aronen HJ, Gazit IE, Louis DN, et al. Cerebral blood volume maps of gliomas: comparison with tumor grade and histologic findings. Radiology. 1994;191(1):41–51. | ||
Donahue KM, Krouwer HG, Rand SD, et al. Utility of simultaneously acquired gradient-echo and spin-echo cerebral blood volume and morphology maps in brain tumor patients. Magn Reson Med. 2000;43(6):845–853. | ||
Sugahara T, Korogi Y, Kochi M, Ushio Y, Takahashi M. Perfusion-sensitive MR imaging of gliomas: comparison between gradient-echo and spin-echo echo-planar imaging techniques. AJNR Am J Neuroradiol. 2001;22(7):1306–1315. | ||
Gee MS, Saunders HM, Lee JC, et al. Doppler ultrasound imaging detects changes in tumor perfusion during antivascular therapy associated with vascular anatomic alterations. Cancer Res. 2001;61(7):2974–2982. | ||
Ostergaard L, Sorensen AG, Chesler DA, et al. Combined diffusion-weighted and perfusion-weighted flow heterogeneity magnetic resonance imaging in acute stroke. Stroke. 2000;31(5):1097–1103. | ||
Quarles CC, Krouwer HG, Rand SD, Schmainda KM. Dexamethasone normalizes brain tumor hemodynamics as indicated by dynamic susceptibility contrast MRI perfusion parameters. Technol Cancer Res Treat. 2005;4(3):245–249. | ||
Boxerman JL, Hamberg LM, Rosen BR, Weisskoff RM. MR contrast due to intravascular magnetic susceptibility perturbations. Mag Reson Med. 1995;34(4):555–566. | ||
Ostergaard L, Weisskoff RM, Chesler DA, Gyldensted C, Rosen BR. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part I: Mathematical approach and statistical analysis. Magn Reson Med. 1996;36(5):715–725. | ||
Ostergaard L, Chesler DA, Weisskoff RM, Sorensen AG, Rosen BR. Modeling cerebral blood flow and flow heterogeneity from magnetic resonance residue data. J Cereb Blood Flow Metab. 1999;19(6):690–699. | ||
Keunen O, Johansson M, Oudin A, et al. Anti-VEGF treatment reduces blood supply and increases tumor cell invasion in glioblastoma. Proc Natl Acad Sci U S A. 2011;108(9):3749–3754. | ||
Cao N, Cao M, Chin-Sinex H, Mendonca M, Ko SC, Stantz KM. Monitoring the effects of anti-angiogenesis on the radiation sensitivity of pancreatic cancer xenografts using dynamic contrast-enhanced CT. Int J Radiat Oncol Biol Phys. 2014;88(2):412–418. | ||
Quarles CC, Schmainda KM. Assessment of the morphological and functional effects of the anti-angiogenic agent SU11657 on 9l gliosarcoma vasculature using dynamic susceptibility contrast MRI. Magn Reson Med. 2007;57(4):680–687. | ||
Markus HS. Cerebral perfusion and stroke. J Neurol Neurosurg Psychiatry. 2004;75(3):353–361. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.