Back to Journals » OncoTargets and Therapy » Volume 16
From Co-Stimulation to Co-Inhibition: A Continuum of Immunotherapy Care Toward Long-Term Survival in Melanoma
Authors Simonetti E, Cutarella S, Valente M, Sani T, Ravara M, Maio M, Di Giacomo AM
Received 16 January 2023
Accepted for publication 3 April 2023
Published 5 April 2023 Volume 2023:16 Pages 227—232
DOI https://doi.org/10.2147/OTT.S368408
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Arseniy Yuzhalin
Elena Simonetti,1 Serena Cutarella,1 Monica Valente,2 Tommaso Sani,1 Matteo Ravara,1 Michele Maio,1– 3 Anna Maria Di Giacomo1– 3
1University of Siena, Siena, Italy; 2Center for Immuno-Oncology, Medical Oncology and Immunotherapy, Department of Oncology, University Hospital, Siena, Italy; 3NIBIT Foundation Onlus, Genoa, Italy
Correspondence: Anna Maria Di Giacomo, Center for Immuno-Oncology, Medical Oncology and Immunotherapy, Department of Oncology, University Hospital of Siena, Viale Bracci, 14, Siena, 53100, Italy, Email [email protected]
Abstract: Harnessing the immune system with immune-checkpoint(s) blockade (ICB) has dramatically changed the treatment landscape of advanced melanoma patients in the last decade. Indeed, durable clinical responses and long-term survival can be achieved with anti-Cytotoxic T-Lymphocyte Antigen-4 (CTLA-4) and anti-Programmed cell Death-1 (PD-1) monoclonal antibodies (mAb) either alone or in combination. Despite these unprecedented results, due to intrinsic or acquired resistance to ICB-based immunotherapy, about half of metastatic melanoma (MM) patients neither respond to therapy nor experience durable clinical benefit or long-term survival. To improve the efficacy of ICB therapy among a larger proportion of MM patients, in addition to the targeting of immune-checkpoint(s) inhibitors (ICI) such as CTLA-4 or PD-1, several co-stimulatory molecules, such as Inducible T-cell COStimulator (ICOS), CD137 and OX40, have been investigated in MM, with initial signs of activity. Thus, a number of MM patients have been exposed to co-inhibitory and co-stimulatory mAb in the course of their disease. Being aware of the clinical outcome of such patients may pave the way to novel and more effective clinical approaches and therapeutic sequences for MM patients. Here we report a paradigmatic clinical case of a cutaneous MM patient who achieved multiple and durable complete responses, leading to an extraordinary long-term survival with sequential ICB therapies, suggesting the possibility to build a highly effective continuum of care with co-inhibitory and co-stimulatory therapeutic mAb.
Keywords: immunotherapy, combinations, anti-PD1, anti-CTLA-4, CD137 agonist, ICOS agonist
Background
Immune check-point blockade (ICB) with monoclonal antibodies (mAb) to co-inhibitory Cytotoxic T-Lymphocyte Antigen-4 (CTLA-4) or Programmed cell Death-1 (PD-1) and their combination is the mainstay in the treatment landscape of metastatic melanoma (MM) patients.1–6 The initial efficacy of the anti-CTLA-4 ipilimumab in a relatively small percentage of MM patients was rapidly improved by targeting the PD-1/PD-L1 pathway, leading to higher objective response rates and better survival.1–6 Furthermore, combined therapy with anti-CTLA-4 and -PD-1 mAb led to an impressive 56% melanoma specific survival at 6.5 years.7
Despite these unprecedented results, primary or acquired resistance can limit the efficacy of these co-inhibitory mAb in a sizeable proportion of MM patients, and the identification of new therapeutic ICB mAb is among the most critical needs in this still deadly disease.8 Along this line, upcoming evidence has shown that mAb targeting co-stimulatory molecules such as Inducible T-cell COStimulator (ICOS), CD137 and OX40, may have great therapeutic potential in MM; however, their clinical application remains limited to clinical trials, and will undoubtedly grow in the near future. Co-stimulatory receptors such as CD28 are constitutively expressed on T lymphocytes while others, including 4–1BB, OX40, and ICOS, are induced by antigen priming.9–11
Among co-stimulatory molecules, ICOS has recently raised great interest due to its potential synergistic activity when combined with ICI.12,13 The ICOS agonist mAb, vopratelimab, has been investigated in combination with anti-PD-1 and anti-CTLA-4 mAb in the Phase I ICONIC trial (NCT02904226).14–16 Furthermore, the first-in-human INDUCE-1 study (NCT02723955) explored the combination of the ICOS agonist mAb feladilimab with anti-PD-1, anti-TIM-3 mAb or chemotherapy, in patients with advanced solid tumors.17,18 However, in spite of the initial results showing safety, tolerability, and clinical activity of ICOS agonists in heavily pretreated patients, more mature data failed to confirm their efficacy; thus, their therapeutic potential is being investigated further.19
Concerning CD137, the agonist mAb urelumab also showed initial encouraging signs of activity in two phase I/II clinical trials in metastatic solid tumors, including MM patients.20 However, clinical development of urelumab was prematurely discontinued due to drug-related hepatotoxic deaths; nevertheless, subsequent analyses demonstrated that urelumab at 0.1 mg/kg every 3 weeks had an acceptable safety profile.20 An additional CD137 agonist utomilumab, showed a good safety profile as single agent in a phase I clinical trial.21 Moreover, the safety and clinical activity of utomilumab, administered at 5 different doses (ranging from 0.45 to 5.0 mg/kg) every 3 weeks in combination with pembrolizumab (2mgs), was confirmed in a phase Ib clinical trial in different tumor histotypes, including MM.22
Though initial and partially limited by their safety profiles, the specific mechanism(s) of action of co-stimulatory ICB mAb seems to hold significant therapeutic potential; however, a proper balancing of co-stimulatory and co-inhibiting strategies can be mandatory for a more rational development strategy of ICB therapy. Along this line, we here report the clinical case of an MM patient achieving multiple complete responses after sequential therapy with co-inhibitory and co-stimulatory mAb.
Case Presentation
This clinical case illustrates the long-term benefit achieved by a stage IV cutaneous melanoma patient who received sequential immunotherapies with co-stimulatory and co-inhibitory mAb, leading to multiple and durable complete responses and to a very long-term survival.
In December 2004, a 72-year-old female was diagnosed with a stage IIIC (pT2bN3), BRAF V600E mutant, cutaneous melanoma of the left thigh, and was treated with a wide local excision and a left inguinal-iliac-obturator lymph node dissection, after a positive sentinel node biopsy. She entered a follow-up program that was negative until September 2007, when a restaging computed tomography (CT) scan identified disease recurrence at the left popliteal and iliac metastatic lymph nodes, with normal Lactate Dehydrogenase levels. In October 2007, the patient was evaluated at our Center for Immuno-Oncology at the University Hospital of Siena, Italy, for enrollment in a randomized, double-blind, Phase III trial (BMS CA184024), comparing ipilimumab 10 mgs i.v. combined with dacarbazine (DTIC) 850 mg/mq i.v. versus DTIC alone, in untreated MM patients. Due to evidence of autoimmune thyroiditis, the patient did not meet the study protocol eligibility criteria and received DTIC 800 mg/mq i.v. every 3 weeks in combination with Thymosin-α (4 cycles), within an Expanded Access Program (EAP).
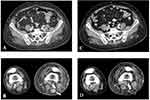
In April 2008, due to evidence of progressive disease (PD) of the pre-existing metastatic lesions, the patient was enrolled in the randomized, open-label, Phase II, BMS CA186-006 (NCT00612664) clinical trial and received the CD137 agonist mAb, urelumab, at 1 mg/kg i.v. every 3 weeks (10 cycles) until November 2008, without treatment-related side effects. Nevertheless, treatment had to be discontinued for the premature study closure due to two fatal cases of drug-related hepatic toxicity. Despite early treatment discontinuation, the patient achieved complete response (CR) in May 2009, 6 months after the last dose of urelumab, as shown by a CT scan (Figure 1). Importantly, she remained in CR for more than 4 years.
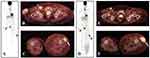
In December 2013, a control [18F] fluorodeoxy-glucose positron emission tomography (FDG-PET) scan revealed a right inguinal metastatic lymph node and a left thigh subcutaneous metastatic lesion; thus, the patient received standard third-line therapy with ipilimumab (3 mgs) i.v. every 3 weeks (4 doses). Therapy was well tolerated, except for a treatment-related (TR)-grade (G) 2 skin toxicity and TRG2 diarrhea, treated with symptomatic therapy and low dose steroids (ie, prednisone 25 mg/die) for 3 weeks, slowly tapered with rapid improvement of side effects. Tumor assessment (TA) performed by FDG-PET scan at week 12 showed a mixed response consisting of a partial regression of the right inguinal node and a progression of the left thigh subcutaneous lesion. Thus, the patient had achieved stable disease (SD) as per immune-related Response Criteria (irRC) and PD as per Response Evaluation Criteria in Solid Tumors (RECIST) v. 1.1 (Figure 2). However, the subsequent TA at week 24, confirmed PD according to RECIST v. 1.1 and irRC criteria (data not shown).
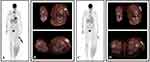
Based on the confirmed PD, in October 2014 the patient received fourth-line therapy with the anti-PD-1 pembrolizumab (2 mgs) i.v. every 3 weeks within an EAP, achieving a new CR at week 24 of therapy (Figure 3). Treatment was well tolerated with evidence of TRG1 arthralgia and TRG1 fatigue after the 4th cycle and TRG1 diarrhea after the 10th cycle, which resolved with symptomatic therapy.
In August 2017, due to the durable CR and to the patient’s desire, treatment with pembrolizumab was permanently discontinued. The last FDG-PET scan performed in December 2022 confirmed CR lasting 91+ months.
Discussion and Conclusions
Understanding the immune mechanisms controlling T-cell immunity has impressively grown over the past two decades, particularly in the field of co-stimulation and co-inhibition that can be therapeutically tilted to enhance immune responses against cancer cells. Thus, despite decades of failures and scepticism about cancer immunotherapy, ICI treatment with anti-CTLA-4 and -PD-1 has proven to be a valuable therapeutic strategy first in melanoma, and subsequently in other tumor types. These unprecedented clinical results are paving the way to the development of additional mAb to different co-inhibitory and co-stimulatory immune molecules. Thus, further exploring novel combinations and sequences of mAb to different ICB is a crucial issue to fully exploit their clinical potential; in this context, even single case experiences may help.
The patient we described here experienced a 4-year CR with urelumab against the co-stimulatory CD137 and, at disease recurrence, a new 7-year ongoing CR with pembrolizumab against the co-inhibitory PD-1. The message derived from this case report suggests that ICB targeting of co-inhibitory and co-stimulatory immune molecules can separately lead to very long disease-free survival, even in the presence of disease recurrence. Whether treatment with the co-stimulatory CD137 first, followed by the co-inhibitory PD-1 has facilitated the very positive outcome of this patient remains to be defined. However, though speculative, an intriguing hypothesis is that an effective anti-tumor immune priming induced by urelumab first followed by ipilimumab, may have facilitated the subsequent effector activity of pembrolizumab.23 Consistent with this notion, CTLA-4 blockade has been postulated to lead to a more favorable tumor milieu through the increase of tumor-infiltrating lymphocytes and interferon-γ-inducible genes that promoted the efficacy of concurrent or sequential anti-PD-1 therapy.24,25 Furthermore, co-targeting of CD137 and CTLA-4 increased the intratumoral CD8/Treg ratio and generated a significantly higher CD4 T-effector/T-reg ratio compared to either molecule alone, in the poorly immunogenic B16 melanoma model.23
Overall, the long-lasting complete responses we observed in this MM patient seem to indicate that the prospective clinical availability of a large array of therapeutic mAb to ICB, with different immune function, may generate extremely long-term survivals, even in elderly patients, and without impairment of their quality of life.
Abbreviations
ICB, Immune Checkpoint Blockade; CTLA-4, Cytotoxic T-Lymphocyte Antigen 4; PD-1, Programmed cell Death 1; mAb, monoclonal Antibody; MM, Metastatic Melanoma; ICI, Immune Checkpoint Inhibitors; ICOS, Inducible T-cell COStimulator; PD-L1, Programmed cell Death Ligand 1; CT, Computed Tomography; DTIC, Dacarbazine; EAP, Expanded Access Program; FDG-PET, [18F] FluoroDeoxy-Glucose Positron Emission Tomography; PD, Progression of Disease; SD, Stable Disease; CR, Complete Response; TR, Treatment Related; TA, Tumor Assessment; irRC, immune-related Response Criteria; RECIST, Response Evaluation Criteria in Solid Tumors.
Ethics Approval and Informed Consent
No institutional approval was required to publish the case details.
Consent for Publication
Informed consent was obtained in both written and verbal forms from patient to publish this case report and any accompanying images.
Acknowledgments
The authors wish to acknowledge the patient who participated in this study and her family.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
AMDG has served as a consultant and/or advisor to Incyte, Pierre Fabre, Glaxo Smith Kline, Bristol-Myers Squibb, Merck Sharp Dohme, SunPharma, Novartis and Sanofi and has received compensated educational activities from Bristol Myers Squibb, Merck Sharp Dohme, Pierre Fabre and Sanofi; MM has served as a consultant and/or advisor to Roche, Bristol-Myers Squibb, Merck Sharp Dohme, Incyte, AstraZeneca, Amgen, Pierre Fabre, Eli Lilly, Glaxo Smith Kline, Sciclone, Sanofi, Alfasigma, and Merck Serono; and owns shares in Epigen Therapeutics, Srl. ES, SC, MV, TS, MR have no conflict of interest to declare.
References
1. Hamid O, Robert C, Daud A, et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol. 2019;30(4):582–588. doi:10.1093/annonc/mdz011
2. Robert C, Long GV, Brady B, et al. Five-year outcomes with nivolumab in patients with wild -type. J Clin Oncol. 2020;38(33):3937–3946. doi:10.1200/JCO.20.00995
3. Maio M, Di Giacomo AM, Robert C, Eggermont AM. Update on the role of ipilimumab in melanoma and first data on new combination therapies. Curr Opin Oncol. 2013;25(2):166–172. doi:10.1097/CCO.0b013e32835dae4f
4. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi:10.1056/NEJMoa1003466
5. Maio M, Grob JJ, Aamdal S, et al. Five-year survival rates for treatment-naive patients with advanced melanoma who received ipilimumab plus dacarbazine in a phase III trial. J Clin Oncol. 2015;33(10):1191–1196. doi:10.1200/JCO.2014.56.6018
6. Schadendorf D, Hodi FS, Robert C, et al. Pooled analysis of long-term survival data from Phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015;33(17):1889–1894. doi:10.1200/JCO.2014.56.2736
7. Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Long-term outcomes with nivolumab plus ipilimumab or nivolumab alone versus ipilimumab in patients with advanced melanoma. J Clin Oncol. 2022;40(2):127–137. doi:10.1200/JCO.21.02229
8. Chen X, Zhang W, Yang W, Zhou M, Liu F. Acquired resistance for immune checkpoint inhibitors in cancer immunotherapy: challenges and prospects. Aging. 2022;14(2):1048–1064. doi:10.18632/aging.203833
9. Melero I, Hirschhorn-Cymerman D, Morales-Kastresana A, Sanmamed MF, Wolchok JD. Agonist antibodies to TNFR molecules that costimulate T and NK cells. Clin Cancer Res. 2013;19(5):1044–1053. doi:10.1158/1078-0432.CCR-12-2065
10. Chester C, Ambulkar S, Kohrt HE. 4-1BB agonism: adding the accelerator to cancer immunotherapy. Cancer Immunol Immunother. 2016;65(10):1243–1248. doi:10.1007/s00262-016-1829-2
11. Sanmamed MF, Pastor F, Rodriguez A, et al. Agonists of co-stimulation in cancer immunotherapy directed against CD137, OX40, GITR, CD27, CD28, and ICOS. Semin Oncol. 2015;42(4):640–655. doi:10.1053/j.seminoncol.2015.05.014
12. Marinelli O, Nabissi M, Morelli MB, et al. ICOS-L as a potential therapeutic target for cancer immunotherapy. Curr Protein Pept Sci. 2018;19(11):1107–1113. doi:10.2174/1389203719666180608093913
13. Fan X, Quezada SA, Sepulveda MA, et al. Engagement of the ICOS pathway markedly enhances efficacy of CTLA-4 blockade in cancer immunotherapy. J Exp Med. 2014;211(4):715–725. doi:10.1084/jem.20130590
14. Burris HA, Callahan MK, Tolcher AW, et al. Phase 1 safety of ICOS agonist antibody JTX-2011 alone and with nivolumab (nivo) in advanced solid tumors; predicted vs observed pharmacokinetics (pK) in iconic. J Clin Oncol. 2017;35(15 Suppl):3033. doi:10.1200/JCO.2017.35.15_suppl.3033
15. Yap TA, Gainor JF, Callahan MK, et al. First-in-human Phase I/II ICONIC trial of the ICOS agonist vopratelimab alone and with nivolumab: ICOS-High CD4 T-cell populations and predictors of response. Clin Cancer Res. 2022;28(17):3695–3708. doi:10.1158/1078-0432.CCR-21-4256
16. Jounce Therapeutics. Jounce therapeutics announces update on vopratelimab program; 2020. Available from: https://ir.jouncetx.com/news-releases/news-release-details/jounce-therapeutics-announces-update-vopratelimab-program/.
17. Maio M, Weber JS, Villar MV, et al. Inducible T cell costimulatory (ICOS) receptor agonist, feladilimab (FE), alone and in combination (combo) with pembrolizumab (PE): results from INDUCE-1 relapsed/refractory (R/R) melanoma expansion cohorts (EC). Cancer Res. 2021;81(13 Suppl):CT033. doi:10.1158/1538-7445.AM2021-CT033
18. GlaxoSmithKline. GSK provides update on feladilimab, an investigational inducible T cell co-stimulatory (ICOS) agonist; 2021. Available from: www.gsk.com/en-gb/media/press-releases/gsk-provides-update-on-feladilimab-an-investigational-inducible-t-cell-co-stimulatory-icos-agonist/.
19. Solinas C, Gu-Trantien C, Willard-Gallo K. The rationale behind targeting the ICOS-ICOS ligand costimulatory pathway in cancer immunotherapy. ESMO Open. 2020;5(1):e000544. doi:10.1136/esmoopen-2019-000544
20. Segal NH, Logan TF, Hodi FS, et al. Results from an integrated safety analysis of urelumab, an agonist anti-CD137 monoclonal antibody. Clin Cancer Res. 2017;23(8):1929–1936. doi:10.1158/1078-0432.CCR-16-1272
21. Segal NH, He AR, Doi T, et al. Phase 1 study of single agent utolimumab PF-05082566, an anti-4-1BB/cd137 agonist in patients with advanced cancer. Clin Cancer Res. 2018;24(8):1816–1823. doi:10.1158/1078-0432.CCR-17-1922
22. Tolcher AW, Sznol M, Hu-Lieskovan S, et al. Phase Ib study of Utolimumab (PF-05082566), a 4-1BB/CD137 Agonist, in combination with pembrolizumab (MK-3475) in patients with advanced solid tumors. Clin Cancer Res. 2017;23(18):5349–5357. doi:10.1158/1078-0432.CCR-17-1243
23. Curran MA, Kim M, Montalvo W, Al-Shamkhani A, Allison JP, von Herrath MG. Combination CTLA-4 blockade and 4-1BB activation enhances tumor rejection by increasing T-cell infiltration, proliferation, and cytokine production. PLoS One. 2011;6(4):e19499. doi:10.1371/journal.pone.0019499
24. Ji RR, Chasalow SD, Wang L, et al. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother. 2012;61(7):1019–1031. doi:10.1007/s00262-011-1172-6
25. Taube JM, Young GD, Mc Miller TL, et al. Differential expression of immune-regulatory genes associated with PD-L1 display in melanoma: implications for PD-1 pathway blockade. Clin Cancer Res. 2015;21(17):3969–3976. doi:10.1158/1078-0432.CCR-15-0244
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.