Back to Journals » Journal of Inflammation Research » Volume 15
From Suspicion to Diagnosis: Analysis on the Clinical Characteristics of 37 Cases of IgG4-Related Disease (IgG4-RD) in Northeast China
Authors Zhang S , Zhang J, Li Y, Jiao J
Received 23 March 2022
Accepted for publication 8 July 2022
Published 6 August 2022 Volume 2022:15 Pages 4487—4497
DOI https://doi.org/10.2147/JIR.S367211
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Shanshan Zhang, Jun Zhang, Yifang Li, Jian Jiao
Department of Gastroenterology & Hepatology, China-Japan Union Hospital, Jilin University, Changchun, People’s Republic of China
Correspondence: Jian Jiao, Email [email protected]
Introduction: IgG4-related disease (IgG4-RD) mimics a variety of disorders, the final diagnosis is heavily dependent on the doctor’s familiarity with the disease, most patients are expected to get a good prognosis by early diagnosis and timely treatment.
Methods: Retrospective analysis was carried out on 147 patients tested for serum IgG4 because of suspected IgG4-RD. These cases were grouped as the IgG4-RD group and non-IgG4-RD group according to the diagnostic criteria proposed by the Japanese IgG4-RD research group and the American College of Rheumatology and the European Union of Rheumatology. Characteristics of these patients were investigated and analyzed.
Results: The onset age of IgG4-RD was 57.29 ± 14.03 years old, male to female ratio of IgG4-RD was 1.31:1. The most commonly affected organs were the pancreas (48.6%), lymph nodes (40.5%) and biliary tract (35.1%), the proportion of patients with simultaneous involvement of multiple organs is as high as 83.2%. A history of allergy is more common in IgG4-RD patients (32.4% vs 14.5%), the optimal critical value of serum IgG4/IgG ratio for diagnosis of IgG4-RD was 0.09 (sensitivity 94.7%, specificity 91.7%) and the optimal threshold for IgG diagnosis of IgG4-RD was 15.25g/L (sensitivity 73.7%, specificity 77.8%) in this study.
Conclusion: IgG4-RD is often manifested as multiple organ involvement, and is most likely to involve the pancreas, biliary tract and lymph nodes. Most patients were diagnosed in other departments instead of rheumatology. Serum IgG4 level, especially IgG4/IgG ratio has a higher predictive value for IgG4-RD. Early diagnosis is the key point to improve the prognosis.
Keywords: IgG4, IgG4-related disease, IgG4-RD, clinical characteristics
Introduction
IgG4-related disease (IgG4-RD) is a rare, not well recognized, kind of immune disease with multiple systemic involvement. It is characterized by focal diffuse inflammatory cell infiltration or organ enlargement in affected organs, usually manifested by elevated serum IgG4 level, infiltration of IgG4-positive plasma cells, fibrotic mass formation, and favorable response to corticosteroids.1 Although elevated serum level of IgG4 is one of the characters of IgG4-RD and can be used as an index for preliminary screening, many studies have found that increased serum IgG4 could also be detected in patients with other systemic disorders like lymphoma, systemic lupus erythematosus(SLE), granulomatosis with polyangiitis, etc. Moreover, IgG4-RD mimics a variety of disorders, such as malignant, infectious and other immune diseases,2 patients may initially see doctors based on the most obvious clinical manifestations, the final diagnosis is heavily dependent on the doctor’s familiarity with the disease. However, awareness of diagnostic and therapeutic measures for the management of patients with IgG4-RD remains limited to tertiary care centers, and the disease is still misdiagnosed as a tumor or inflammatory and infectious diseases. Analyzing the clinical features of the disease in clinical practice may facilitate its early recognition and establish personalized follow-up and treatment strategies.
In this study, we collected and analyzed 147 patients tested for serum IgG4 because of suspected IgG4-RD in terms of clinical presentation, serological features and response to treatment, in order to better understand the natural history of this specific disorder and prompt its early recognition.
Patients and Methods
Retrospective analysis was carried out on 147 patients tested for serum IgG4 because of suspected IgG4-RD. These cases were grouped as the IgG4-RD group and non-IgG4-RD group according to the following two standards: (1) The comprehensive diagnostic criteria of IgG4-RD proposed by the Japanese IgG4-RD research group (2011).3 (2) The classification criteria of IgG4-RD proposed by the American College of Rheumatology and the European Union of Rheumatology (2019).4
The clinical data of 147 cases, including gender, age of onset, history of allergy, smoking and drinking, history of hypertension and diabetes, symptoms at onset, the department of first visit, the number and location of affected organs, methods of treatment and prognosis were collected. Lab test results including serum level of IgG4, total immunoglobulin, complement C3, autoimmune antibody, C-reactive protein (CRP), procalcitonin (PCT), fasting blood glucose, lipids, amylase, routine blood test, liver and renal function, and erythrocyte sedimentation rate (ESR) and tumor markers were recorded in detail. Imaging data including CT, ultrasound, PET-CT, MRI, and histopathological and immunehistochemical results were collected to determine the type and number of organs involved. Serum levels of IgG4 were detected by scattering immunoturbidimetric assay.
The study complied with the Declaration of Helsinki and was approved by the Medical Ethics Committee of the China Japan Friendship Hospital of Jilin University (No. 2019-NSFC-008). Informed consent forms for data collection were kept in the patient’s medical records.
Statistics
SPSS 26.0 software was used for statistical analysis. Clinical data and serological results of patients were analyzed by descriptive statistics. Data, in accordance with the normal distribution, were calculated by average ± standard deviation (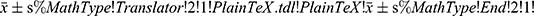 ). Data not conforming to normal distribution are represented by median (quaternary) [M(P25, P75)]. T-test was used to analyze the comparison between two groups in accordance with normal distribution, Mann–Whitney U-test was used to analyze the comparison between two groups in non-normal distribution. The Kruskal–Wallis test was used to compare the differences between groups with non-normal distribution. “Rate”, “constituent ratio” and “relative ratio” were used to describe the enumeration data. Differences were analyzed using the chi-square test or Fisher’s exact test (n < 40). Spearman correlation analysis was used for correlation analysis. The predictive value of some serological indicators in the diagnosis of IgG4-RD was evaluated by receiver operating curve (ROC). For all tests, a P-value < 0.05 was considered significant.
). Data not conforming to normal distribution are represented by median (quaternary) [M(P25, P75)]. T-test was used to analyze the comparison between two groups in accordance with normal distribution, Mann–Whitney U-test was used to analyze the comparison between two groups in non-normal distribution. The Kruskal–Wallis test was used to compare the differences between groups with non-normal distribution. “Rate”, “constituent ratio” and “relative ratio” were used to describe the enumeration data. Differences were analyzed using the chi-square test or Fisher’s exact test (n < 40). Spearman correlation analysis was used for correlation analysis. The predictive value of some serological indicators in the diagnosis of IgG4-RD was evaluated by receiver operating curve (ROC). For all tests, a P-value < 0.05 was considered significant.
Results
Initial Symptoms and First-Visit-Departments of the 37 Patients Finally Diagnosed as IgG4-RD
There was a great variability in IgG4-RD patients’ initial symptoms that indicates there is no typical clinical manifestation. A total of 14 cases (37.8%) initially presented with abdominal pain, which is the most common initial symptom of these patients. Besides, a number of patients initially presented with foamy urine, frequent urination, edema in both lower extremities, swelling and pain in multiple joints, fatigue, and numbness of the limbs, details are shown in Figure 1.
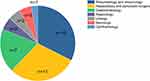
IgG4-RD is a disease associated with autoimmune pathogenesis, while a large number of patients are not first diagnosed in the rheumatology/immunology department. This work further showed that only 32.4% of the patients were referred to the rheumatology/immunology department at their first visit to the hospital, most of them consulted doctors in the corresponding departments according to their initial symptoms, as shown in Figure 2.
Involved Organs in 37 Patients Diagnosed as IgG4-RD
The number of organs involved in the IgG4-RD group ranged from 1 to 7 (with a median of 3), which further confirmed that it is a multiorgan involved disease. The pancreas and biliary tract involvement often coexisted, presented as pancreatitis, jaundice, abdominal pain, uneven stricture and dilation of the bile duct, cholangitis and cholecystitis. Enlargement of the lymph nodes usually emerged as superficial and deep lymphadenopathy in multiple locations throughout the body, especially in the vicinity of the affected organs, details are illustrated in Figure 3.
Correlation Analysis Between Serum IgG4 Level and the Number of Involved Organs in IgG4-RD Patients
Two of 37 patients eventually were diagnosed as IgG4-RD on the basis of postoperative pathological results, that had early surgical treatment due to the strong suspicion of neoplasmic disease but without serum IgG4 level determination. Serum levels of IgG4 was measured in the remaining 35 cases, with a mean value of 7.64 (2.10, 8.94) g/L, no significant correlation was found between serum IgG4 level and the number of organs involved (P = 0.149). A scatter diagram is presented in Figure 4.
Comparison on Serum IgG4 Levels in IgG4-RD Patients with Different Serum EOS Levels
Peripheral blood eosinophil count is usually associated with immune abnormalities. 35 IgG4-RD patients were divided into three groups according to the peripheral blood eosinophil count, elevated EOS group, lowered EOS group and normal EOS group (normal range of EOS is defined as 0.05–0.5×109/L), serum IgG4 levels in these groups were compared, results demonstrated that serum IgG4 level was higher in the elevated EOS group, which suggested that there may be some correlation between them in the pathogenesis of this disease (Figure 5).
Comparison of Patient’s History and General Data Between the IgG4-RD Group and Non-IgG4-RD Group
A total of 147 cases who had suspected IgG4-RD were included, the patient’s history and general data between the IgG4-RD group and non-IgG4-RD group were compared, as shown in Table 1.
 |
Table 1 Comparison of Patient’s History and General Data Between IgG4-RD Group and Non-IgG4-RD Group |
Twelve patients (32.4%) in the IgG4-RD group had a history of allergies, which was intensely higher than that in the non-IgG4-RD group (P = 0.017). There was no significant difference in the distribution of gender and age between these two groups (P = 0.139 and 0.089, respectively). No statistical difference was also found between the two groups in the history of drinking and smoking (P = 0.132 and 0.089, respectively), similarly, no conspicuous difference in patients whether complicated with hypertension (P = 0.211), but statistically remarkable differences were found in the presence or absence of diabetes between the two groups (P = 0.002).
Comparison of Serum Level of IgG4 and Laboratory Test Results Between IgG4-RD Group and Non-IgG4-RD Group
Average serum levels of IgG4 were 3.59 (2.10, 8.94) g/L and 0.53 (0.29, 1.66) g/L in the IgG4-RD group and non-IgG4-RD group, respectively (p < 0.001), and 85.7% patients had elevated serum IgG4 ≥ 1.35 g/L in the IgG4-RD group, higher than that in the non-IgG4-RD group (85.7% vs 29.1%, P < 0.001), which suggested that elevated serum IgG4 level was the characteristic of most IgG4-RD patients. Statistical differences could be detected in the serum levels of IgG, IgE, EO%, EOS, UREA, CREA, AST, GLB and fasting glucose between these two groups, the specific data is listed in Table 2.
 |
Table 2 Comparison of Laboratory Test Results Between IgG4-RD Group and Non IgG4-RD Group |
Predictive Value of Serum IgG4 Level in the Diagnosis of IgG4-RD
ROC analysis on the predictive value of serum IgG4 level in the diagnosis of IgG4-RD showed the sensitivity and specificity was 85.7% and 71.0% respectively at the cutoff value of IgG4 > 1.35 g/L, which is the standard recommended by the current guidelines. Further analysis showed that the optimal cutoff value of serum IgG4 level for the diagnosis of IgG4-RD was 2.01 g/L with a sensitivity of 80.0% and specificity of 88.3%, Youden index was 0.683, AUC was 0.854 (95% CI 0.771~0.938, P < 0.001), as shown in Figure 6A.
Predictive Value of Serum Levels of Immunoglobulin Subclasses and IgG4/IgG Ratio in the Diagnosis of IgG4-RD
ROC analysis showed that both the levels of IgG and IgG4/IgG ratio have a predictive value for the diagnosis of IgG4-RD, while the levels of serum IgA and IgM have little significance for IgG4-RD diagnosis. The cutoff value of serum total IgG level for the diagnosis of IgG4-RD was 15.25 g/L with a sensitivity of 73.7% and specificity of 77.8%. The cutoff value of serum IgG4/IgG ratio for the diagnosis of IgG4-RD was 0.09 with a sensitivity of 94.7% and specificity of 91.7%. IgG4/IgG ratio had a higher predictive value for the diagnosis of IgG4 than IgG4 level, as shown in Figure 6B.
Therapeutic Measures and Prognosis of 37 Patients Confirmed with IgG4-RD
Nearly half of the patients received glucocorticoid (methyl prednisolone or prednisone acetate pieces) treatment, of which 13 cases were treated with glucocorticoid, 7 cases were treated with glucocorticoid combined with immunosuppressant (cyclophosphamide, methotrexate, fluorine milt), 1 case converted to rituxan due to the poor efficacy of glucocorticoid, and another 1 patient underwent local lesion resection in addition to glucocorticoid therapy. While 4 cases were treated with local lesion resection, 1 patient was treated with PTCD drainage because of biliary tract involvement, 1 patient with biliary tract involvement was stented under ERCP, however, 9 patients refused glucocorticoid therapy and received only symptomatic supportive therapy regarding IgG4-RD.
No fatal cases were discovered after 27 months of follow-up, in the follow-up group, the serum IgG4 levels of 8 patients who were treated with glucocorticoid had largely decreased, 7 of which were back to normal. Related symptoms were significantly improved in all these patients, 2 patients relapsed after glucocorticoid tapered and needed a small dose of glucocorticoid to maintain remission.
Discussion
IgG4-RD is a kind of fibro-inflammatory disorder and has been gradually increasing in concern in recent years, while the understanding of IgG4-RD is still limited and the pathogenesis is also unclear. Due to the limited knowledge and its infrequency, large-scale epidemiological investigation is lacking, most of the current studies are derived from single center or special case reports.
Unlike some autoimmune diseases, such as Sjogren’s syndrome, systemic lupus erythematosus, and rheumatoid arthritis, which usually happen in females, typically IgG4-RD occurs mostly in males.5 The results of this study also support that IgG4-RD is more likely to occur in the middle-aged and elderly male population. The male-to-female ratio was 1.31:1, and the onset age ranged from 25 to 79 (57.29 ± 14.03), 70.3% of them were over 50 years old at the time of onset.
Broadly speaking, IgG4-RD is a multisystemic immune disease, while only a small number of patients are first diagnosed in the department of rheumatology and immunology. The initial symptom of IgG4-RD vary because it may extensively involve any organ of the body. According to the order of occurrence rate, the initial symptom of the cases in this study were abdominal pain (37.8%), jaundice (21.6%), lymph node enlargement (21.6%), submandibular gland enlargement (13.5%), Low back pain (13.5%), eyelid swelling (13.5%), parotid gland enlargement (10.8%), anorexia and emaciation (10.8%). Only 32.4% of these patients were diagnosed in the department of rheumatology and immunology, and the remaining patients were identified in the departments corresponding to the affected organs. IgG4-RD patients who need to be confirmed with a tumor could be misdiagnosed and result in unnecessary local excision, while those who are undiscovered may blunder away the best time for early treatment. For these reasons, raising the awareness of IgG4-RD among doctors of all specialties is conducive to avoid the misdiagnosis of IgG4-RD.
Wallace et al recently applied latent classification analysis (LCA) to the largest international cohort of IgG4-RD patients, a cohort of patients identified during the development and validation of the 2019 ACR/EULAR Classification Criteria for IgG4-RD grading criteria. These investigators identified four IgG4-RD phenotypes with epidemiological and serological characteristics. The phenotypes include: pancreato-hepato-biliary disease; retroperitoneal fibrosis and/or aortitis; head and neck-limited disease; and classic Mikulicz’s syndrome with systemic involvement.6 This classification helps to improve the identification ability of doctors in related departments.
It should also be noted that multiple organ involvement is a prominent clinical characteristic of IgG4-RD. This study showed that only 16.2% of the patients were involved with a single organ, 83.8% of the patients were involved with two or more organs. Among them, pancreas was the most commonly affected, followed by biliary tract, retroperitoneal, submandibular gland and parotid gland, and most of the cases were accompanied with lymph node involvement, these results are consistent with the previous study.7 While there was no significant correlation between serum IgG4 level and the number of affected organs in this work, which was different from Kamisawa T’s study, he reported that the more organs involved, the higher the IgG4 levels,8 thus further study and exploration with large sample data is absolutely necessary to be carried out in the future. Our study also found that gender differences existed in patients with pancreatic involvement, with a significantly higher difference of pancreatic involvement in male patients than female patients (p = 0.02). While Liu and Wallace et al reported that patients with head and neck limited disease were far more likely to be female and Asian than were patients in the other types.9,10
The pathogenesis of IgG4-RD is still unclear, it has been proposed that allergic mechanisms drive IgG4-RD. It was reported that serum IgE and eosinophils are elevated in 30% to 40% of cases, regardless of an underlying atopic background, and their increase correlates even better with disease activity in many of these patients than do IgG4 concentrations.11,12 Kamisawa et al13 reported that eosinophils were elevated in nearly half of patients with IgG4-RD. While Emanuel Della Torre et al evaluated atopy, peripheral blood eosinophilia and serum IgE concentrations in a large cohort of patients with IgG4-RD in whom a wide range of organs were affected by disease, results demonstrated that the majority of patients with IgG4-RD are nonatopic, which suggested that processes were inherent to IgG4-RD itself rather than atopy, this contributes to the eosinophilia and IgE elevation observed in the absence of atopy.14 In this paper, the serum IgG4 level of IgG4-RD patients with elevated eosinophils was higher than that of IgG4-RD patients with normal eosinophils (p < 0.05), this suggested that the elevated levels of these two indicators may be equally attributable to the immune abnormalities of IgG4-RD itself. In addition, we found that there was a statistical difference in the proportion of patients with diabetes mellitus between IgG4-RD and non-IgG4-RD patients, which may be related to more pancreatic involvement in IgG4-RD or an affected pancreas was more likely to capture the attention of a doctor for IgG4 testing.
The significance of serum IgG4 level in the diagnosis of IgG4-RD has been an ongoing issue, there is no doubt that serum IgG4 levels in the IgG4-RD group were significantly higher than those in the non-IgG4-RD group. However, elevated serum IgG4 is not a necessary feature of IgG4-RD, it was reported that nearly 50% of patients with active IgG4-RD had normal serum IgG4 levels.15–17 IgG4 ≥ 1.35 g/L is the threshold value for IgG4-RD diagnosis recommended by present guidelines, so we analyzed the predictive value of serum IgG4 level in the diagnosis of IgG4-RD, results showed that the optimal cutoff value of IgG4 was 2.01 g/L, which was higher than the recommended value of the guideline (IgG4 ≥ 1.35 g/L). In addition, it was reported that quantitative polymerase chain reaction of the IgG4:IgG RNA ratio on peripheral blood seemed to accurately distinguish IgG4 related cholangitis from hepatobiliary malignancies and inflammatory processes with a sensitivity of 94% and a specificity of 99%.18 The predictive value of IgG4/IgG ratio and Ig subclass for the diagnosis of IgG4-RD was also analyzed in this study, ROC analysis showed that serum IgG4/IgG ratio may be of good value in predicting the diagnosis of IgG4-RD. The optimal cut-off value was 0.09 g/L, the sensitivity and the specificity was 94.7% and 91.7% respectively. However, either optimal cutoff value of IgG4 or the predictive value of IgG4/IgG depends on larger population surveys to comfirm.19
Imaging methodology is necessary for the diagnosis of IgG4-RD. Due to the lack of typical clinical manifestations and hidden organs, such as retroperitoneal, pituitary, meningeal, etc. maybe being involved, imaging examination is especially useful in analyzing affected organs where pathology is difficult to obtain. Ultrasound can be used to find the involvement of glands and superficial lymph nodes. CT can be used to evaluate the lesions of substantive organs; MRI may be used in the evaluation of pituitaritis, meninges and vasculitis, it is more sensitive than CT in the diagnosis of soft tissue inflammation and vascular lesions. MRCP is useful when the biliary tract system is involved; PET-CT is mainly used to exclude malignant tumors and distinguish infectious and non-infectious diseases.20–22 It has been reported that compared with ultrasound and CT, PET-CT is more helpful to discover the involved organs in IgG4-RD, it also has advantages in assessing the activity of the disease, judging the efficacy of treatment and monitoring the recurrence,23,24 however, the high price and radiation exposure are its disadvantages. In this study, 2 patients without any complaints of discomfort were found to be abnormal by imaging analyses during routine physical examination, they were finally confirmed as IgG4-RD and accepted glucocorticoid treatment. Therefore, the existence of IgG4-RD can not be ignored even in the population of routine physical examination.
Up to now, glucocorticoid is the first choice for the treatment of IgG4-RD. The majority of patients responded well and the clinical symptoms were quickly relieved. However, the efficacy varies with the degree of fibrosis in the affected organs, the more severe the fibrosis, the worse the efficacy.1,25 For irreversible organ damage or obstruction, such as ureteral obstruction caused by retroperitoneal fibrosis or biliary tract obstruction due to pancreatic fibrosis, surgery and interventional therapy might be unavoidable. It was reported that IgG4-RD lesions are more likely to shrink early in the presence of a prominent lymphoplasmacytic infiltrate (inflammatory phase) than at later stages when both inflammatory cells and myofibroblasts are rare (fibrotic phase), indicating that a “window of therapeutic opportunity” for preventing irreversible organ damage exists,26 therefore, early diagnosis and timely treatment are important factors to determine the prognosis of IgG4-RD.
Current guidelines recommended an initial dose of prednisone of 30–40 mg daily for 2–4 weeks, followed by a reduction of 5 mg every 1–2 weeks, with a maintenance dose of 2.5–5.0 mg/d,27 clinicians should consider the relapsing-remitting nature of IgG4-RD and the potential side effects of glucocorticoids. It was reported that head and neck manifestations of IgG4-RD seem to be more challenging to treat and more prone to relapse in a shorter period of time, leading to higher exposure to glucocorticoids over time. Anti-rheumatic drugs (DMARDs), such as azathioprine, mycophenolate mofetil, methotrexate, leflunomide, tacrolimus, ciclosporin A, iguratimod, and cyclophosphamide can be added to first line steroid therapy to improve the likelihood of obtaining disease remission, but little evidence exists for the additional efficacy of these drugs and most data derive from retrospective studies. Recently, with the understanding of the pathophysiology of IgG4-RD, novel potential biological therapies are already in clinical trials, which not only helps to improve the outcomes, but also avoid the side effects caused by long-term use of glucocorticoids, especially for those refractory patients.28–30
In this study, 56.6% of patients were treated with glucocorticoids, 35.1% of whom were treated with glucocorticoids alone. Follow-up observations showed that all these patients achieved good results, and serum IgG4 levels also decreased after treatment. One patient achieved effective clinical remission after switching to rituximab because of resistance to glucocorticoids. While the determination of drug withdrawal indicators, prevention of disease recurrence, and the development of individualized treatment plans according to the patient’s condition are still issues that need to be discussed in the future.
In conclusion, the limitations of this study are that it is a retrospective analysis, the number of cases is quite small although they come from different clinical departments, and not all patients have undergone histopathological examination, the comparison of patients before and after treatment is not completely parallel, and the follow-up time is limited. All these shortcomings need further large sample, multi-center and prospective clinical research to improve, but after all, through a rough understanding, this paper provides a basis for improving the awareness and understanding of IgG4-RD in clinical departments.
Disclosure
Authors have no conflict of interest to declare
References
1. Kamisawa T, Zen Y, Pillai S, Stone JH. IgG4-related disease. Lancet. 2015;385(9976):1460–1471. doi:10.1016/S0140-6736(14)60720-0
2. Umehara H, Okazaki K, Masaki Y, et al. A novel clinical entity, IgG4-related disease (IgG4RD): general concept and details. Mod Rheumatol. 2012;22(1):1–14. doi:10.3109/s10165-011-0508-6
3. Umehara H, Okazaki K, Masaki Y, et al. Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD), 2011. Mod Rheumatol. 2012;22(1):21–30. doi:10.3109/s10165-011-0571-z
4. Wallace ZS, Naden RP, Chari S, et al. The 2019 American College of Rheumatology/European League Against Rheumatism classification criteria for IgG4-related disease. Ann Rheum Dis. 2020;79(1):77–87. doi:10.1136/annrheumdis-2019-216561
5. Lanzillotta M, Campochiaro C, Mancuso G, et al. Clinical phenotypes of IgG4-related disease reflect different prognostic outcomes. Rheumatology. 2020;59(9):2435–2442. doi:10.1093/rheumatology/keaa221
6. Wallace ZS, Zhang Y, Perugino CA, et al. Clinical phenotypes of IgG4-related disease: an analysis of two international cross-sectional cohorts. Ann Rheum Dis. 2019;78:406–412. doi:10.1136/annrheumdis-2018-214603
7. Inoue D, Yoshida K, Yoneda N, et al. IgG4-related disease: dataset of 235 consecutive patients. Medicine. 2015;94(15):e680. doi:10.1097/MD.0000000000000680
8. Kamisawa T, Okamoto A, Funata N. Clinicopathological features of autoimmune pancreatitis in relation to elevation of serum IgG4. Pancreas. 2005;31(1):28–31. doi:10.1097/01.mpa.0000167000.11889.3a
9. Liu Y, Xue M, Wang Z, et al. Salivary gland involvement disparities in clinical characteristics of IgG4-related disease: a retrospective study of 428 patients. Rheumatology. 2020;59:634–640. doi:10.1093/rheumatology/kez280
10. Wallace ZS, Zhang Y, Perugino CA, Naden R, Choi HK. Stone JHACR/ EULAR IgG4-RD Classification Criteria Committee. Clinical phenotypes of IgG4-related disease: an analysis of two international crosssectional cohorts. Ann Rheum Dis. 2019;78:406–412.
11. Culver EL, Sadler R, Bateman AC, et al. Increases in IgE, Eosinophils, and Mast Cells Can be Used in Diagnosis and to Predict Relapse of IgG4-Related Disease. Clin Gastroenterol Hepatol. 2017;15:1444–1452. doi:10.1016/j.cgh.2017.02.007
12. Wallace ZS, Mattoo H, Mahajan VS, et al. Predictors of disease relapse in IgG4-related disease following rituximab[Oxford]. Rheumatology. 2016;55:1000–1008. doi:10.1093/rheumatology/kev438
13. Kamisawa T, Anjiki H, Egawa N, Kubota N. Allergic manifestations in autoimmune pancreatitis. Eur J Gastroenterol Hepatol. 2009;21(10):1136–1139. doi:10.1097/MEG.0b013e3283297417
14. Della Torre E, Mattoo H, Mahajan VS, Carruthers M, Pillai S, Stone JH. Prevalence of atopy, eosinophilia, and IgE elevation in IgG4-related disease. Allergy. 2014;69(2):269–272. doi:10.1111/all.12320
15. Wallace ZS, Deshpande V, Mattoo H, et al. IgG4-Related Disease: clinical and Laboratory Features in One Hundred Twenty-Five Patients. Arthritis Rheumatol. 2015;67(9):2466–2475. doi:10.1002/art.39205
16. Sato Y, Kojima M, Takata K, et al. Multicentric Castleman’s disease with abundant IgG4-positive cells: a clinical and pathological analysis of six cases. J Clin Pathol. 2010;63(12):1084–1089. doi:10.1136/jcp.2010.082958
17. Hara S, Kawano M, Mizushima I, et al. A condition closely mimicking IgG4-related disease despite the absence of serum IgG4 elevation and IgG4-positive plasma cell infiltration. Mod Rheumatol. 2016;26(5):784–789. doi:10.3109/14397595.2014.916836
18. Doorenspleet ME, Hubers LM, Culver EL, et al. Immunoglobulin G4(+) B-cell receptor clones distinguish immunoglobulin G 4-related disease from primary sclerosing cholangitis and biliary/pancreatic malignancies. Hepatology. 2016;64:501–507. doi:10.1002/hep.28568
19. Carruthers MN, Khosroshahi A, Augustin T, Deshpande V, Stone JH. The diagnostic utility of serum IgG4 concentrations in IgG4-related disease. Ann Rheum Dis. 2015;74(1):14–18. doi:10.1136/annrheumdis-2013-204907
20. Fonti R, Conson M, Del Vecchio S. PET/CT in radiation oncology. Semin Oncol. 2019;46(3):202–209. doi:10.1053/j.seminoncol.2019.07.001
21. Haroon A, Zumla A, Bomanji J. Role of fluorine 18 fluorodeoxyglucose positron emission tomography-computed tomography in focal and generalized infectious and inflammatory disorders. Clin Infect Dis. 2012;54(9):1333–1341. doi:10.1093/cid/cis193
22. Yao Y, Ou X. [The application of fluorine-18-fluorodeoxyglucose positron emission tomography/computed tomography in inflammation and infectious disease]. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2020;37(4):730–735. Chinese. doi:10.7507/1001-5515.202002030
23. Zhang J, Chen H, Ma Y, et al. Characterizing IgG4-related disease with 18F-FDG PET/CT: a prospective cohort study. Eur J Nucl Med Mol Imaging. 2014;41(8):1624–1634. doi:10.1007/s00259-014-2729-3
24. Pan Q, Luo Y, Zhang W. Recurrent Immunoglobulin G4-Related Disease Shown on 18F-FDG and 68Ga-FAPI PET/CT. Clin Nucl Med. 2020;45(4):312–313. doi:10.1097/RLU.0000000000002919
25. Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012;366(6):539–551. doi:10.1056/NEJMra1104650
26. Lanzillotta M, Mancuso G, Della-Torre E. Advances in the diagnosis and management of IgG4 related disease. BMJ. 2020;16(369):m1067. doi:10.1136/bmj.m1067
27. Khosroshahi A, Wallace ZS, Crowe JL, et al. International Consensus Guidance Statement on the Management and Treatment of IgG4-Related Disease. Arthritis Rheumatol. 2015;67(7):1688–1699. doi:10.1002/art.39132
28. Ramadass T, Balaji V, Sheba SKJ, Vali Ahmed KS, Abdurahiman R. IgG4-Related Disease (IgG4-RD) Presenting as a Mass in the Carotid Triangle Masquerading Paraganglioma. Indian J Otolaryngol Head Neck Surg. 2017;69(2):259–262. doi:10.1007/s12070-016-0976-2
29. Sarkar A, Pitchumoni CS. The protean manifestations of IgG4-RD in gastrointestinal disorders. Dis Mon. 2015;61(12):493–515. doi:10.1016/j.disamonth.2015.09.008
30. Ebbo M, Grados A, Samson M, et al. Long-term efficacy and safety of rituximab in IgG4-related disease: data from a French nationwide study of thirty-three patients. PLoS One. 2017;12(9):e0183844. doi:10.1371/journal.pone.0183844
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.