Back to Journals » Cancer Management and Research » Volume 12
Full-Core Biopsy Systems Take Larger Liver Tissue Samples with Lower Fragmentation Rates Than Conventional Side-Notch Systems: A Randomized Trial
Authors Schaible J, Utpatel K, Verloh N , Einspieler I , Pregler B, Zeman F, Wiggermann P, Schreyer AG, Stroszczynski C, Beyer LP
Received 24 March 2019
Accepted for publication 16 November 2019
Published 13 February 2020 Volume 2020:12 Pages 1121—1128
DOI https://doi.org/10.2147/CMAR.S209824
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Rituraj Purohit
Jan Schaible,1,* Kirsten Utpatel,2,* Niklas Verloh,1 Ingo Einspieler,1 Benedikt Pregler,1 Florian Zeman,3 Philipp Wiggermann,4 Andreas G Schreyer,5 Christian Stroszczynski,1 Lukas P Beyer6
1Department of Radiology, University Medical Center Regensburg, Regensburg, Germany; 2Institute of Pathology, University of Regensburg, Regensburg, Germany; 3Center for Clinical Studies, University Medical Center Regensburg, Regensburg, Germany; 4Department of Radiology, Municipal Hospital Braunschweig, Braunschweig, Germany; 5Department of Radiology, Brandenburg University Hospital, Brandenburg Medical School, Brandenburg, Germany; 6Department of Diagnostic and Interventional Radiology, Clinic Ernst von Bergmann, Potsdam, Germany
*These authors contributed equally to this work
Correspondence: Lukas P Beyer
Department of Diagnostic and Interventional Radiology, Clinic Ernst von Bergmann, Potsdam 14467, Germany
Tel +49 331 2413 6702
Email [email protected]
Background: The aim of this study was to compare the histopathological quality and physical features of the specimen of a full-core end-cut biopsy system with that of the standard side-notch system for liver biopsies.
Methods: A full-core end-cut 16G biopsy device and a standard side-notch 16G needle were used to take biopsies of unclear liver lesions. Patients were randomized in two groups of 16 patients each. The primary endpoint of this prospective study was the core length measured using a dedicated microscope imaging software. Secondary endpoints were the quality of the specimen rated by an independent pathologist unaware of the device (scale from 1 to 5; with 1 as best and 5 as worst), the core diameter (determined by the microscopic imaging software) and presence of fragmentation (evaluated by the pathologist).
Results: For the full-core (FC) and side-notch (SN) groups, the mean core length was similar with 13,599 μm and 11,570 μm (p=0.131), respectively. The quality of the specimen was significantly better in the FC-group with an average rating of 1.68 vs 2.50 (p=0.009). The fragmentation rate in the FC-group was statistically significantly lower at 2/27 (7%) than in the SN-group at 13/33 (39%) (p=0.021). The diameter in the FC-group was 1042 μm vs 930 μm in SN-group (p=0.018).
Keywords: liver biopsy, full-core, side-notch, liver tumor, biopsy system
Introduction
The diagnostic informative value of a liver biopsy is directly correlated with the sampling size and quality. To reduce the risk of misinterpretation and to increase the inter-observer variability, liver specimens are considered to be of high quality if the punch-cylinder is longer than 15 mm, or in case of a tumor-free biopsy, 10 portal fields should be recognizable.1,2
Differences in material quality might lead to variations in the histological diagnoses of liver fibrosis, especially when diagnosing the stage of liver fibrosis.3,4
The dominating needle-design for soft tissue biopsies has been the side-notch needle for more than three decades.5 It consists of an inner stylet with a side notch and an outer cutting needle. Because of the inner stylet, the full diameter of the cannula cannot be filled.
More recently, end-cut, full-core needle devices have been developed in which the entire lumen and almost the whole length of the advancement of the needle are used to capture the specimen. A coaxial pincer, which slides through a slit at the tip of the device, cuts and encloses the full-core specimen.6 In this study, a new full-core device with the aforementioned features was used.
Obviously, one of the main objectives of a valuable biopsy system is to gain as much tissue as possible with the smallest possible trauma. While the end-cut needle has been shown to be safe for biopsy of organs like lung or liver,6 the adequacy of the specimen using the full-core and side-notch design for those organs has not been examined yet.
Therefore, the objective of this prospective randomized study was to compare the specimen of a full-core, end-cut device to a side-notch device regarding the histopathological quality and physical features.
Materials and Methods
Study Design and Participant Selection
In a prospective, randomized two-armed study carried out between April and November 2017, biopsies of malignant liver tumors were performed with either a full-core (FC) or side-notch (SN) system.
The ethics committee of the University Regensburg approved this study (approval no. 16-101-0137). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all patients. All biopsies were performed by one operator. All patients had one or more unclear liver lesions confirmed by contrast-enhanced magnetic resonance imaging (n = 18), computed-tomography (n = 8) or both (n = 6) and the indication for biopsy was based only on clinical criteria. The following exclusion criteria were applied: coagulation disorders not amenable to substitution; clinical need to use a larger or smaller diameter than 16-gauge of the biopsy device; refusal to participate in the study. Subjects with all the following characteristics were eligible for study enrolment: Male patients and non-pregnant, non-lactating females aged ≥18 years of age, INR<1.4, thrombocyte count >10×109/l, informed consent signed.
After signing the informed consent, patients were randomized either to the FC or SN group for a total of 32 patients (Figure 1). The allocations were sealed in consecutively numbered opaque envelopes and assigned by the study nurse. Once the patient was included in the trial, he/she was then irreversibly randomized by opening the next sealed envelope containing his/her assignment. Patients were only included and randomized once.
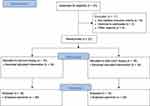 |
Figure 1 CONSORT flow diagram displaying the progress of all participants through the trial. |
Biopsy
Forty-three patients were assessed for eligibility. Eleven patients were excluded from the study. Nine patients met exclusion criteria. Two patients refused to participate in the study. After randomization liver biopsy was performed in 16 patients using the 16-gauge full-core, end-cut biopsy device (full-sCore®, Möller Medical GmbH, Fulda, Germany; FC-group) and in 16 patients using the 16-gauge side-notch biopsy device (Coaxial SABD Biopsy Device, Argon Medical Devices Inc., Athens, TX, USA; SN-group) (Figure 2).
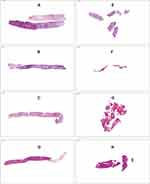
A total of 62 specimens were collected, 29 in the FC-group and 33 in the SN-group. The different number of samples can be explained by the macroscopic quality of the samples. If the interventionist believed it was not a representative sample or normal liver tissue, he could take further biopsies. All liver specimens were formalin-fixed, paraffin-embedded and stained with haematoxylin and eosin using routine methods. The specimens were evaluated by an independent experienced pathologist (K.U.) unaware of the used device. The overall diagnostic quality of the specimen was assessed (ie if the specimen is sufficient to establish a diagnosis) using a subjective grading from 1 (very good) to 5 (insufficient) (Figure 3, Table 1). Samples were evaluated as fragmented if more than one core per specimen was present (Figure 4). Since all samples were processed in the same way, the effect of tissue handling post biopsy can be neglected. The length and diameter of the specimen were measured using Nikon NIS-Element Microscope Imaging Software version 5.02.
 |
Table 1 Definition of the Quality Scores Used from 1 (Very Good) to 5 (Insufficient) |
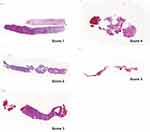 |
Figure 3 Example images of the various quality levels. The definition of the individual scores can be found in Table 1. |
All liver biopsies were performed under inpatient conditions, the standard procedure in our clinic, which ensures overnight monitoring. All adverse events, ie all deviations from the normal post-interventional course, were documented.
Power Analysis
The primary endpoint was the length of the specimen. Currently, there are no reliable data available with respect to the diagnostic valence of the specimen provided by full-core devices for organs other than prostate or kidney. Therefore, based on the previous reports for the prostate, we assumed the specimen provided by the end-cut device was 4.7 mm greater than in the biopsies from the side-notch device with a standard deviation of 5.2.7 With an α of 0.05 and a power level of 0.9, a sample size of at least 15 patients per group is needed to reach the primary endpoint.
Statistics
Quality of the specimen, length and diameter were compared between the two randomized groups by using mixed linear models. The proportion of fragmented specimen between both groups were compared by using a generalized linear-mixed model. Both models account for the correlations between specimen within the same patient by adding patient as a random factor. A p-value of ≤0.05 was considered statistically significant. SAS 9.4 (SAS Institute Inc., Cary, NC, USA) and the procedures PROC MIXED and PROC GLIMMIX were used to perform the statistical analyses.
Results
Patient and Tumor Characteristics
After randomization, the biopsy was performed in 16 patients using the side-notch (SN) device and in 16 patients using the full-core (FC) device. No peri-interventional complications were noted. The baseline characteristics of the two groups are shown in Table 2.
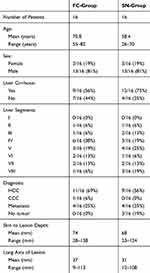 |
Table 2 Comparison of the Patient and Tumor Characteristics of the Full-Core and Side-Notch Groups |
Diagnostic Value and Physical Features of the Specimen
The diagnostic quality of the specimen, rated by the blinded pathologist, was significantly better in FC-group (p=0.009). The average quality of the FC-group was 1.68 compared to 2.50 in SN-group. All specimens in FC-group were at least rated 3 (“satisfactory”) for diagnostic purpose, whereas 5 specimens were rated only 4 (“sufficient”) and one specimen even 5 (“insufficient”) in the SN-group. Considering only patients with liver cirrhosis, similar values for diagnostic quality were found compared to the study population with 1.94 for the FC-group and 2.72 for the SN-group (p=0.020). There was no significant difference in length of the specimen (p=0.131) whereas FC-group showed a significantly larger diameter of the specimen compared to SN-group (p=0.018). Finally, there were only two fragmented specimens in FC-group versus 13 in the SN-group (p=0.021). The fragmented samples in the FC-group were 1 HCC in cirrhotic liver and 1 metastasis of colorectal cancer. The fragmented samples in the SN-group were 7 HCC in cirrhotic liver and 6 metastases (2 colorectal cancer, 3 breast cancer, 1 neuroendocrine tumor). The physical features and diagnostic quality of the specimen are summarized in Table 3.
 |
Table 3 Physical Features and Diagnostic Quality of the Specimen |
Discussion
Percutaneous biopsy of soft tissues and bones is an essential and minimally invasive tool for obtaining tissue for histopathological examination and other tests, and quality of the specimen is crucial for informative value. Besides the dominating needle-design which has been the side-notch needle for the last three decades, a novel full-core needle device has been recently developed. Although, contrary to our expectations, the length of the specimen was not greater with the full-core device, our investigation showed significantly better results using the full-core device in terms of the diameter of the specimen, fragmentation of the specimen and overall diagnostic value.
Obviously, one of the main objectives of a good biopsy system is to gain as much tissue as possible with the smallest possible trauma. In this respect, full-core needles have been shown to be superior to side-notch needles for prostate7 and renal biopsy.8 Although simple end-cutting systems with a beveled 45° convex tip have already been developed in the past, the fragmentation rate of these systems was significantly higher than with side-notch systems, but yielding larger specimen volumes.9
While the end-cut needle has also been shown to be safe for biopsy of other organs like lung or liver,6 the adequacy of the specimen using the full-core and side-notch design for those organs has not been examined yet. Therefore, the objective of this prospective study was to compare the diagnostic valence of the specimen of a full-core, end-cut device compared with a side-notch device in a randomized and controlled manner.
The full-core device used in this comparison (Möller Medical GmbH, Fulda, full-sCore®) is a disposable biopsy needle which is equipped with a full-core cutting technique to be used with a reusable biopsy device (Möller Medical GmbH, Fulda, BLUE).
The full-core system operates with an end-cutting technique at the cannula’s very front (distal) tip. This end-cutting technique does not require a window in the cannula’s wall for proper tissue cutting. Therefore, the complete length of the cannula penetrating the lesion allows tissue sampling.
The inner mandrin’s beveled edge facet-cut is placed opposite to the outer cannula’s special tip cut. Consequently, bruising of the tissue samples is avoided which may explain the lower fragmentation rate in our results. Another possible explanation for the considerable difference in the fragmentation rate is the significantly smaller diameter in SN biopsies combined with a reduced reticulin fiber network in HCC, which leads to higher tissue fragility.10
Contrary to side-notch devices, where the mandrin’s side notch fills with tissue, the full-core device offers the complete volume of the cannula for tissue sampling resulting in a larger diameter of the specimen.
This study is limited by the fact that the biopsy dispatching physician cannot be blinded against the biopsy system for obvious reasons and the different number of samples between the two groups as explained above. Although a further limitation is certainly the small number of patients, we were still able to show significant differences between the systems.
From the authors’ point of view, it is regrettable that, especially in the case of new technical developments in the interventional field, no solid studies are usually carried out to evaluate the possible advantages and disadvantages. We, therefore, believe that our study is important because of its high quality due to the blinding of the pathologist and the prospective randomized design. We hope that with our study we can stimulate further, preferably multi-center, studies to compare different biopsy systems.
Conclusion
The aim of our work was to evaluate the theoretical advantages of full-score systems in clinical practice using the biopsy of liver tumors as an application. We were able to show that. In summary, we were able to show that the full-core end-cut biopsy system provided better-evaluated specimen with more available material compared to a conventional side-notch system of identical size.
Disclosure
The abstract of this paper was presented at the CIRSE 2018 conference as a poster presentation with interim findings. The poster’s abstract was published in CardioVascular and Interventional Radiology, October 2018, Volume 41, Supplement 3.
University Hospital Regensburg received financial support for the study by Möller Medical. The execution of the study, data analysis and preparation of the manuscript were carried out by the authors exclusively. The authors report no other conflicts of interest in this work.
References
1. Regev A, Berho M, Jeffers LJ, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614–2618. doi:10.1111/ajg.2002.97.issue-10
2. Germani G, Hytiroglou P, Fotiadu A, Burroughs AK, Dhillon AP. Assessment of fibrosis and cirrhosis in liver biopsies: an update. Semin Liver Dis. 2011;31:082–090. doi:10.1055/s-0031-1272836
3. Maharaj B, Leary WP, Naran AD, et al. Sampling variability and its influence on the diagnostic yield of percutaneous needle biopsy of the liver. Lancet (London, England). 1986;1:523–525. doi:10.1016/S0140-6736(86)90883-4
4. Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495–500. doi:10.1056/NEJM200102153440706
5. Lindgren PG. Percutaneous needle biopsy. A new technique. Acta Radiol Diagn (Stockh). 1982;23:653–656. doi:10.1177/028418518202300621
6. Diederich S, Padge B, Vossas U, Hake R, Eidt S. Application of a single needle type for all image-guided biopsies: results of 100 consecutive core biopsies in various organs using a novel tri-axial, end-cut needle. Cancer Imaging. 2006;6:43–50. doi:10.1102/1470-7330.2006.0008
7. Ubhayakar GN, Li WY, Corbishley CM, Patel U. Improving glandular coverage during prostate biopsy using a long-core needle: technical performance of an end-cutting needle. BJU Int. 2002;89:40–43. doi:10.1046/j.1464-410X.2002.02531.x
8. Constantin A, Brisson M-L, Kwan J, Proulx F. Percutaneous US-guided renal biopsy: a retrospective study comparing the 16-gauge end-cut and 14-gauge side-notch needles. J Vasc Interv Radiol. 2010;21:357–361. doi:10.1016/j.jvir.2009.11.005
9. Bateson MC, Hopwood D, Duguid HL, Bouchier IA. A comparative trial of liver biopsy needles. J Clin Pathol. 1980;33:131–133. doi:10.1136/jcp.33.2.131
10. Kim H, Park YN. Role of biopsy sampling for diagnosis of early and progressed hepatocellular carcinoma. Best Pract Res Clin Gastroenterol. 2014;28:813–829. doi:10.1016/j.bpg.2014.08.012
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.