Back to Journals » Vascular Health and Risk Management » Volume 17
Fractional Flow Reserve: Patient Selection and Perspectives
Authors Peper J , Becker LM , van Kuijk JP, Leiner T, Swaans MJ
Received 13 August 2021
Accepted for publication 30 November 2021
Published 14 December 2021 Volume 2021:17 Pages 817—831
DOI https://doi.org/10.2147/VHRM.S286916
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Daniel Duprez
Joyce Peper,1,2,* Leonie M Becker,1,2,* Jan-Peter van Kuijk,1 Tim Leiner,2 Martin J Swaans1
1Department of Cardiology, St. Antonius Hospital, Nieuwegein, The Netherlands; 2Department of Radiology, University Medical Center Utrecht, Utrecht, The Netherlands
*These authors contributed equally to this work
Correspondence: Joyce Peper
Department of Cardiology, St. Antonius Hospital, Koekoekslaan 1, Nieuwegein, 3435 CM, The Netherlands
Tel +31 88 320 09 22
Email [email protected]
Abstract: The aim of this review was to discuss the current practice and patient selection for invasive FFR, new techniques to estimate invasive FFR and future of coronary physiology tests. We elaborate on the indication and application of FFR and on the contraindications and concerns in certain patient populations.
Keywords: coronary artery disease, fractional flow reserve, patient selection, revascularisation
Introduction
Coronary artery disease (CAD) remains the main cause of morbidity and mortality in the world. Improvement in the diagnostic and therapeutic pathways can improve the overall health status and reduce the economic burden of CAD.1–4 Optimal medical therapy (OMT) is considered the foundation to treat symptoms inherent to CAD and to prevent major cardiovascular events.5 Additional revascularization procedures, percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG), lead to a reasonable increase in quality of life and/or life expectancy.6–8 However, adequate patient selection for revascularization procedures of coronary lesions is of growing importance due to the rapidly increasing burden of CAD.
Invasive coronary angiography (ICA) is generally used to diagnose significant CAD, although the correlation between stenosis severity, blood flow and patient prognosis is complicated.9,10 The association between visual assessment of a lesion and its physiological significance is poor.1 Therefore, numerous coronary physiology tests can be integrated into the diagnostic strategy to assess coronary blood flow through exercise or pharmacological stimulation. Fractional flow reserve (FFR), invasive coronary physiology measurements with coronary guidewires with a pressure sensor located near the tip, has become routine in most catheterization labs.2,11,12 FFR is defined as the ratio of the measured pressure distal of a coronary stenosis (Pd) in relation to the pressure proximal to the stenosis, usually aortic pressure (Pa) or alternatively the pressure in a healthy proximal coronary segment (FFR = Pd divided by Pa). It was originally defined as the ratio of the maximal flow before and after a stenosis. However, pressure measurements are less complicated to perform and show a (near) linear correlation with the blood flow. The linear correlation between pressure and flow is only accurate when pressure measurements are performed while the coronary resistance is minimal. Hyperemia is necessary to minimize this resistance. The most frequently used drug to induce hyperemia is adenosine, administered by either continuous intravenous infusion (140 μg/kg/min) or intracoronary bolus.12 In clinical decision-making, FFR is used as a dichotomous variable with a value ≤0.80 as indication for revascularization and >0.80 as indication for a conservative approach.13 In the current European Society of Cardiology (ESC) guideline, FFR has a class 1A recommendation for identification of hemodynamically relevant coronary lesions in stable patients when evidence of ischemia is not available (Table 1).1
 |
Table 1 Current Guidelines for FFR |
In this review, we elaborate on the current applications and patient selection for invasive FFR, new techniques to estimate invasive FFR and future of coronary physiology tests.
Practical Aspects and Patient Selection
Methodology of FFR
To ensure optimal reliability and minimal risk of FFR-measurements in all circumstances, a correct and standardized methodology has to be applied. In 2017, an expert consensus document by Achenbach et al summarized the recommendations for performing and interpreting FFR.14 In brief, the patient should have a large venous access in the cubital vein or more proximal with sufficient flow to ensure timely arrival of the hyperemic agents, which generally have a short half-life. Since FFR-catheters are foreign material, sufficient antithrombotic prophylaxis should be administered. Generally, there is no procedure-related reason to prefer either transfemoral or transradial access as long as there is sufficient space for at least a 5F guide catheter. Use of guide catheters is recommended to ensure that wire-related dissections (a rare but serious complication of FFR) can be treated immediately. The catheters should be without side-holes, as these can influence both deliverance of the hyperemic agent and pressure calibrations. All calibrations should be performed before and after FFR-measurements to ensure artifact-free measurements. During pressure equalizations, it is important that the introducer is removed, the hemostatic valve is completely closed and, if possible, other wires than the pressure wire are removed to prevent artifacts. Pressure curves should be stable and free of artifacts for at least three to five heartbeats. To prevent spasms and minimize resistance, intracoronary nitroglycerin should be administered before advancing the FFR-wire. During FFR-measurements, it needs to be ensured that the catheter does not occlude the ostium or more proximal stenoses since the reduced coronary flow can lead to falsely elevated FFR-values. During PCI, it is important to ensure the FFR-wire is not jailed by the stent.14
Choice of Hyperemic Agents
The expert consensus document by Achenbach et al elaborated on the choice of hyperemic agents as well.14 In short, hyperemia can be induced by intravenous or intracoronary administered medications.14 Both procedures have two options. Intravenously, operators can choose between adenosine 140 µg/kg/min or regadenoson 400µg. Intracoronary, operators can choose between adenosine 40–200µg rapid bolus, usually 40µg for the right coronary system and 80µg for the left coronary system, or papaverine, 8mg for the right coronary system and 12mg for the left coronary system.
Generally, adenosine is the first choice for both intravenous and intracoronary administration as it is the most thoroughly tested drug. Because it has a very short half-life, administration should be as proximal as possible and with sufficient flow speed. Intravenous adenosine takes effect after approximately one minute. Intravenous regadenoson might have fewer side effects for COPD-patients. It takes effect after a little over half a minute, reaches its peak effect half a minute after this and then fades out over 10 minutes. Intracoronary papaverine takes effect after approximately 15 seconds and lasts 40–60 seconds. Papaverine has not been thoroughly studied and might have a higher rate of side effects. It is therefore not recommended as routine hyperemia drug but might be a good choice when adenosine is contraindicated.14
Current Indications and Applications of FFR
FFR can be used in both stable coronary artery disease and acute coronary syndrome, although the direct indication differs. Moreover, FFR can be helpful as predictor for cardiovascular events and in the assessment of hemodynamic relevance of a myocardial bridge. We discuss these indications and applications separately below.
Stable CAD and FFR
ICA with FFR is the current standard for diagnosing hemodynamically significant CAD and determining the need for revascularization. FFR is indicated in all patients with intermediate coronary lesions visible during ICA, which is defined as a 40–90% stenosis in the European guidelines. The current guidelines are based on several trials published in the past decades.1,2,11,12 The DEFER trial was the first trial to evaluate the clinical impact of FFR-guided revascularization in patients with intermediate stenoses. Their results showed that PCI for stenosis with FFR-values <0.75 improves angina pectoris and associated symptoms.15 The second trial on FFR-guided treatment was the FAME-trial. It was found that FFR-guided treatment using a different cut-off value of 0.80 is associated with a significant reduction in invasive procedures and major adverse cardiac events (MACE - a composite of death, myocardial infarction and repeat revascularization) until 2 years after PCI compared to ICA-guided treatment.16 The FAME-2 trial was developed to determine whether this difference could be attributed to the FFR-guided strategy but was terminated prematurely. An unacceptable higher number of MACE in the OMT-group was observed, driven by the high incidence of urgent revascularization procedures.17 These results were confirmed after 5 years of follow-up.18
For patients without hemodynamically significant CAD, adding PCI to OMT does not lead to a further reduction in MACE.2,12 Moreover, the residual anatomic disease burden was not associated with target vessel failure 2 years after PCI.11 The DEFER trial shows, after 15 years of follow-up, that patients in whom PCI was deferred based on non-significant FFR had less myocardial infarctions than patients who had received PCI despite non-significant FFR. These results suggest that PCI of functionally non-significant lesions actually worsens long-term outcomes.19
Multiple studies have investigated the use of FFR for treatment guidance in intermediate LMCA stenoses to decide between surgical revascularization and OMT. These studies found that revascularization (usually by CABG in these studies) can be safely deferred for patients with FFR-values >0.80. No difference was found in survival rates between patients with FFR >0.80 treated with OMT and patients with FFR ≤0.80 that received CABG. FFR is a safe and helpful tool in the decision-making process regarding LMCA stenoses and is recommended for patients with intermediate LMCA stenoses to avoid unnecessary revascularization procedures.20,21
Acute Coronary Syndrome
PCI is the quickest and most readily available revascularization strategy, and therefore the method of choice in patients with acute coronary syndromes (ACS).22 FFR is not recommended as functional assessment strategy for the culprit lesion. Microcirculatory debris after the culprit lesion causes elevated microvasculature resistance, leading to a false elevation of the FFR-value.23 However, FFR is accurate for the assessment of additional lesions in the presence of multivessel disease (MVD).24 The PRAMI-trial, the COMPLETE trial and CvLPRIT-trial all showed that full revascularization of all angiographically significant stenoses of ST-elevation myocardial infarction (STEMI) patients led to a significant reduction in MACE during follow-up compared to culprit-only treatment.25–27 Moreover, the Compare-Acute trial and the DANAMI-3-PRIMULTI trial used an FFR-based treatment strategy for STEMI patients with MVD and also showed significantly less MACE after full revascularization, especially due to more urgent revascularizations after culprit-only treatment.22,28 A FAME-sub study showed that FFR-guided revascularization is sufficient, and revascularization of lesions that were only angiographically significant did not further reduce the risk of MACE.29 Therefore, the 2018 ESC guidelines on revascularization recommend full revascularization of all significant lesions in ACS-patients with MVD.
Post-PCI FFR
Assessing stenoses by anatomic severity only is unreliable, and this extends to the assessment of PCI results.30 Optimal post-PCI FFR-values, generally meaning FFR ≥0.90, are associated with better outcomes, such as a lower rate of MACE and angina recurrence.30,31 With angiographically satisfactory PCI-results, assessment of post-PCI FFR shows suboptimal FFR-values in 30–65% of the patients, and impaired FFR-values (FFR ≤0.80) in up to 20% of the patients.30–33 Post-PCI FFR-values are influenced by various factors, such as diffuse CAD without focal lesions, the presence of residual lesions unsuitable for PCI, an initial narrow or short stent trajectory, stent malposition or suboptimal expansion, edge dissection and plaque protrusion.30,31,33 Additionally, improved coronary flow after PCI might show other stenoses in the same coronary artery to be functionally significant as well, especially more distal lesions.31
Multiple studies have assessed the prevalence and subsequent treatment of suboptimal and impaired FFR-values after PCI.30–33 Treatment is generally performed by post-dilatation or additional stenting, sometimes aided by intracoronary imaging techniques such as IVUS and OCT.31 While additional treatments generally improve the post-PCI FFR-value, optimal FFR-values are only obtained in a minority of treated lesions.30–33 Limited evidence is available, whereas the only randomized controlled trial showed no significant difference in outcomes between physiology-guided and FFR-guided assessment of PCI-results.33
It is likely that the factors that influence post-PCI FFR-values are also risk factors for future atherosclerosis and target vessel failure, especially diffuse coronary disease and residual lesions. The cause for suboptimal and impaired post-PCI FFR-values is often located outside the stent.31 It might be that patients at higher risk for future MACE or target vessel failure are also more likely to have suboptimal post-PCI FFR-values, regardless of any existing causal relationship. Although theoretically likely, it is currently unknown whether additional treatments based on suboptimal or impaired post-PCI FFR lead to better long-term outcomes.31 More randomized controlled trials with long follow-up duration are needed to assess this. Therefore, current evidence does not support FFR-measurements post-PCI.
Global FFR as Independent Predictor
Global FFR is defined as the sum of the FFR-values in the three major coronary arteries (right coronary artery (RCA), left anterior descending artery (LAD), circumflex artery (Cx).13 A retrospective study compared global FFR-values among patients of the FAME trials without hemodynamically significant lesions. Lower global FFR-values (<2.80) were associated with a higher MACE-rate compared to intermediate (2.80–2.88) and high (>2.88) global FFR-values, mainly driven by a higher rate of acute revascularization. No relation was found between the 5-year outcome and the presence or number of angiographically significant lesions or whether or not patients had undergone PCI.13
Assessment of Hemodynamic Significance of Myocardial Bridging
Myocardial bridging is a congenital anomaly in which part of a coronary artery is covered by overlying myocardium.34 This results in vessel compression during systole and might cause angina and myocardial ischemia in the absence of coronary artery disease.34,35 Intravascular ultrasound is the standard for the detection of myocardial bridging. Only in a minority of cases, the bridge can be identified by the characteristic “milking effect” during regular ICA.34,36 Since regular FFR is based on mean pressures over the full cardiac cycle, and systolic pressure overshooting occurs in myocardial bridging, FFR is not reliable.37 Newer techniques as iFR can overcome these limitations. iFR will be discussed in the chapter “advances in non and less invasive FFR”.
Patient Selection
The guidelines currently recommend measuring FFR in all patients with intermediate coronary lesions observed during ICA. However, several comorbidities might influence the performance and safety of FFR. The most important ones are discussed below.
Elderly Patients
With increasing age comes increased microvascular dysfunction and coronary flow velocity, leading to a reduction of coronary perfusion pressure and diastolic coronary filling. Theoretically, this would affect the assessment of FFR and impact its accuracy. Multiple studies have shown that FFR-values increase with age, independently of other factors such as stenosis severity.38–40 The difference between resting pressure and pressure during maximal hyperemia is lower in elderly patients.38,40 A significantly higher FFR has been observed for similar degrees of stenosis and lesion length in older patients (>70 years of age) compared to younger patients (<70 years). It is hypothesized that this can be attributed to the reduced hyperemia response, meaning reduced vasodilatory response to adenosine. This hypothesis is supported by a significantly larger decrease of FFR-values in younger patients after administration of increasing doses of adenosine.39 These differences might lead to undertreatment of coronary lesions in elderly patients when using FFR. On the other hand, the possible impact of diseased microvasculature on FFR-measurements is subject of continued debate. It has been argued that myocardial resistance should still be minimal in the presence of a diseased microvasculature, and therefore coronary pressure should be proportionate to coronary flow regardless of vasodilatory response.41
The impact of the earlier mentioned differences on treatment decisions, clinical condition and MACE was not mentioned in the reporting studies. However, a FAME-substudy found that, while FFR-values are less likely to be abnormal in elderly patients for any given stenosis degree, FFR-guided PCI was equally beneficial compared to angiography-guided PCI for elderly patients (>65 years of age) with MVD as for younger patients with MVD.40 Therefore, the use of FFR-guidance in the treatment of CAD is currently recommended in elderly patients. Currently, the FIRE-trial (NCT03772743) is investigating the impact of full FFR-guided revascularization in elderly patients with MVD on MACE.42
Diabetes Mellitus
Visually estimating CAD-severity is even more challenging in patients suffering from diabetes mellitus (DM) because of diffuse and accelerated atherosclerosis. Additionally, diabetics more frequently suffer from microvascular dysfunction, leading to elevated pressure in the microcirculation, which theoretically leads to elevated FFR-values. Because of this, the accuracy of FFR has been questioned in diabetic patients.43,44 Studies comparing FFR-values between diabetic and non-diabetic patients with similar Quantitative Coronary Analysis (QCA) results demonstrated that assessment of functional severity of coronary stenosis using FFR was reliable in diabetic patients. They did however observe suboptimal FFR-measurements in diabetic patients with HbA1c >7%, which occurs in patients with chronically high plasma glucose (ie, uncontrolled DM).43,45 Deferral of PCI in diabetics has been shown to be safe when FFR-values indicate a hemodynamic non-significant stenosis, but a tendency towards more target lesion revascularizations has been reported.46 A secondary analysis of the DEFINE-FLAIR trial described an almost twofold increase of the risk of MACE for diabetic patients compared to non-diabetic patients, mainly due to more nonfatal myocardial infarction and unplanned revascularizations. No significant interaction between the revascularization strategy and the presence of diabetes for risk of death, cardiovascular death and unplanned revascularization was observed.47
Based on current evidence, FFR is a safe and reliable technique in diabetic patients, although reduced vasodilatory response might lead to falsely elevated FFR-values in uncontrolled diabetes with high HbA1c levels. FFR-guided PCI in diabetic patients is associated with similar benefits as FFR-guided PCI in non-diabetics when compared to angiography-based revascularization.
Left Ventricular Hypertrophy
Both increasing and decreasing effects on measured FFR-values have been posed in patients with left ventricular hypertrophy (LVH) on FFR-accuracy. Based on hemodynamic theory there should be an inverse relationship between the mass of myocardium supported by a coronary segment and the FFR-value of this segment, where a similar stenosis produces a lower FFR-value in the presence of a larger myocardial mass.48,49 It has been shown that a stenosis in a coronary segment that supports a large amount of myocardium (eg, proximal LAD) is more likely to have an FFR-value ≤0.80 than a stenosis of similar degree in a segment supporting a small amount of myocardium (eg, distal RCA). These findings support the hemodynamic theory, although it has not been assessed in patients with severe LVH.48 On the other hand, LVH is associated with microvascular dysfunction and decreased coronary flow. Diastolic dysfunction, which is often present in some degree in LVH, leads to increased extravascular compression of the microcirculation and thus elevates FFR. These factors might balance one another’s effects, explaining why various other studies investigating the impact of LVH on FFR found no difference between patients with and without LVH.49,50 The current evidence therefore supports the use of FFR in patients with LVH.
Heart Failure and Impaired Left Ventricular Function
Most research on the combination of heart failure and CAD has been conducted on patients with ischemic cardiomyopathy. Successful revascularization in patients with ischemic cardiomyopathy based on untreated CAD might improve left ventricular function (LVF) and prognosis, especially when hibernating myocardium is present.2 Guidelines suggest a similar approach regarding assessment of CAD-severity in patients with heart failure.51 However, there is a theoretical concern that FFR is less accurate in patients with reduced left ventricular ejection fraction (LVEF). This is based on the possible effects that increased left ventricular end-diastolic pressure, elevated venous pressure and reduced mass of viable myocardium could have on coronary pressure and flow.52 A sub-analysis of the FAME study, comparing patients with preserved LVEF first to those with LVEF <50%, and subsequently <40%, showed no impact of reduced LVEF on the FFR-value unless there was a severe stenosis (>90%) in the measured segment. The reduction of MACE and its individual components in the FFR-guided arm was similar for patients with reduced LVEF compared to those with preserved LVEF.52 Moreover, FFR guided treatment was associated with lower rates of major adverse cardiovascular and cerebrovascular events (MACCE) and less invasive treatment procedures. Patients more frequently underwent PCI instead of CABG and were more often deferred to OMT.53 Additionally, it has been shown that the differences in FFR-values in patients with elevated right atrial pressures had negligible clinical impact and were usually within the limits of the test–retest repeatability.54 In conclusion, current evidence does not support or show any proof that the mentioned theoretical concerns influence the accuracy of FFR in patients with reduced LVEF.
Severe Aortic Valve Stenosis
Since CAD is common in patients with severe aortic stenosis, coronary angiography before valve replacement is recommended to assess whether revascularization is needed.2 The accuracy of FFR-measurements in patients with severe aortic stenosis is unclear. It is hypothesized that the higher ventricular pressures, lower aortic pressures, lower coronary flow and microvasculatory dysfunction that are associated with aortic stenosis affect FFR-values.1,55 Additionally, the hemodynamic stability in patients with severe aortic stenosis is fragile, which might cause medical professionals to avoid the use of vasoactive medication and invasive intracoronary pressure measurements.55 Use of FFR in patients with aortic valve stenosis is associated with downgrading of CAD-severity, more revascularization procedures with PCI instead of CABG and deferred valve replacement. FFR-guided CABG in patients with aortic stenosis is associated with less venous grafts and fewer anastomoses. The rates of MACE after 5 years of follow-up appear to be comparable for FFR-guidance and angiography-guidance in patients with aortic stenosis.56 Generally, in patients with FFR-values ≤0.80 pre-TAVI, indicating hemodynamically significant stenosis, FFR tends to be even lower after the procedure. For patients with FFR-values >0.80 pre-TAVI, FFR tends to increase after the procedure.57 FFR-changes after TAVI appear to be generally mild, but borderline negative values might become positive after TAVI in a minority of patients and vice versa. A study comparing FFR-values pre- and post-TAVI found that negative FFR-values pre-TAVI became positive post-TAVI in 6% of the patients, and borderline positive FFR-values (0.75–0.80) pre-TAVI became negative after TAVI in 5% of the patients.57 However, a decreased FFR post-TAVI with further reduction after long-term follow-up has also been found and was attributed to increased hyperemic coronary flow velocity. Other physiological tests remained unchanged after TAVI.58 This suggests that severe aortic stenosis causes falsely elevated FFR-measurements, leading to underestimation of the stenosis severity. Since no randomized controlled trials have been performed, the 2018 ESC-guidelines on revascularization stated that the available evidence is insufficient to support invasive functional assessment of coronary lesions in patients with severe aortic stenosis.1
Impact of FFR on Revascularization Strategy
The advantage of an FFR-guided revascularization strategy compared to ICA-guided revascularization has been sufficiently proven by the large, randomized trials described in this review. This advantage is present regardless of comorbidities. For CABG, however, the benefit of FFR-guidance is still controversial. Additionally, some lesions are more challenging to approach for either revascularization strategy. Below we discuss the place of FFR in CABG decision-making and its reliability in the treatment of bifurcation lesions.
CABG
As mentioned before, visual assessment of the coronary arteries is not sufficient to assess the hemodynamic significance of a coronary lesion.1 However, several meta-analyses did not find a difference in MACE between FFR-guided and ICA-guided CABG. They did find that FFR guided CABG reduces the complexity of the surgery. The FFR-guided patients received a lower number of anastomoses, more arterial grafts and more frequently underwent off-pump procedures, which are associated with a reduction of (short-term) complications.59–62 Moreover, a significant improvement in graft patency compared to ICA-guided CABG is observed.59 A possible explanation for the apparent lack of benefits for FFR-guided CABG can be found in a process called surgical collateralization – grafting might protect against the effects of progression of coronary lesions in proximal segments.1,61 Grafting of non-critically diseased coronary arteries is associated with a higher rate of graft closure due to competitive flow in the grafted coronary artery.1,21 Functional testing may help guide the surgical revascularization strategy in ambiguous lesions.1 Since FFR-guided CABG led to less complex procedures, FFR could be a useful tool for Heart Team discussions to choose the best and least invasive surgical approach for each individual patient.61
The FUTURE-trial aimed to evaluate the role of FFR in determining a treatment strategy by assessing clinical outcomes and cost-effectiveness of FFR-guided treatment versus angiography-guided treatment for patients with intermediate coronary lesions.63,64 In the FFR-guided group, less patients received PCI and more patients received medical therapy compared to the angiography-guided group. The rate of CABG was similar.12 The FUTURE-trial was designed to show a 30% relative risk reduction of MACE for FFR-guidance.63,64 However, it was terminated prematurely because of an unexplained but significant higher mortality rate in the FFR-guided group (17 deaths versus 7 deaths among a combined number of 937 patients).12,64 While the composite endpoint of death, myocardial infarction, repeat revascularization and stroke was not different between both groups after 2 years of follow-up, the risk of mortality remained significantly higher in the FFR-guided arm.64 An exploratory analysis showed that patients in the FFR-group had more severe CAD and more three-vessel disease. Since the FUTURE-trial is the only large trial in which FFR-guidance was associated with a higher mortality rate, and the low overall mortality rate meant one or two additional deaths could make the difference between significance and non-significance, it is suspected that the findings are due to chance.64
Bifurcation Lesions
The treatment of bifurcation lesions is complex and associated with higher event rates.65,66 The use of FFR in diagnosing significant bifurcation- and side branch lesions is safe and leads to less side branch interventions with similar clinical outcomes. FFR shows functional significant stenosis in only a minority of the angiographically significant side branches, and points out functional significance in angiographically non-significant side branches.67–70 However, a functionally significant stenosis proximal or distal of the side branch lesion can influence the measured FFR-value, leading to a falsely lowered or elevated FFR, respectively. Considering this influence, appropriate timing and placement of FFR-measurements is important when evaluating side branch lesions.67–70
Contraindications and Concerns
Despite the class I recommendation for the use of FFR in the ESC guidelines, it is performed in only 10–20% of the patients with intermediary stenoses.1 During ICA, most coronary lesions are only assessed visually, despite the poor correlation between visual estimation and functional significance. Factors that might contribute to this low adoption rate are discussed below.
Assumptions Made in FFR-Development
One of the initial assumptions in the development of invasive techniques, among which FFR, is that functional significant CAD leads to a higher risk of MACE and mortality if left untreated. This assumption is supported by the results of the FAME2-trial, which was prematurely ended due to a higher rate of MACE for patients treated with OMT compared to those treated with FFR-guided PCI.17 However, the ISCHEMIA-trial showed no difference in MACE comparing initial OMT-based treatment to direct invasive testing and revascularization and the ORBITA-trial found no difference in MACE and angina pectoris between patients receiving FFR-guided PCI and those receiving sham-PCI.7,71 In the FAME-2 trial, there was no difference in deaths and myocardial infarction between groups. The higher rate of MACE in the FAME-2 trial was due to a higher rate of unplanned revascularizations.17 These results however shed more doubt on the usefulness of revascularization, than on FFR. Since an FFR-guidance usually leads to more conservative treatment strategies, this seems an argument in favor of FFR. Additionally, since the studies that show more urgent revascularizations were usually not blinded, this could mean that operators are more likely to perform PCI after initial conservative treatment because they felt that treatment of CAD was warranted despite the initial allocation. On the other hand, in the ISCHEMIA-trial, patients in the OMT-group did have more angina pectoris. Improvement of the quality of life is an important outcome of the efficacy of treatment, which supports revascularization for significant CAD.7
The pathway that led to the development of FFR has been cause for concern for some. The recognized shortcomings of diagnostic ICA led to the development of cardiac stress tests, to assess whether cardiac ischemia was present. The interpretation of these stress tests was made in relation to ICA images. To directly assess the hemodynamic significance of specific stenoses visualized during ICA, FFR was developed after this, based on ICA images and calibrated against cardiac stress tests.72 It has been argued that the current recommendation to treat coronary lesions that result in FFR-values ≤0.80 defines CAD as a localized instead of diffuse disease.13,72 However, when using FFR as guidance for treatment a dichotomization is necessary to define a treatment threshold. Despite the concerns regarding the development, the consistent positive outcomes for patients when using FFR as gatekeeper support its strong recommendation in current guidelines.
Factors Influencing Medical Professionals
The effect of various factors on the adoption rate of FFR was investigated in a brief online survey among 104 interventional cardiologists from various countries. The mean adoption rate of coronary physiology for all PCIs among these cardiologists was 26.9%, without significant effects of age and years of experience. The investigators found that ease of use and knowledge about guidelines had a positive effect on the adoption rate of coronary physiology, while constraints due to competing tasks, finances or time had a negative impact. Motivation, considered importance of guideline adherence, and training played no significant role.73
The significant positive impact of knowledge about guidelines shows the importance of the current class 1A recommendation for coronary physiology in guidelines.1,2 Concerning time constraints, studies have demonstrated that FFR reduces procedural time. The additional time required to perform the analysis is more than balanced by less invasive treatment after performing FFR, ie, less time spent on stenting. Financial constraints lie in the high cost of the pressure wire, which is approximately 600–800 euros, and the additional expense of adenosine. However, it seems reasonable to assume that the reduced procedure rate after FFR balances the additional costs tied to coronary physiology assessment.73
Changes in Coronary Flow Post-Myocardial Infarction
Shortly after myocardial infarction, debris and post-ischemic effects such as platelet plugging, thrombus embolization, coronary vasospasm, endothelial dysfunction and vascular stunning, affect the flow and pressure in the coronary arteries and -microcirculation. Additionally, coronary blood flow is lower in both culprit- and non-culprit vessels after recent myocardial infarction, especially when supporting infarcted myocardium. However, the FFR-values of vessels that support recently infarcted myocardium are similar to the FFR-values of control vessels that did not experience ischemia.41,74 Currently, it is unknown how long these effects remain present after myocardial infarction.75
Coronary Tortuosity
Theoretically, increased coronary tortuosity is related to a larger decrease in coronary blood pressure, although the effect of coronary tortuosity on coronary blood supply at rest appears to be minimal.76–78 Patients with tortuous coronary arteries without visible CAD have normal FFR-values. They do appear to have lower CFR-rates and higher IMR compared to reference values for normal coronary arteries. Lower coronary flow is associated with falsely lowered FFR-values, while microvascular dysfunction is associated with falsely higher FFR-values. These factors might influence the accuracy of FFR-measurements in the presence of coronary tortuosity, although it is also possible that these effects balance each other. However, no studies were found investigating the effect of tortuous coronary arteries on FFR accuracy in diseased coronary arteries. Currently, FFR is considered safe and reliable in the presence of coronary tortuosity.77
Contraindications for FFR
There are no absolute contraindications for FFR-measurements.2,23 Second- and third degree atrioventricular blocks, sick sinus syndrome without pacemaker, prolonged QT-interval, severe hypotension, heart failure and obstructive pulmonary diseases are relative contraindications for intravenous administration of adenosine.79 However, the clinical condition of the patient and the expected benefit of the procedure should be taken into account, similar to the considerations of ICA. Additionally, invasive diagnostic procedures should not be performed if there are no invasive therapeutic options feasible.23
Advances in Non and Less Invasive FFR
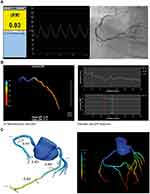
Although invasive FFR is a well described and evaluated diagnostic test, there are multiple limitations and concerns resulting in the underuse of FFR. Besides invasive FFR, several advancements for less- or even non-invasive FFR have been made. Those newer, less invasive techniques have been developed to overcome previously stated objections regarding FFR. They therefore have the potential to be implemented in the current workflow and can eliminate the need for invasive flow- or pressure measurements. Figure 1 gives an overview of different less- and non-invasive FFR techniques.
Non-Hyperemic Pressure Ratios
In adition to measurements under hyperemic conditions as performed with FFR, various alternatives have been developed using pressure wires. These alternatives measure the pressure gradient across a coronary stenosis without the need for hyperemia. Non-hyperemic pressure ratios (NHPR) can be obtained using whole-cycle Pd/Pa at rest or using sub-cycle measurements as instantaneous wave-free ratio (iFR - Philips),80 resting full-cycle ratio (RFR - Abbott),81 diastolic hyperemia-free ratio (DFR - Boston Scientific)82 and the diastolic pressure ratio (dPR – OpSens).83 A cut-off value of ≤0.89 is used as revascularization threshold.84
The first and best validated NHPR is iFR. It is based on the hypothesis that during a specific time interval during the cardiac cycle, the diastolic “wave-free period”, coronary flow is not influenced by (de)compression of the microcirculation and microvascular resistance is minimized.85 iFR has similar diagnostic performance compared to FFR when using different reference tests for myocardial ischaemia. Both iFR and FFR have different strengths and weaknesses. Whereas FFR has a stronger theoretical framework and is better validated, iFR has shorter procedural times and avoids the side effects, costs and contraindications of adenosine.86 Previous randomized controlled trials as the DEFINE-FLAIR and the iFR SWEDEHEART demonstrated non-inferiority of iFR to FFR with respect to the rate of major adverse cardiac events at 12 months.84,87
Although iFR does not require a hyperemic agent, it does require an invasive procedure with FFR-wire.
FFR-CT
The developments in computational flow dynamics (CFD) of the past decade give the opportunity for non-invasive ischemia detection using anatomical CCTA data only. Computed tomography–derived FFR (FFR-CT) enables to simulate coronary blood flow and obtain functional information on the hemodynamically severity of a stenosis. Anatomical models derived from CCTA are used as input for FFR-CT. Advanced calculations based on CFD are made to estimate the flow in the coronary arteries. To shorten the calculation time of the simulation models, several assumptions on boundary conditions (eg, viscosity of blood, inflow conditions), modeling microvascular resistance and compliance are made.4 The first and only commercially available FFR-CT software is developed by HeartFlow Inc (Redwood City, CA, USA). HeartFlow offers a solution in which an artificial intelligence–powered offsite algorithm and trained analysts use the sent CCTA-images to create the HeartFlow analysis. This color-coded three-dimensional model provides functional information about the patient’s coronary arteries. Previous trials – the DISCOVER-FLOW, DEFACTO and NXT – showed improved diagnostic accuracy in the detection of hemodynamic significant CAD of HeartFlow FFR-CT compared to CCTA.88–90 Beyond the HeartFlow application, other vendors as Siemens Healthineers, Canon Medical Systems (formerly Toshiba Medical Systems Corp), Philips Healthcare and various research groups developed FFR-CT algorithms.91–98 These algorithms are not yet commercially available. A recent meta-analysis showed similar performance in terms of diagnostic performance measurements (ie, sensitivity, specificity and accuracy) for both HeartFlow and the other FFR-CT algorithms.99 No randomized trials on clinical events are available yet. Multiple clinical trials are recruiting patients, eg, the iCORONARY trial (NCT04939207).
Invasive Coronary Angiography-Based FFR
More invasive than FFR-CT, but less invasive than FFR is invasive coronary angiography-based FFR. Angiography-based FFR uses three-dimensional reconstructions of images of the coronary arteries acquired by ICA.100 This reconstruction of the artery of interest can be constructed of multiple images of the same artery in different angles using three-dimensional quantitative coronary analysis (3D-QCA). This reconstruction is used as input for advanced calculations enable to estimate the flow in the target vessel and across the lesion. The ratio between the calculated flow proximal and distal to the lesions serves as proxy for invasive FFR.
Multiple vendors offer software for angiography-based FFR, although the most widely evaluated and used angiography-based FFR is the Quantitative Flow Ratio (QFR) by Medis Medical Imaging Systems (Leiden, the Netherlands).101,102 QFR can be performed on-site and requires some user interaction, for example for frame selection, indicating the start and endpoint of target vessel and lumen contouring. Previous trials as the FAVOR II China, FAVOR II Europe–Japan and the WIFI II demonstrated excellent diagnostic performance using invasive FFR as reference standard.103–105 Besides commercially available QFR, other angiography-based methods as vFAI, vFFR, FFRangio, QCA-TP and CAAS-vFFR have been developed.106–110 Similar to FFR-CT, no randomized controlled trials on clinical endpoints are available.
Future Perspectives
While invasive FFR will remain an important determinant of the treatment indication of CAD, we expect that the newer less- and noninvasive techniques will play an increasingly important role. However, an advantage of ischemia-detection during ICA is that PCI can be performed immediately.2,24 Currently, many practical objections have contributed to the low adoption rate of invasive FFR-measurements. The new techniques render many objections against FFR-measurements moot as they have a lower to no procedure-related complication rate, are less invasive for the patient, come with lower cost of time and resources and some techniques do not even require additional appointments or catheterization lab capacity. Nowadays, these techniques have mainly been tested in an experimental setting, directly comparing the results against ICA with or without invasive FFR. The cost-effectiveness and long-term safety of basing treatment decisions solely on these FFR-based techniques will have to be confirmed, preferable in large randomized trials, before they can assume the position of the new standard.
Abbreviations
ACS, acute coronary syndrome; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CCTA, coronary computed tomography angiography; CFD, computational flow dynamics; CFR, coronary flow reserve; Cx, circumflex (artery); ESC, European Society of Cardiology; ICA, invasive coronary angiography; iFR, instantaneous wave-free ratio; IMR, microcirculatory resistance; FFR, fractional flow reserve; FFR-CT, computed tomography-derived FFR; LAD, left anterior descending (artery); LMCA, left main coronary artery; LVEF, left ventricular ejection fraction; LVH, left ventricular hypertrophy; LVF, left ventricular function; MACCE, major adverse cardiovascular and cerebrovascular events; MACE, major adverse cardiac events; MRI, magnetic resonance imaging; MVD, multivessel disease; NSTE-ACS, non-ST elevation acute coronary syndrome; NSTEMI, non-ST elevation myocardial infarction; OMT, optimal medical therapy; PCI, percutaneous coronary intervention; QFR, quantitative flow ratio; RCA, right coronary artery; STEMI, ST-elevation myocardial infarction.
Disclosure
Prof. Dr Tim Leiner reports grants from ZonMW, outside the submitted work; in addition, Prof. Dr Tim Leiner has a patent 11004198 licensed to Pie Medical B.V. Dr Martin J Swaans reports personal fees from Abbott Vascular, personal fees from Boston Scientific, personal fees from Philips Healthcare, personal fees from Edwards Lifesciences, personal fees from Bioventrix inc., outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87–165. doi:10.1093/eurheartj/ehy394
2. Neumann FJ, Sechtem U, Banning AP, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407–477. doi:10.1093/eurheartj/ehz425
3. Kandaswamy E, Zuo L. Recent advances in treatment of coronary artery disease: role of science and technology. Int J Mol Sci. 2018;19(2):424. doi:10.3390/ijms19020424
4. Huang AL, Maggiore PL, Brown RA, et al. CT-Derived Fractional Flow Reserve (FFRCT): from gatekeeping to roadmapping. Can Assoc Radiol J. 2020;71(2):201–207. doi:10.1177/0846537119893752
5. Boden WE, O’Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. New Engl J Med. 2007;356(15):1503–1516. doi:10.1056/NEJMoa070829
6. Lukkarinen H, Hentinen M. Treatments of coronary artery disease improve quality of life in the long term. Nurs Res. 2006;55(1):26–33. doi:10.1097/00006199-200601000-00004
7. Maron DJ, Hochman JS, Reynolds HR, et al. Initial invasive or conservative strategy for stable coronary disease. New Engl J Med. 2020;382(15):1395–1407. doi:10.1056/NEJMoa1915922
8. Gibbons RJ, Chatterjee K, Daley J, et al. ACC/AHA/ACP–ASIM guidelines for the management of patients with chronic stable angina: executive summary and recommendations. Circulation. 1999;99(21):2829–2848. doi:10.1161/01.CIR.99.21.2829
9. Mancini GBJ, Hartigan PM, Shaw LJ, et al. Predicting outcome in the COURAGE trial (Clinical outcomes utilizing revascularization and aggressive drug evaluation). JACC Cardiovasc Interv. 2014;7(2):195–201. doi:10.1016/j.jcin.2013.10.017
10. Topol EJ, Nissen SE. Our preoccupation with coronary luminology. Circulation. 1995;92(8):2333–2342. doi:10.1161/01.CIR.92.8.2333
11. Lee JM, Hwang D, Choi KH, et al. Prognostic impact of residual anatomic disease burden after functionally complete revascularization. Circ Cardiovasc Interv. 2020. doi:10.1161/CIRCINTERVENTIONS.120.009232
12. Okutucu S, Cilingiroglu M, Feldman MD. Physiologic assessment of coronary stenosis: current status and future directions. Curr Cardiol Rep. 2021;23(7):1–10. doi:10.1007/s11886-021-01521-3
13. Fournier S, Collet C, Xaplanteris P, et al. Global fractional flow reserve value predicts 5-year outcomes in patients with coronary atherosclerosis but without ischemia. J Am Heart Assoc. 2020;9(24). doi:10.1161/JAHA.120.017729
14. Achenbach S, Rudolph T, Rieber J, et al. Performing and interpreting fractional flow reserve measurements in clinical practice: an expert consensus document. Interv Cardiol Rev. 2017;12(2):97–109. doi:10.15420/icr.2017:13:2
15. Bech GJW, de Bruyne B, Pijls NHJ, et al. Fractional flow reserve to determine the appropriateness of angioplasty in moderate coronary stenosis: a randomized trial. Circulation. 2001;103(24):2928–2934. doi:10.1161/01.CIR.103.24.2928
16. Tonino PAL, de Bruyne B, Pijls NHJ, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. New Engl J Med. 2009;360(3):213–224. doi:10.1056/NEJMoa0807611
17. de Bruyne B, Fearon WF, Pijls NHJ, et al. Fractional flow reserve–guided PCI for stable coronary artery disease. New Engl J Med. 2014;371(13):1208–1217. doi:10.1056/nejmoa1408758
18. Xaplanteris P, Fournier S, Pijls NHJ, et al. Five-year outcomes with PCI guided by fractional flow reserve. New Engl J Med. 2018;379(3):250–259. doi:10.1056/nejmoa1803538
19. Zimmermann FM, Ferrara A, Johnson NP, et al. Deferral vs. performance of percutaneous coronary intervention of functionally non-significant coronary stenosis: 15-year follow-up of the DEFER trial. Eur Heart J. 2015;36(45):3182–3188. doi:10.1093/eurheartj/ehv452
20. Lindstaedt M, Yazar A, Germing A, et al. Clinical outcome in patients with intermediate or equivocal left main coronary artery disease after deferral of surgical revascularization on the basis of fractional flow reserve measurements. Am Heart J. 2006;152(1):
21. Hamilos M, Muller O, Cuisset T, et al. Long-term clinical outcome after fractional flow reserve-guided treatment in patients with angiographically equivocal left main coronary artery stenosis. Circulation. 2009;120(15):1505–1512. doi:10.1161/CIRCULATIONAHA.109.850073
22. Smits PC, Abdel-Wahab M, Neumann FJ, et al. Fractional flow reserve–guided multivessel angioplasty in myocardial infarction. New Engl J Med. 2017;376(13):1234–1244. doi:10.1056/nejmoa1701067
23. Collet JP, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42(14):1289–1367. doi:10.1093/eurheartj/ehaa575
24. Ntalianis A, Sels JW, Davidavicius G, et al. Fractional flow reserve for the assessment of nonculprit coronary artery stenoses in patients with acute myocardial infarction. JACC Cardiovasc Interv. 2010;3(12):1274–1281. doi:10.1016/j.jcin.2010.08.025
25. Gershlick AH, Khan JN, Kelly DJ, et al. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol. 2015;65(10):963–972. doi:10.1016/j.jacc.2014.12.038
26. Wald DS, Morris JK, Wald NJ, et al. Randomized trial of preventive angioplasty in myocardial infarction. New Engl J Med. 2013;369(12):1115–1123. doi:10.1056/nejmoa1305520
27. Mehta SR, Wood DA, Storey RF, et al. Complete revascularization with multivessel PCI for myocardial infarction. New Engl J Med. 2019;381(15):1411–1421. doi:10.1056/nejmoa1907775
28. Engstrøm T, Kelbæk H, Helqvist S, et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3 - PRIMULTI): an open-label, randomised controlled trial. Lancet. 2015;386(9994):665–671. doi:10.1016/S0140-6736(15)60648-1
29. Kobayashi Y, Nam CW, Tonino PAL, et al. The prognostic value of residual coronary stenoses after functionally complete revascularization. J Am Coll Cardiol. 2016;67(14):1701–1711. doi:10.1016/j.jacc.2016.01.056
30. Agarwal SK, Kasula S, Hacioglu Y, Ahmed Z, Uretsky BF, Hakeem A. Utilizing post-intervention fractional flow reserve to optimize acute results and the relationship to long-term outcomes. JACC Cardiovasc Interv. 2016;9(10):1022–1031. doi:10.1016/j.jcin.2016.01.046
31. Ding D, Huang J, Westra J, et al. Immediate post-procedural functional assessment of percutaneous coronary intervention: current evidence and future directions. Eur Heart J. 2021;42(27):2695–2707. doi:10.1093/eurheartj/ehab186
32. Azzalini L, Poletti E, Demir OM, et al. Impact of post-percutaneous coronary intervention fractional flow reserve measurement on procedural management and clinical outcomes: the REPEAT-FFR study. J Interv Cardiol. 2019;31(8):229–234.
33. Collison D, Didagelos M, Aetesam-ur-Rahman M, et al. Post-stenting fractional flow reserve vs coronary angiography for optimization of percutaneous coronary intervention (TARGET-FFR). Eur Heart J. 2021:1–13. doi:10.1093/eurheartj/ehab449
34. Pargaonkar VS, Kimura T, Kameda R, et al. Invasive assessment of myocardial bridging in patients with angina and no obstructive coronary artery disease. EuroIntervention. 2021;16(13):1070–1078. doi:10.4244/EIJ-D-20-00779
35. Hakeem A, Cilingiroglu M, Leesar MA. Hemodynamic and intravascular ultrasound assessment of myocardial bridging: fractional flow reserve paradox with dobutamine versus adenosine. Catheter Cardiovasc Interv. 2010;75(2):229–236. doi:10.1002/ccd.22237
36. Tsujita K, Maehara A, Mintz GS, et al. Comparison of angiographic and intravascular ultrasonic detection of myocardial bridging of the left anterior descending coronary artery. Am J Cardiol. 2008;102(12):1608–1613. doi:10.1016/j.amjcard.2008.07.054
37. Escaned J, Cortés J, Flores A, et al. Importance of diastolic fractional flow reserve and dobutamine challenge in physiologic assessment of myocardial bridging. J Am Coll Cardiol. 2003;42(2):226–233. doi:10.1016/S0735-1097(03)00588-6
38. Jin X, Lim HS, Tahk SJ, et al. Impact of age on the functional significance of intermediate Epicardial artery disease. Circ J. 2016;80(7):1583–1589. doi:10.1253/circj.CJ-15-1402
39. Verdoia M, Gioscia R, Nardin M, et al. Impact of age on the functional evaluation of intermediate coronary stenoses with instantaneous wave-free ratio and fractional flow reserve. Angiology. 2021;72(1):62–69. doi:10.1177/0003319720947578
40. Lim HS, Tonino PAL, de Bruyne B, et al. The impact of age on fractional flow reserve-guided percutaneous coronary intervention: a FAME (Fractional flow reserve versus angiography for multivessel evaluation) trial substudy. Int J Cardiol. 2014;177(1):66–70. doi:10.1016/j.ijcard.2014.09.010
41. McClish JC, Ragosta M, Powers ER, et al. Effect of acute myocardial infarction on the utility of fractional flow reserve for the physiologic assessment of the severity of coronary artery narrowing. Am J Cardiol. 2004;93(9):1102–1106. doi:10.1016/j.amjcard.2004.01.035
42. Biscaglia S, Guiducci V, Santarelli A, et al. Physiology-guided revascularization versus optimal medical therapy of nonculprit lesions in elderly patients with myocardial infarction: rationale and design of the FIRE trial. Am Heart J. 2020;229:100–109. doi:10.1016/j.ahj.2020.08.007
43. Sahinarslan A, Kocaman SA, Olgun H, et al. The reliability of fractional flow reserve measurement in patients with diabetes mellitus. Coron Artery Dis. 2009;20(5):317–321. doi:10.1097/MCA.0b013e32832c8ca3
44. Di Gioia G, Flores NS, Franco D, et al. Coronary artery bypass grafting or fractional flow reserve-guided percutaneous coronary intervention in diabetic patients with multivessel disease. Circ Cardiovasc Interv. 2020;(October):153–171. doi:10.1161/CIRCINTERVENTIONS.120.009157
45. Yanagisawa H, Chikamori T, Tanaka N, Usui Y, Takazawa K, Yamashina A. Application of pressure-derived myocardial fractional flow reserve in assessing the functional severity of coronary artery stenosis in patients with diabetes mellitus. Circ J. 2004;68:11. doi:10.1253/circj.68.993
46. Domínguez-Franco AJ, Jiménez-Navarro MF, Muñoz-García AJ, Alonso-Briales JH, Hernández-García JM, Galván EDT. Long-term prognosis in diabetic patients in whom revascularization is deferred following fractional flow reserve assessment. Revista Española de Cardiología. 2008;61(4):352–359. doi:10.1016/s1885-5857(08)60144-9
47. Lee JM, Choi KH, Koo BK, et al. Comparison of major adverse cardiac events between instantaneous wave-free ratio and fractional flow reserve-guided strategy in patients with or without type 2 diabetes: a secondary analysis of a randomized clinical trial. JAMA Cardiol. 2019;4(9):857–864. doi:10.1001/jamacardio.2019.2298
48. Leone AM, de Caterina AR, Basile E, et al. Influence of the amount of myocardium subtended by a stenosis on fractional flow reserve. Circ Cardiovasc Interv. 2013;6(1):29–36. doi:10.1161/CIRCINTERVENTIONS.112.971101
49. Sabbah M, Nepper-Christensen L, Lønborg J, et al. Fractional flow reserve-guided PCI in patients with and without left ventricular hypertrophy: a DANAMI-3-PRIMULTI substudy. EuroIntervention. 2021;16(7):584–590. doi:10.4244/eij-d-19-00577
50. Chhatriwalla AK, Ragosta M, Powers ER, et al. High left ventricular mass index does not limit the utility of fractional flow reserve for the physiologic assessment of lesion severity. J Invasive Cardiol. 2006;18(11):544–549.
51. Ponikowski P, Voors AA, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur J Heart Fail. 2016;18(8). doi:10.1002/ejhf.592
52. Kobayashi Y, Tonino PAL, de Bruyne B, et al. The impact of left ventricular ejection fraction on fractional flow reserve: insights from the FAME (Fractional flow reserve versus angiography for multivessel evaluation) trial. Int J Cardiol. 2016;204(2016):206–210. doi:10.1016/j.ijcard.2015.11.169
53. Di Gioia G, de Bruyne B, Pellicano M, et al. Fractional flow reserve in patients with reduced ejection fraction. Eur Heart J. 2020;41(17):1665–1672. doi:10.1093/eurheartj/ehz571
54. Toth GG, de Bruyne B, Rusinaru D, et al. Impact of right atrial pressure on fractional flow reserve measurements comparison of fractional flow reserve and myocardial fractional flow reserve in 1600 coronary stenoses. JACC Cardiovasc Interv. 2016;9(5):453–459. doi:10.1016/j.jcin.2015.11.021
55. Mejia-Renteria H, Nombela-Franco L, Paradis JM, et al. Angiography-based quantitative flow ratio versus fractional flow reserve in patients with coronary artery disease and severe aortic stenosis. EuroIntervention. 2021;16(4):E285–E292. doi:10.4244/eij-d-19-01001
56. Di Gioia G, Pellicano M, Toth GG, et al. Fractional flow reserve-guided revascularization in patients with aortic stenosis. Am J Cardiol. 2016;117(9):1511–1515. doi:10.1016/j.amjcard.2016.02.023
57. Pesarini G, Scarsini R, Zivelonghi C, et al. Functional assessment of coronary artery disease in patients undergoing transcatheter aortic valve implantation: influence of pressure overload on the evaluation of lesions severity. Circ Cardiovasc Interv. 2016;9(11):1–10. doi:10.1161/CIRCINTERVENTIONS.116.004088
58. Vendrik J, Ahmad Y, Eftekhari A, et al. Long-term effects of transcatheter aortic valve implantation on coronary hemodynamics in patients with concomitant coronary artery disease and severe aortic stenosis. J Am Heart Assoc. 2020;9(5). doi:10.1161/JAHA.119.015133
59. Changal K, Patel M, Salman FNU, Nazir S, Gupta R. Meta-analysis comparing angiography-guided versus ffr-guided coronary artery bypass grafting. Am J Cardiol. 2020;135:184–185. doi:10.1016/j.amjcard.2020.09.002
60. Timbadia D, Ler A, Sazzad F, Alexiou C, Kofidis T. FFR-guided versus coronary angiogram-guided CABG: a review and meta-analysis of prospective randomized controlled trials. J Card Surg. 2020;35(10):2785–2793. doi:10.1111/jocs.14880
61. Bruno F, D’Ascenzo F, Marengo G, et al. Fractional flow reserve guided versus angiographic guided surgical revascularization: a meta-analysis. Catheter Cardiovasc Interv. 2020. doi:10.1002/ccd.29427
62. Fournier S, Toth GG, de Bruyne B, et al. Six-year follow-up of fractional flow reserve-guided versus angiography-guided coronary artery bypass graft surgery. Circ Cardiovasc Interv. 2018;11(6):1–7. doi:10.1161/CIRCINTERVENTIONS.117.006368
63. Wermers J, Torguson R. FUTURE trial does not indicate advantage for FFR over angiography alone in multivessel disease patients. Available from: https://www.crtonline.org/news-detail/future-trial-does-not-indicate-advantage-ffr-over-.
64. Neale T. FUTURE results confirm more deaths with FFR-guided treatment choice. Available from: https://www.tctmd.com/news/future-results-confirm-more-deaths-ffr-guided-treatment-choice.
65. Koo BK, Park KW, Kang HJ, et al. Physiological evaluation of the provisional side-branch intervention strategy for bifurcation lesions using fractional flow reserve. Eur Heart J. 2008;29(6):726–732. doi:10.1093/eurheartj/ehn045
66. Koo BK, Waseda K, Kang HJ, et al. Anatomic and functional evaluation of bifurcation lesions undergoing percutaneous coronary intervention. Circ Cardiovasc Interv. 2010;3(2):113–119. doi:10.1161/CIRCINTERVENTIONS.109.887406
67. Ahn JM, Lee JY, Kang SJ, et al. Functional assessment of jailed side branches in coronary bifurcation lesions using fractional flow reserve. JACC Cardiovasc Interv. 2012;5(2):155–161. doi:10.1016/j.jcin.2011.10.015
68. Koo BK, Kang HJ, Youn TJ, et al. Physiologic assessment of jailed side branch lesions using fractional flow reserve. J Am Coll Cardiol. 2005;46(4):633–637. doi:10.1016/j.jacc.2005.04.054
69. Koo BK. Physiologic evaluation of bifurcation lesions using fractional flow reserve. J Interv Cardiol. 2009;22(2):110–113. doi:10.1111/j.1540-8183.2009.00437.x
70. Kang SJ, Ahn JM, Kim WJ, et al. Functional and morphological assessment of side branch after left main coronary artery bifurcation stenting with cross-over technique. Catheter Cardiovasc Interv. 2014;83(4):545–552. doi:10.1002/ccd.25057
71. Al-Lamee R, Thompson D, Dehbi HM, et al. Percutaneous coronary intervention in stable angina (ORBITA): a double-blind, randomised controlled trial. Lancet. 2018;391:10115. doi:10.1016/S0140-6736(17)32714-9
72. Soares A, Brown DL. The fallacies of fractional flow reserve. Int J Cardiol. 2020;302(2020):34–35. doi:10.1016/j.ijcard.2019.12.040
73. Demir OM, Schrieken C, Curio J, Rahman H. Behavioural determinants impacting the adoption rate of coronary physiology. Int J Cardiol. 2021;330:12–14. doi:10.1016/j.ijcard.2021.02.008
74. de Waard GA, Hollander MR, Teunissen PFA, et al. Changes in coronary blood flow after acute myocardial infarction: insights from a patient study and an experimental porcine model. JACC Cardiovasc Interv. 2016;9(6):602–613. doi:10.1016/j.jcin.2016.01.001
75. Thim T, Götberg M, Fröbert O, et al. Agreement between nonculprit stenosis follow-up iFR and FFR after STEMI (iSTEMI substudy). BMC Res Notes. 2020;13(1):1–3. doi:10.1186/s13104-020-05252-6
76. Li Y, Shi Z, Cai Y, et al. Impact of coronary tortuosity on coronary pressure: numerical simulation study. PLoS One. 2012;7(8):3–8. doi:10.1371/journal.pone.0042558
77. Li Y, Zhang X, Dai Q, Ma G. Coronary flow reserve and microcirculatory resistance in patients with coronary tortuosity and without atherosclerosis. J Int Med Res. 2020;48(9). doi:10.1177/0300060520955060
78. Xie X, Wang Y, Zhu H, Zhou H, Zhou J. Impact of coronary tortuosity on coronary blood supply: a Patient-Specific Study. PLoS One. 2013;8(5):1–10. doi:10.1371/journal.pone.0064564
79. Nederland Z. Farmacotherapeutisch Kompas: adenosine. Available from: https://www.farmacotherapeutischkompas.nl/bladeren/preparaatteksten/a/adenosine#contra-indicaties.
80. Sen S, Escaned J, Malik IS, et al. Development and validation of a new adenosine-independent index of stenosis severity from coronary wave-intensity analysis: results of the ADVISE (ADenosine vasodilator independent stenosis evaluation) study. J Am Coll Cardiol. 2012;59(15):1392–1402. doi:10.1016/j.jacc.2011.11.003
81. Svanerud J, Ahn JM, Jeremias A, et al. Validation of a novel non-hyperaemic index of coronary artery stenosis severity: the Resting Full-cycle Ratio (VALIDATE RFR) study. EuroIntervention. 2018;14(7):806–814. doi:10.4244/EIJ-D-18-00342
82. Johnson NP, Li W, Chen X, et al. Diastolic pressure ratio: new approach and validation vs. the instantaneous wave-free ratio. Eur Heart J. 2019;40(31):2585–2594. doi:10.1093/eurheartj/ehz230
83. Van’t Veer M, Pijls NHJ, Hennigan B, et al. Comparison of different diastolic resting indexes to iFR: are they all equal? J Am Coll Cardiol. 2017;70(25):3088–3096. doi:10.1016/j.jacc.2017.10.066
84. Davies JE, Sen S, Dehbi HM, et al. Use of the instantaneous wave-free ratio or fractional flow reserve in PCI. New Engl J Med. 2017;376(19):1824–1834. doi:10.1056/NEJMoa1700445
85. Sen S, Escaned J, Malik IS, et al. Development and validation of a new adenosine-independent index of stenosis severity from coronary wave–intensity analysis. J Am Coll Cardiol. 2012;59(15):1392–1402. doi:10.1016/j.jacc.2011.11.003
86. de Waard G, Di Mario C, Lerman A, Serruys P, van Royen N. Instantaneous wave-free ratio to guide coronary revascularisation: physiological framework, validation and differences from fractional flow reserve. EuroIntervention. 2017;13(4):450–458. doi:10.4244/EIJ-D-16-00456
87. Götberg M, Christiansen EH, Gudmundsdottir IJ, et al. Instantaneous wave-free ratio versus fractional flow reserve to guide PCI. New Engl J Med. 2017;376(19):1813–1823. doi:10.1056/NEJMoa1616540
88. Koo BK, Erglis A, Doh JH, et al. Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms. J Am Coll Cardiol. 2011;58(19):1989–1997. doi:10.1016/j.jacc.2011.06.066
89. Nakazato R, Park HB, Berman DS, et al. Noninvasive fractional flow reserve derived from computed tomography angiography for coronary lesions of intermediate stenosis severity. Circ Cardiovasc Imaging. 2013;6(6):881–889. doi:10.1161/CIRCIMAGING.113.000297
90. Gaur S, Achenbach S, Leipsic J, et al. Rationale and design of the HeartFlowNXT (HeartFlow analysis of coronary blood flow using CT angiography: neXt sTeps) study. J Cardiovasc Comput Tomogr. 2013;7(5):279–288. doi:10.1016/j.jcct.2013.09.003
91. van Hamersvelt RW, Voskuil M, de Jong PA, Willemink MJ, Išgum I, Leiner T. Diagnostic performance of on-site coronary CT angiography–derived fractional flow reserve based on patient-specific lumped parameter models. Radiology. 2019;1(4):e190036. doi:10.1148/ryct.2019190036
92. Donnelly PM, Kolossváry M, Karády J, et al. Experience with an on-site coronary computed tomography-derived fractional flow reserve algorithm for the assessment of intermediate coronary stenoses. Am J Cardiol. 2018;121(1):9–13. doi:10.1016/j.amjcard.2017.09.018
93. Coenen A, Lubbers MM, Kurata A, et al. Fractional flow reserve computed from noninvasive CT angiography data: diagnostic performance of an on-site clinician-operated computational fluid dynamics algorithm. Radiology. 2015;274(3):674–683. doi:10.1148/radiol.14140992
94. Ihdayhid AR, Sakaguchi T, Linde JJ, et al. Performance of computed tomography-derived fractional flow reserve using reduced-order modelling and static computed tomography stress myocardial perfusion imaging for detection of haemodynamically significant coronary stenosis. Eur Heart J Cardiovasc Imaging. 2018;19(11):1234–1243. doi:10.1093/ehjci/jey114
95. Ko BS, Cameron JD, Munnur RK, et al. Noninvasive CT-derived FFR based on structural and fluid analysis: a comparison with Invasive FFR for detection of functionally significant stenosis. JACC Cardiovasc Imaging. 2017;10(6):663–673. doi:10.1016/j.jcmg.2016.07.005
96. Renker M, Schoepf UJ, Wang R, et al. Comparison of diagnostic value of a novel noninvasive coronary computed tomography angiography method versus standard coronary angiography for assessing fractional flow reserve. Am J Cardiol. 2014;114(9):1303–1308. doi:10.1016/j.amjcard.2014.07.064
97. Yang L, Xu L, He J, et al. Diagnostic performance of a fast non-invasive fractional flow reserve derived from coronary CT angiography: an initial validation study. Clin Radiol. 2019;74(12):
98. Peper J, Schaap J, Kelder JC, et al. Added value of computed tomography fractional flow reserve in the diagnosis of coronary artery disease. Sci Rep. 2021;11(1):1–9. doi:10.1038/s41598-021-86245-8
99. Celeng C, Leiner T, Maurovich-Horvat P, et al. Anatomical and functional computed tomography for diagnosing hemodynamically significant coronary artery disease: a meta-analysis. JACC Cardiovasc Imaging. 2019;12(7):1316–1325. doi:10.1016/j.jcmg.2018.07.022
100. Tu S, Barbato E, Köszegi Z, et al. Fractional flow reserve calculation from 3-dimensional quantitative coronary angiography and TIMI frame count. JACC Cardiovasc Interv. 2014;7(7):768–777. doi:10.1016/j.jcin.2014.03.004
101. Collet C, Onuma Y, Sonck J, et al. Diagnostic performance of angiography-derived fractional flow reserve: a systematic review and Bayesian meta-analysis. Eur Heart J. 2018;39(35):3314–3321. doi:10.1093/eurheartj/ehy445
102. Tu S, Westra J, Yang J, et al. Diagnostic accuracy of fast computational approaches to derive fractional flow reserve from diagnostic coronary angiography: the international multicenter FAVOR Pilot Study. JACC Cardiovasc Interv. 2016;9(19):2024–2035. doi:10.1016/j.jcin.2016.07.013
103. Xu B, Tu S, Qiao S, et al. Diagnostic accuracy of angiography-based quantitative flow ratio measurements for online assessment of coronary stenosis. J Am Coll Cardiol. 2017;70(25):3077–3087. doi:10.1016/j.jacc.2017.10.035
104. Westra J, Andersen BK, Campo G, et al. Diagnostic performance of in‐procedure angiography‐derived quantitative flow reserve compared to pressure‐derived fractional flow reserve: the FAVOR II Europe‐Japan Study. J Am Heart Assoc. 2018;7(14). doi:10.1161/JAHA.118.009603
105. Westra J, Tu S, Winther S, et al. Evaluation of coronary artery stenosis by quantitative flow ratio during invasive coronary angiography. Circ Cardiovasc Imaging. 2018;11(3). doi:10.1161/CIRCIMAGING.117.007107
106. Masdjedi K, van Zandvoort LJC, Balbi MM, et al. Validation of a three-dimensional quantitative coronary angiography-based software to calculate fractional flow reserve: the FAST study. EuroIntervention. 2021;16(7):591–599. doi:10.4244/eij-d-19-00466
107. Seike F, Uetani T, Nishimura K, et al. Correlation between quantitative angiography–derived translesional pressure and fractional flow reserve. Am J Cardiol. 2016;118(8):1158–1163. doi:10.1016/j.amjcard.2016.07.026
108. Papafaklis MI, Muramatsu T, Ishibashi Y, et al. Fast virtual functional assessment of intermediate coronary lesions using routine angiographic data and blood flow simulation in humans: comparison with pressure wire - fractional flow reserve. EuroIntervention. 2014;10(5):574–583. doi:10.4244/EIJY14M07_01
109. Morris PD, Ryan D, Morton AC, et al. Virtual fractional flow reserve from coronary angiography: modeling the significance of coronary lesions. Results from the VIRTU-1 (VIRTUal fractional flow reserve from coronary angiography) study. JACC Cardiovasc Interv. 2013;6(2):149–157. doi:10.1016/j.jcin.2012.08.024
110. Tröbs M, Achenbach S, Röther J, et al. Comparison of fractional flow reserve based on computational fluid dynamics modeling using coronary angiographic vessel morphology versus invasively measured fractional flow reserve. Am J Cardiol. 2016;117(1):29–35. doi:10.1016/j.amjcard.2015.10.008
111. Levine GN, Bates ER, Blankenship JC, et al. ACCF/AHA/SCAI guideline for percutaneous coronary intervention a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124(23):574–651. doi:10.1161/CIR.0b013e31823ba622
112. Lotfi A, Jeremias A, Fearon WF, et al. Expert consensus statement on the use of fractional flow reserve, intravascular ultrasound, and optical coherence tomography: a consensus statement of the society of cardiovascular angiography and interventions. Catheter Cardiovasc Interv. 2014;83(4):509–518. doi:10.1002/ccd.25222
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.