Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Four Cases of Port-Wine Birthmark Treated with Hematoporphyrin Monomethyl Ether-Mediated Photodynamic Therapy After Radioactive Nuclide Patch Therapy
Authors Liu X , Yang L , Zhang Q , Yang F , Jiang X
Received 26 April 2023
Accepted for publication 22 June 2023
Published 28 June 2023 Volume 2023:16 Pages 1667—1675
DOI https://doi.org/10.2147/CCID.S418019
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Anne-Claire Fougerousse
Xu Liu,1,* Lihua Yang,1,* Qian Zhang,1 Fengjuan Yang,1 Xian Jiang1,2
1Department of Dermatology, West China Hospital, Sichuan University, Chengdu, People’s Republic of China; 2Laboratory of Dermatology, Clinical Institute of Inflammation and Immunology, Frontiers Science Center for Disease-Related Molecular Network, West China Hospital, Sichuan University, Chengdu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xian Jiang, West China Hospital, Sichuan University, #37 Guoxue Alley, Wuhou District, Chengdu, Sichuan, 610041, People’s Republic of China, Tel +86-28-85423315, Fax +86-28-85422560, Email [email protected]
Abstract: Port-wine birthmark (PWB) are congenital vascular malformations that commonly occur on the face and neck, with an incidence of 0.3– 0.5% in the general population, causing significant negative psychological effects and economic burden to patients. Nevertheless, amidst the plethora of different treatment methods for PWB, choosing the option that best suits the patient’s need can be a challenge. In recent years, traditional treatment methods for PWB have been replaced by new therapies, and radioactive nuclide patch therapy is one of them. A panel of experts sought to describe herein 4 clinical cases, illustrating the PDT can demonstrate good precision and efficacy in the treatment of PWB. The research findings show the 4 patients in this group had a history of treatment with radioactive isotope patches. After 2– 3 sessions of HMME-PDT, all cases achieved satisfactory results, the color of the red skin lesions significantly faded, and the area of the lesions decreased noticeably. Superficial tissue ultrasound showed a reduction in lesion thickness before and after treatment. In summary, for cases where the efficacy of PWB treatment with radioactive isotope patches is inadequate, Photodynamic therapy (PDT) can be used as a treatment reference.
Keywords: port-wine birthmark, PWB, photodynamic therapy, PDT, hematoporphyrin monomethyl ether, radioactive nuclide patch therapy
Introduction
Port-wine birthmark (PWB) are congenital vascular malformations that commonly occur on the face and neck,1 with an incidence of 0.3–0.5% in the general population, causing significant negative psychological effects and economic burden to patients. In recent years, traditional treatment methods for PWB have been replaced by new therapies, and radioactive nuclide patch therapy is one of them. The principle of this therapy is to use the sensitivity of skin capillaries to beta-rays, and to achieve therapeutic purposes by inducing changes such as atrophy and occlusion of blood vessels in the lesion area under irradiation. Photodynamic therapy (PDT) is a relatively new treatment method, and HMME-PDT is currently the most commonly used PDT treatment for PWB in China. Literature review of radioactive nuclide patch therapy for PWB in PubMed and Wanfang databases shows that there are poor therapeutic effects and scar risks in treating large areas and thickened lesions of PWB.2–4 Our department treated a total of nine patients with PWB who had unsatisfactory therapeutic effects after radioactive nuclide patch therapy from 2018 to 2023. Among them, four cases achieved satisfactory results with HMME-PDT, and the results are reported below.
Materials and Methods
Nine patients were included in this study, consisting of eight females and one male, with an age range of 8 to 36 years and a mean age of 21 years. The lesion sites were located on the jaw and face in eight cases and on the arm in one case. The size of the lesions varied from 2x2cm² to 12x8cm². The shape of the lesions differed according to the disease course and presented as flat, mildly elevated, pink, or purplish-red. Prior to treatment, all patients had received radioactive nuclide patch therapy (Sr90) 2 to 30 years ago, but the red skin lesions did not fade significantly, and different degrees of scars and pigmented spots were left behind. The Visual Analog Scale (VAS) scores were between 0 and 1, and the Vancouver Scar Scale (VSS) scores ranged from 0 to 6. Skin microscopy revealed varying degrees of brown pigmentation and white structureless areas. The thickness of the skin lesions measured by superficial tissue ultrasound ranged from 0 to 7.0 mm (see Table 1).
 |
Table 1 Clinical Data and Treatment of HMME-PDT of 4 Patients with PWB |
Given the increasing number of available treatments for PWB with varying efficacy and safety profiles, it is critical to understand the level of trade-offs that patients are willing to make between benefits and risks. After communication, 4 patients are willing to accept PDT.
Example 1: A 17-year-old female presented on June 28, 2021 with “irregular redness on the left side of the face”. The redness has been present since birth, and has gradually increased in size and darkened in color as she aged, spreading from the area around the eye to the middle and outer parts of the same side of the face, with a tendency to spread to the surrounding area. The patient received treatment with radioactive nuclide (Sr90) paste during the ages of 1–5, with little improvement in the redness, which has mildly darkened over the past ten years, but with no further increase in size. There is no family history of a similar condition, and the patient’s parents are not closely related. The patient was born full-term, and the mother had no history of infection or trauma during pregnancy. Physical examination showed a large irregular red patch on the left side of the face around the eye, which did not extend beyond the midline and was slightly elevated above the skin surface, non-pulsatile, non-blanching, and with normal skin temperature. The lesion had scars and pigmentation spots with a VAS score of 1 and a VSS score of 5. Dermatoscopy revealed brown reticular pigmentation, reticular and branched vessels, and some areas of white structureless areas. Superficial tissue ultrasound showed slightly thickened skin in the area of the “redness” on the left side of the face, with a maximum thickness of approximately 3.0mm (compared to 2.7mm on the opposite side), and no obvious blood flow signal or definite mass in the skin or subcutaneous tissue. The diagnosis was fresh hemangioma and scar, and after 3 sessions of HMME-PDT treatment, the erythema significantly faded in the treated area without any adverse reactions, and the scar and pigmentation spots remained unchanged. Patients are satisfied with the current evaluation of the current treatment effect, and considering the cost of treatment and the necessity of treatment, we are not planning a fourth PDT (See Figure 1).
 |
Figure 1 Various stages of treatment for Case 1. (A) Baseline; (B) 6M: Before the second treatment; (C) 12M: Before the third treatment; (D) 14M: Treatment endpoint. |
Example 2: A 21-year-old female patient presented with “irregular erythema on the left side of the face” on April 18th, 2022. The erythema has been present since birth and has gradually increased in size and darkened in color as she grew older, spreading around the left orbit to the middle of the same side of the face. The patient underwent treatment with radioactive isotopes (Sr90) for the erythema when she was between 1 and 5 years old and reported partial improvement, but no significant changes in color or size of the erythema have occurred in the past 10 years. The patient denied any similar family history and her parents were not closely related. There was no significant history of infection or trauma during the mother’s pregnancy, and the patient was delivered at full term without complications. Physical examination showed a large irregular erythema located on the left side of the face and around the left orbit, which did not cross the midline, was not raised above the skin surface, did not pulsate, faded slightly under pressure, and had normal skin temperature. Scarring and pigmentation were visible at the lesion, with a Visual Analog Scale (VAS) score of 1 and Vancouver Scar Scale (VSS) score of 5. Dermoscopy revealed a brown reticular pigmentation, reticular and branched vessels, and white structureless areas in some regions. Superficial tissue ultrasound showed a slight increase in skin thickness (up to 1.9 mm) at the erythema site on the left side of the face, compared to 1.6 mm on the opposite side, with no significant blood flow signals or definite masses in the skin or subcutaneous layers. The diagnosis was fresh hemangioma and scarring. After three sessions of HMME-PDT treatment, the erythema significantly faded in the treated area with no obvious adverse reactions, while the scarring and pigmentation remained unchanged. Patients are satisfied with the current evaluation of the current treatment effect, and considering the cost of treatment and the necessity of treatment, we are not planning a fourth PDT (See Figure 2).
 |
Figure 2 Various stages of treatment for Case 2. (A) Baseline; (B) 12M: Before the second treatment; (C) 24M: Before the third treatment; (D) 26M: Treatment endpoint. |
Example 3: A 17-year-old female patient presented on April 18, 2022, with “redness on the left hand and left forearm”. The redness has been present since birth and has increased in proportion to the patient’s age, with no significant changes in color. The patient received treatment with radioactive nuclide plaster (Sr90) during the age of 1–5, and reported a mild fading of the redness. Over the past 10 years, there has been no significant change in the color or size of the redness. The patient denied any similar family history and her parents were not closely related. There was no history of significant infection or trauma during the mother’s pregnancy, and the patient was born at full term by vaginal delivery. Physical examination: no significant abnormalities were found during a systemic examination, but examination of the affected area showed irregular redness on the left hand and left forearm, without protrusion from the skin surface or pulsation, slight fading of color when pressed, and normal skin temperature. Scarring and pigmentation were observed at the affected area, with a VAS score of 0 and a VSS score of 2. Dermoscopy revealed brown reticular pigmentation, reticular and branching vessels, and some areas with white non-structured areas. Superficial tissue ultrasound showed that the skin layer at the “redness” of the left arm was slightly thickened, with a maximum thickness of about 1.8 mm (the opposite skin layer was about 1.6 mm), with no obvious blood flow signals and no definite mass echo in the skin and subcutaneous layers. The diagnosis was fresh red mole and scar. After three HMME-PDT treatments, good efficacy, the color significantly faded in the treated area, and there were no significant adverse reactions. There was no significant change in scarring and pigmentation. Patients are satisfied with the current evaluation of the current treatment effect, and considering the cost of treatment and the necessity of treatment, we are not planning a fourth PDT (See Figure 3).
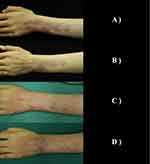 |
Figure 3 Various stages of treatment for Case 3. (A) Baseline; (B) 2M: Before the second treatment; (C) 4M: Before the third treatment; (D) 6M: Treatment endpoint. |
Example 4: An 18-year-old female patient presented on July 5, 2021, with “redness on the right jaw”. The redness has been present since birth and has increased in proportion to the patient’s age, with no significant changes in color. The patient received treatment with radioactive nuclide plaster (Sr90) during the age of 1–5, and reported no significant fading of the redness. Over the past 10 years, there has been no significant change in the color or size of the redness. The patient denied any similar family history and her parents were not closely related. There was no history of significant infection or trauma during the mother’s pregnancy, and the patient was born at full term by vaginal delivery. Physical examination: no significant abnormalities were found during a systemic examination, but examination of the affected area showed irregular redness on the right jaw, without protrusion from the skin surface or pulsation, slight fading of color when pressed, and normal skin temperature. Dermoscopy revealed reticular and punctate vessels, and superficial tissue ultrasound showed that the skin layer at the “redness” of the right jaw was slightly thickened, with a maximum thickness of about 1.5 mm (the opposite skin layer was about 1.3 mm), with no obvious blood flow signals and no definite mass echo in the skin and subcutaneous layers. The diagnosis was fresh red mole. After two HMME-PDT treatments, good efficacy, the color significantly faded in the treated area, and there were no significant adverse reactions. Patients are satisfied with the current evaluation of the current treatment effect, and considering the cost of treatment and the necessity of treatment, we are not planning a third PDT (See Figure 4).
 |
Figure 4 Various stages of treatment for Case 4. (A) Baseline; (B) 6M: Before the second treatment; (C) 8M: Treatment endpoint. |
Instrument: LED-IE type LED therapeutic instrument (Wuhan Ya Ge Optic And Electronic Technique CO, LTD, Wuhan, People’s Republic of China); output wavelength: green light 530 ± 10nm; output power density: ≥50mW/cm2; vertical lifting range of the main therapeutic head: 0 ~ 35cm ± 5cm; horizontal rotation angle of the main therapeutic head: less than 175° in the vertical direction; timing range: 0 ~ 120 minutes continuously adjustable; power supply: AC220V, 50Hz. Photosensitizer name: Injection-grade hematoporphyrin monomethyl ether (HMME) (Taizhou Fudan-Zhangjiang Pharmaceutical Co, Ltd, Taizhou, People’s Republic of China).
Treatment Method
Four patients with poorly effective bright red nevus after radioisotope patch therapy were tested negative for HMME skin test. The patient received intravenous injection of 80mg/kg HMME and the affected area was exposed to green light at 85 nm LED, with an irradiation power density between 105J/cm², 88mw/cm²-115J/cm², 95mw/cm². Each light spot was irradiated for 20–25 minutes. After 2–3 treatments, clinical efficacy and post-treatment reactions of each treated area were observed.
Post-Treatment Care
The main manifestation of HMME-PDT is pain. Local cold therapy were used to reduce pain and cool the skin.5 Oral analgesics relieve pain after HMME-PDT, common oral analgesics include NSAIDS and central analgesics.6 Patients were advised to avoid strong light and sunshine, apply sunscreen, wear a hat, sunglasses, and cover up for at least 14 days post treatment.7
Clinical Efficacy Evaluation
Digital photographs before and after treatment were taken using the same light source. After two-three treatment sessions, two dermatologists (not participating in the study) independently reviewed the photos. Efficacy evaluation standards: excellent efficacy, the color nearly or completely resolved in the treated area (≥ 90% improvement); good efficacy, the color significantly faded in the treated area (≥ 60 to < 90% improvement); fair, the color partially faded in the treated area (≥ 20 to < 60% improvement); no improvement, the color was mostly unchanged in the treated area (< 20% improvement).8
Results
This study included a total of four patients, all of whom were female. The average age was 18 years old. They were all clinically diagnosed with PWB. Among them, two patients had lesions on the left side of the face, one patient had a lesion on the left forearm, and one patient had a lesion on the right side of the jaw. All 4 patients in this group had a history of treatment with radioactive isotope patches. After 2–3 sessions of HMME-PDT, the color of the red skin lesions significantly faded, and the area of the lesions decreased noticeably. Superficial tissue ultrasound showed a reduction in lesion thickness before and after treatment, with no significant changes in scars or pigment deposition (see Table 2).
 |
Table 2 Demographic Information and Clinical Data of 9 PWB Patients |
Discussion
Currently, laser therapy is the preferred treatment for PWB, which is safer and more effective than skin grafting, cryotherapy, and other treatments. The most commonly used laser for PWB is pulsed dye laser (PDL).9 Although PDL is the gold standard for PWB treatment, but according to clinical application, PDL has limited efficacy and high recurrence rate.10,11 It is estimated that less than 10% of PWB patients will achieve complete clearance after PDL therapy.12 In 2002, Ho et al performed research in which 107 patients with PWB were treated with laser (wavelength: 532 nm and 585 nm) and found that Chinese patients had lower sensitivity to laser treatment, with higher complication rates and more treatment sessions required to achieve maximum blanching.13 A retrospective study of Treatment of PWB with pulsed dye laser 848 cases showed that effect of PDL was associated with five factors of PWB patients, including age at treatment, location, and size of lesions, presence of existing hyperplastic lesions, and the number of treatments.14 The cases reported here had a history of treatment with radioactive isotope patches, large lesion areas, and deep lesions, making PDT a more prominent treatment option than PDL in their case selection.
The principle of PDT is to administer a photosensitizer intravenously and use a specific wavelength of laser to activate the photosensitizer located in the target tissue, which produces reactive oxygen species (ROS) that damage the endothelial cells in blood vessels, causing irreversible vascular contraction and achieving the purpose of treating PWB.15 A retrospective study of 216 children with PWB treated with HMME-PDT showed that PDT had a significant effect on the treatment of PWB, with excellent and good efficacy rates of 25.46% and 35.65%, respectively.16 Wang conducted a meta-analysis of the safety and efficacy of PDT in treating PWB, which showed that the estimated percentage of individuals achieving a 60% improvement in the 26 studies included in the analysis was 51.5%, and the percentage achieving an improvement of ≥75% was 20.5%.17
This research has some limitations. The number of cases involved in the treatment was relatively small, and the evaluation of the treatment relied mainly on visual assessments. Additionally, the treatment and follow-up periods varied due to individual patient circumstances. In the future, we hope to employ more objective assessment tools and establish standardized case-control trials with a larger sample size to further demonstrate the value of HMME-PDT in PWB treatment.
Conclusion
In summary, PDT can demonstrate good precision and efficacy in the treatment of PWB. For cases where the efficacy of PWB treatment with radioactive isotope patches is inadequate, PDT can be used as a treatment reference. However, attention should be paid to how to avoid the formation of scars and pigment deposition in PWB treatment with radioactive isotope patches, and the treatment plan for existing scars and pigment deposition is worth considering.
Ethics Approval and Consent for Publication
This case series has been performed in accordance with the principles stated in the Declaration of Helsinki. Written informed consent, provided by the Taylor & Francis group® for publication of this case report and including photography and medical information, were signed by the patients. Institutional approval was not required to publish the case details.
Acknowledgments
There are no acknowledgments here.
Funding
This study was supported by the 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (ZYJC21036) and the National Natural Science Foundation of China (82073473) and the National Natural Science Foundation of China (82273559).
Disclosure
The authors have no conflicts of interest to declare in this work.
References
1. Finley JL. Port-wine stains. morphologic variations and developmental lesions. Arch Dermatol. 1984;120(11):1453–1455. doi:10.1001/archderm.1984.01650470059013
2. Lu T, Qin M. Radionuclide Therapeutics. TianJin: Tianjin Science and Technology Press; 1994:215–216.
3. Li Z, Pan F, Chang Y, et al. Laser treatment of dye after radionuclide application: 6 cases of white atrophy-like scars. Laser J. 2001;22(3):77. doi:10.3969/j.issn.0253-2743.2001.03.055
4. Li Q, Yang L, Rong S, et al. Efficacy of 90 strontium patch in the treatment of port-wine stains. Med J Natl Defending Forces Southwest China. 2017;27(9):971–973. doi:10.3969/j.issn.1004-0188.2017.09.022
5. Stangeland KZ, Kroon S. Cold air analgesia as pain reduction during photodynamic therapy of actinic keratosis. J Eur Acad Dermatol Venereol. 2012;26:849–854. doi:10.1111/j.1468-3083.2011.04167.x
6. Huang N, Zeng J, Liang J, et al. A randomized, double-blind, placebo-controlled study of oral oxycodone plus Acetaminophen for the treatment of pain in photodynamic therapy on port wine stains. Photodiagnosis Photodyn Ther. 2014;11(2):134–140. doi:10.1016/j.pdpdt.2014.03.004
7. Zhang XY, Al-Odaini N, Fan RG, et al. The efficacy of hematoporphyrin monomethyl ether photodynamic therapy in adult patients with port-wine stains: a retrospective study. Dermatol Ther. 2022;12(4):861–869. doi:10.1007/s13555-022-00699-w
8. Zhao Y, Tu P, Zhou G, et al. Hemoporfin photodynamic therapy for portwine stain: a randomized controlled trial. PLoS One. 2016;11(5):0156219.
9. Fölster-Holst R, Shukla R, Kassir M, et al. Treatment update of port-wine stain: a narrative review. J Drugs Dermatol. 2021;20(5):515–518. PMID: 33938700. doi:10.36849/JDD.5005
10. Zhang MW, Lin Q, Lin T, et al. Hematoporphyrin monomethyl ether photodynamic therapy for the treatment of facial port-wine stains resistant to pulsed dye laser. Photodiagnosis Photodyn Ther. 2020;31:101820. doi:10.1016/j.pdpdt.2020.101820
11. Zhang B, Zhang TH, Huang Z, Li Q, Yuan KH, Hu ZQ. Comparison of pulsed dye laser (PDL) and photodynamic therapy (PDT) for treatment of facial port-wine stain (PWS) birthmarks in pediatric patients. Photodiagnosis Photodyn Ther. 2014;11(4):491–497. doi:10.1016/j.pdpdt.2014.06.004
12. Liu X, Li Q, Zhao L, Yang X. Jiang pathogenesis of port-wine stains: directions for future therapies. Int J Mol Sci. 2022;23(20). doi:10.3390/ijms232012139
13. Ho WS, Chan HH, Ying SY, Chan PC. Laser treatment of congenital facial port wine stains: long-term efficacy and complication in Chinese patients. Lasers Surg Med. 2002;30(1):44–47.
14. Shi W, Wang J, Lin Y, et al. Treatment of port wine stains with pulsed dye laser: a retrospective study of 848 cases in Shandong Province, People’s Republic of China. Drug Des Devel Ther. 2014;8:2531–2538. doi:10.2147/DDDT.S71710
15. Xu X, Lin L, Li B. Automatic protocol for quantifying the vasoconstriction in blood vessel images. Biomed Opt Express. 2020;11(4):2122–2136. doi:10.1364/BOE.387080
16. Zhang XY, Al-Odaini N, Zheng WJ, et al. The relationship between the effectiveness of HMME-PDT and the dermoscopic features of port-wine stains in Chinese pediatric patients: a retrospective study. Dermatol Ther. 2022;12(7):1671–1683. doi:10.1007/s13555-022-00757-3
17. Wang L, Li L, Huang C. Efficacy of photodynamic therapy in the treatment of port wine stains: a systematic review and meta-analysis. Front Med. 2023;10:1111234. doi:10.3389/fmed.2023.1111234
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
