Back to Journals » International Journal of Nanomedicine » Volume 18
Fortification of Iron Oxide as Sustainable Nanoparticles: An Amalgamation with Magnetic/Photo Responsive Cancer Therapies
Authors Rethi L, Rethi L, Liu CH , Hyun TV, Chen CH , Chuang EY
Received 11 January 2023
Accepted for publication 10 May 2023
Published 4 October 2023 Volume 2023:18 Pages 5607—5623
DOI https://doi.org/10.2147/IJN.S404394
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Phong A Tran
Lekha Rethi,1,2,* Lekshmi Rethi,2,* Chia-Hung Liu,3,* Tin Van Hyun,4,5 Chih-Hwa Chen,1,2,6 Er-Yuan Chuang1,2,7
1Graduate Institute of Biomedical Materials and Tissue Engineering, College of Biomedical Engineering, Taipei Medical University, Taipei, 11031, Taiwan; 2International PhD Program in Biomedical Engineering, College of Biomedical Engineering, Taipei Medical University, Taipei, 11031, Taiwan; 3Department of Urology, School of Medicine, College of Medicine, Taipei Medical University, Taipei, 11031, Taiwan; 4International PhD Program in Medicine, College of Medicine, Taipei Medical University, Taipei, 11031, Taiwan; 5Department of Interventional Cardiology, Thong Nhat Hospital, Ho Chi Minh City, 700000, Vietnam; 6Department of Orthopedics, Taipei Medical University – Shuang Ho Hospital, New Taipei City, Taiwan; 7Cell Physiology and Molecular Image Research Center, Wan Fang Hospital, Taipei Medical University, Taipei, Taiwan
*These authors contributed equally to this work
Correspondence: Er-Yuan Chuang, Email [email protected]
Abstract: Due to their non-toxic function in biological systems, Iron oxide NPs (IO-NPs) are very attractive in biomedical applications. The magnetic properties of IO-NPs enable a variety of biomedical applications. We evaluated the usage of IO-NPs for anticancer effects. This paper lists the applications of IO-NPs in general and the clinical targeting of IO-NPs. The application of IONPs along with photothermal therapy (PTT), photodynamic therapy (PDT), and magnetic hyperthermia therapy (MHT) is highlighted in this review’s explanation for cancer treatment strategies. The review’s study shows that IO-NPs play a beneficial role in biological activity because of their biocompatibility, biodegradability, simplicity of production, and hybrid NPs forms with IO-NPs. In this review, we have briefly discussed cancer therapy and hyperthermia and NPs used in PTT, PDT, and MHT. IO-NPs have a particular effect on cancer therapy when combined with PTT, PDT, and MHT were the key topics of the review and were covered in depth. The IO-NPs formulations may be uniquely specialized in cancer treatments with PTT, PDT, and MHT, according to this review investigation.
Keywords: cancer, hyperthermia, iron oxide, magnetic hyperthermia, photodynamic therapy, photothermal therapy, near-infrared
Introduction
Cancer is one of the world’s most common diseases, and it is now regarded as the most dangerous. It is a chronic sickness caused by abnormal cell development in practically any part of the body, and it necessitates long-term therapy. This can develop beyond its normal boundaries and spread to nearby organs, a process known as metastasis.1 Surgery, chemotherapy, and radiation therapy are all part of the treatment. However, due to the intense therapy, survivor suffers from various physical and psychological side effects, limiting its effectiveness. The current treatment method focuses on hypothermia, which uses heat to kill diseased tissues by raising the temperature to 45°C.2 Cancerous tissues are targeted for destruction while healthy tissues are spared. Magnetic hyperthermia therapy (MHT), photothermal therapy (PTT), and photodynamic therapy (PDT) are cancer ablation therapies that involve hyperthermia.3
The development of nanomaterials-based cancer therapy and integrated nanodevices for detection has always been the subject of current nanotechnology advancements. As a result, NPs enabled advanced, sensitive, highly precise imaging in cancer treatment and the delivery of anticancer drugs to tumor targets. The intrinsically special magnetic or optical characteristics of NPs make their use ideal for numerous imaging modalities. Due to their great sensitivity, small size, and unique composition, NPs make excellent contrast agents. On the surface of NPs, suitable targeting ligands are frequently conjugated. By integrating different functional materials, multifunctional NPs can be created, enabling simultaneous multimodal imaging and therapy, or theranostics. Current research on NPs has shed light on novel oncogenic targets, systemic cancer therapy, and the development of new targeted medicines. Nanomaterials like Au NPs, liposomes, carbon nanotubes, polymeric micelles, graphene, ferrous or ferric oxide NPs, and quantum dots are frequently used in biomedical applications. Because of recent developments in NP-based applications, human exposure to manufactured NPs is unavoidable.4–8 In hyperthermia treatment, metal NPs such as gold NPs, SPIONs, or polymer NPs such as polypyrrole are often employed.9,10 These NPs are introduced into the body and Thermal treated by near-infrared (NIR) radiation to target cancer tissues.11 NPs absorb light energy and convert it to heat energy, which aids in the destruction of cancer tissues and subsequent eradication. IO-NPs (IO-NPs) are receiving increased recognition in the biomedical field due to their unique physicochemical characteristics (Figure 1). IO-NPs are better for MHT, PTT, and PDT because of their physiochemical properties. In comparison to gadolinium, which have magnetic but highly toxic, iron NPs have a significant superparamagnetic characteristic and have lower toxicity.12 IO-NPs are frequently employed in hyperthermia treatment. IO-NPs-containing ferrofluid is generally administered into the tumor. In the case of MHT, they are heated in the presence of an alternating high-frequency magnetic field. The heat released by the NPs disperses the temperature, assisting in the destruction of malignant cells while causing very minor damage to normal cells.13,14
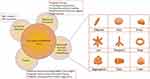 |
Figure 1 Developments of IO-NPs for potential medical technological applications and cancer therapy. |
The focus of this review is on IO-NPs NPs (IO-NPs) and their applications in MHT, PDT, and PTT. Furthermore, the advantages and disadvantages of different types of IO-NP magnetic nanoformulations in terms of phototherapeutic effects are compared in the context of magnetic hyperthermia, PDT, and PTT in current clinical settings.
Cancer Therapy and Hyperthermia
Apoptosis and other thermally induced metabolic processes, such as heating tumor cells to a temperature of 40–45 °C, are used in hyperthermic therapy to kill cancer cells. All hyperthermia-related events work to change the extracellular milieu by triggering immune responses and forcing tumor cells to switch to an anaerobic metabolic mode. According to the location of the application, hyperthermia can be divided into the following groups.
Whole Body Hyperthermia
Depending on how it is applied, this procedure could be invasive or non-invasive. While non-invasive hyperthermia entails raising the temperature using hot air, hot wax, or RF or IR irradiation, these methods are ineffective for treating deep tumors. Invasive hyperthermia requires heating blood extracorporeally.
Regional Hyperthermia
Regional cancers are heated either through non-invasive way like ultrasound or non-ionizing electromagnetic radiation (EMR), or (ii) via invasive way like thermal conduction or magnetic implants.
Local Hyperthermia
Using the invasive or non-invasive techniques outlined above, this technique is utilized to heat tiny tumors to a depth of 4 cm.15
Photothermal Agents
Photothermal agent (PA) is required to turn light into heat. A good PA must be able to absorb NIR radiation, have a diameter around 30 and 200 nm for promoting long circulation and enhanced tumor accumulation, have minimal toxicity and maximum biocompatibility, be able to absorb NIR radiation, and have a high absorption cross-section to maximize light-to-heat conversion. PA is currently available in a wide variety of compounds with various compositions, structures, shapes, and surface coatings. Organic and inorganic biomedical materials can be used to categorize PA. Although there are many other forms of inorganic materials, metallic nanostructures and carbon-based materials are the most common. Polymer NPs and organic dyes are the two organic components that stand out in the heterogeneous category.16–20
The local surface plasmon resonance is a distinctive photophysical phenomenon that occurs in metallic nanostructures, often known as plasmonics (LSPR). The electromagnetic radiation’s oscillating magnetic field causes the conduction-band electrons on a particle’s surface to oscillate in unison when it interacts with a particulate plasmonic material. This oscillation suggests the generation of heat. The oscillation’s amplitude is greatest at a certain wavelength known as LSPR. Nanomaterials based on gold are among the most studied examples. For PTT ablation, targeted drug administration, controlled drug release, and other forms of treatment for tumor models in vivo, gold nanorods, nanoshells, nanostars, nanocages, and “cluster”-shapes have been employed.20,21 Au NPs are extremely adaptable in biophysical applications due to their apparent chemical characteristics, such as surface chemistry, zeta potential, and electron placement. Physical characteristics including size and form have an impact on cellular uptake overall, with sphere-shaped NPs being favored over nanorods in comparison. Nanorods are thought to be inhibitory while nanospheres are thought to be far more stimulating. Due to its high surface-to-volume ratios and low toxicity, the spherical shape is suitable Irradiation is used to mold NPs into nanorods when they are exposed to a laser.20–22
Due to the wide range of nonmetallic materials, electronic transition materials are also being tested as PA. PTT transduction is triggered by the electrons' ability to change between molecular and atomic orbital energy levels, where the energy gap is comparable to the NIR range of light. Organic chemicals, namely NIR dyes, are very desirable candidates for PTT when compared to inorganic PA because of their photophysical characteristics and accessibility for large-scale chemical synthesis. Additionally, organic dyes are capable of incorporating a variety of specialized molecules, including antibodies, double-stranded DNA, proteins, DNA primers, amino acids, and chemical small molecules. These molecules have all been used in molecular imaging.20,23,24
After choosing a PA to be utilized in PTT, it must be administered either intravenously through targeted administration or directly, or intratumorally. When injected intravenously, which is the most likely case, the PA must be absorbed into the tumour utilising two distinct strategies: active and passive targeting. To accomplish active targeting, the surface of the PA must be functionalized with a peptide or antibody that can be selectively recognised by proteins overexpressed in tumour cells. It is necessary to functionalize the interface of the PA with antibody or polypeptide that can be selectively recognized by proteins expressed in tumoral cells to achieve active targeting. Biological targeting is the term for this kind of targeting. The improved permeability and retention effect, on the other hand, is the foundation for passive targeting (EPR). NPs of a specific size range (usually 20–300 nm) preferentially aggregate in tumor tissue as a result of anomalies in the tumor vasculature. As a result, size has a big impact on how PA behaves pharmacologically in the body. Large PA molecules (>40 nm at least in one dimension) have difficulty penetrating deeply into tumor tissues and being eliminated by the body after treatment, which can reduce therapeutic effects and raise the risk of toxicity.25–29
The particle size can be decreased to solve this issue. It has been shown that sub-10 nm NPs can enter the deep region of tumors, can integrate themselves more effectively than larger tumor cells, and can also leave the body quickly. Depending on the laser dosage, type, and exposure time, photothermally caused cell death can occur either by apoptosis or necrosis.17,20,30,31
Iron Oxide Nanoparticles (IO-NPs)
The term “nanoparticle (s)” (NP(s)) refers to particles with a diameter of less than 100 nm. Different materials can be used to make NPs for different purposes. The size, shape, and phase of NPs might vary depending on their purpose and use. The current research is more focused on the enhancement of drug delivery systems and the high concentration of drug release in the infected site with lower toxicity, as a result of the expanding research interest in therapeutic or diagnostic NPs. To generate heat evenly, the NPs employed for HTP should be tumor attached, nominally interfering, and homogeneously distributed. To avoid destroying normal tissues in the surrounding area, the NPs should only be targeted and deposited on tumor cells.
Hyperthermia has been treated using a variety of NPs, including Au NPs, polypyrrole NPs, and IO-NPs NPs. Iron NPs have lately been researched for biomedical uses after proving to be very effective materials in therapeutic applications. The utilization of iron NPs for hyperthermia was proved by the influential work of Jordan et al in 1993.32–38 They are a promising choice for application in particle sizes ranging from 15nm to 200nm because of their excellent superparamagnetic property, biocompatibility, and biodegradability.39,40 Iron particles are widely recognized as a great material for magnetic resonance imaging (MRI). They give high tissue contrast and astonishing picture resolution, revealing anatomical and morphological aspects of the entire human body. Several studies have recently demonstrated that iron NPs can be used in PTT in both vitro and in vivo.37,41–43
The different chemical compositions of IO-NPs include magnetite (Fe3O4) and maghemite(-Fe2O3) most frequently a non-stoichiometric combination of the two. In other words, superparamagnetic NPs become magnetic in the presence of an external magnet but return to a non-magnetic condition when the external magnet is removed. Both oxides exhibit superparamagnetic behaviour below a certain size (25 nm for magnetite, 30 nm for maghemite). IO-NPs with magnetic properties and a biocompatible coating have been used to increase the contrast in magnetic resonance imaging (MRI).20,44–46
Because of the biodegradability, biocompatibility, ease of synthesis, and ease of tuning and functionalization for particular applications, magnetite cores have a significant potential for use in oncological treatment. Furthermore, superparamagnetic behavior will be seen in spherical magnetite NPs whose diameters are less than about 20 nm. This behavior is used to improve contrast in magnetic resonance imaging (MRI). The magnetite core of most superparamagnetic IO-NPs nanoparticle (SPION) conjugates naturally provides MRI contrast, and the biocompatible coating offers plenty of functional groups for conjugating additional tumor targeting and therapeutic moieties. As some magnetite-based NP formulations, such as Feraheme®, Feridex I.V.®, and Gastromark®, have already been given approval by the Food and Drug Administration (FDA) for application in humans to be iron deficiency therapeutics and to be MRI contrast agents, an expansion of various NP topologies for applications beyond than enhancing MRI contrast, such as cancer therapies by drug delivery, biotherapeutic transport, magnetic hyperthermia, and photothermal ablation. Researchers may quickly and efficiently construct customised NP formulations with a full range of therapeutic and diagnostic capabilities using a library of synthesis techniques and discrete nanoscale modules with responsibilities particular to cancer theranostics. The significance of the possibility for IO-NPs NPs to be used in highly customised therapy is highlighted by this concept. 47–51
Maghemite is a reddish-brown oxide with a structure like magnetite that is frequently created by oxidizing other Fe oxides, particularly magnetite. With a density of 4600 kg/m3 and a bulk saturation magnetization value of *76 to 80 emu g-1, maghemite is also ferrimagnetic. All or most of the Fe is present as Fe 3+, having cation vacancies accounting for the oxidation of Fe 2+, which is the main distinction from Fe3O4. Because of the huge interface area (relative to volume) available for chemical reaction, IO-NPs are easily oxidized to the more stable once exposed to gas.52,53
IO-NPs are used to treat cancer by triggering noninvasive cell death while leaving normal cells intact due to the effective synthesis of magnetic NPs to generate heat under an applied magnetic field. Because IO-NPs have a high magnetic saturation, they are a more reliable choice.54 The use of NIR irradiation has been employed to investigate and develop IO-NPs as a promising possibility for cancer treatment.55,56 Several recent investigations have shown that mixing MHT with chemotherapy results in cancer cell execution. Due to its response to external magnetic fields, as well as the potential of targeted molecules and biocompatibility, superparamagnetic IO-NPs NPs (SPIONs) have proved their effectiveness in MHT. SPION is more appealing for biomedical applications because of its intrinsic superparamagnetic characteristics. A typical SPION has an IO-NP core that acts as a contrast agent in MRI and as a carrier in targeted cancer treatment, while the outside surface provides active sites for the interaction of cancer cell surface receptors with SPION. Gilchrist et al made the first demonstration of the use of IONPs for cancer treatment using magnetic hyperthermia in 1957.54,57–61
The ability to tune surface characteristics and surface functionalization with biomolecules has made IO-NPs extremely appealing. These factors are allowing IO-NPs to carve out a niche for themselves in the realm of nanocomposites and electronics. They are employed in nanomedicines for imaging modalities, sensing, and treatments because of their biocompatibility. Surface modification of IO-NPs can increase a variety of attributes, including chemical dispersion, biocompatibility, physiochemical and mechanical capabilities, and surface activity.62–65
Therapy Outcomes of Iron Oxide Nanoparticles (IO-NPs)
Clinical Target for IO-NPs
Due to their strong magnetic characteristics, IO-NPs are widely used in biological applications. They are a good contender for a variety of applications because of their tailored medication delivery and magnetic separation capabilities. Their properties are put to use in preclinical research such as imaging contrast enhancement, MHT, cell tracking, and a variety of diagnostics and treatments66–69 (Figure 2).
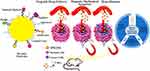 |
Figure 2 SPIONs as magnetic therapeutic agents and the functions performed for tissue engineering and cancer treatment. Reprinted with permission from Nabavinia M, Beltran-Huarac J. Recent progress in iron oxide nanoparticles as therapeutic magnetic agents for cancer treatment and tissue engineering. ACS Appl Bio Mater. 2020;3(12):8172–8187. Copyright © 2020, American Chemical Society.69 |
Despite their vast properties, IO-NPs can only be used in clinical processes due to their surface chemistry, size, and biodistribution.70 Their biodistribution investigations revealed a significant buildup in the liver and spleen, even though the literature states that kidneys are responsible for their removal. Magnetic NPs can be employed for diagnostic and cancer therapy because of their structural, electrical, optical, and magnetic capabilities, as well as their capacity to connect tumor-targeting ligands.37
In the biomedical field, magnetic NPs are employed as a medication delivery and release agent, MHT, MRI contrast agent, and magnetic partition agent. Magnetic NPs in the field of cellular therapy perform duties such as cell labeling, tissue healing, cell partition and handling, and magneto reception.37,71–73 In the form of chemotherapeutic drugs, IO-NPs have been used as nanocarriers in vaccination. In skin care, they are excellent antimicrobial and antibacterial agents, as well as age-defying.74–76
FDA Approved IO-NPs and Their Applications
The FDA has approved the IO-NPs as an MRI contrast agent. Even though some IO-NPs, such as SPIONS, have been approved, they are not permitted to be administered to the body without restriction. Because of the documented toxicity of uncoated SPIONs and various surface-layered IO-NPs, this is the case. SPIONs caused cytoskeleton disruption, oxidative stress induction, free radical generation, nullifying mitochondrial function, DNA damage, alteration of cell signaling, and other harmful factors to cell behavior through various mechanisms such as cytoskeleton disruption, oxidative stress induction, free radical generation, nullifying mitochondrial function, DNA damage, alteration of cell signaling, and so on, all of which were caused by the magnetic cores and coating, which were unstable in biological media and serum. Feraheme, Sinerem, Endorem Feridex, Clariscan, Resovist, and Resovist S Supravist are among the FDA-approved IONPs.77–82
The Nanoparticle and MHT, PTT, PDT
For effective local tumor ablation, the MHT utilizes heat generated by an alternating magnetic field (AMF). When magnetic NPs and an external AMF accumulate in the target site, heat is commonly generated. Furthermore, all magnetic NPs can generate heat, but the amount of heat generated is dependent on the materials used and the AMF. Foucault’s currents also known as Eddy current, frictional heating, relaxation, and hysteretic losses all aid in heat generation in magnetic hyperthermia. MHT, in combination with chemotherapy and smart NPs, is a successful treatment for tumor eradication. The IO-NPs nanocubes are subjected to a dual-mode treatment that combines MHT and PTT. When nanocubes coated with PEG-Gallol were exposed to AMF and NIR irradiation, the heating impact was two to five times more than when MHT was used alone. Apoptosis and collagen fiber degradation has been demonstrated to be useful in ablating tumors.38,83,84
Phototherapy is the use of nanomaterials triggered by precise wavelengths of light and vibrational energy to generate heat to cure ailments safely and effectively. Because they use NPs and NIR, PTT, and PDT are the most prevalent treatments for effective cancer treatment. The mechanism of cancer treatment using PDT is that photoactivated material produces reactive oxygen species, which causes oxidative stress in cells, DNA and protein denaturation, and cell death. The tissue has a low absorption of NIR laser.85–87
The photosensitizer is excited with NIR in PTT-based cancer therapeutic technique. When triggered, the photosensitizer is energized by vibrational energy and releases heat up to 42 ° C, killing cancer cells. This technique makes use of a non-toxic but effective hyperthermia approach. Since photosensitizers are so important, nanoformulations and NIR absorption after they have collected on the tumor are crucial because they allow faster cancer ablation and heat conversion. Cell membrane damage, cancer cell DNA denaturation, and angiogenesis obstruction are the most relevant factors that contribute towards cell death.88–90
There is a need to boost the effectiveness and discernment of energy to thermal transduction, aka a photothermal agent into the tumor, to provide an effective treatment. Nowadays, a diverse spectrum of photothermal agents is widely available. Even if it’s PTT or PDT, two well-known minimally invasive cancer therapies, the role of NPs is unavoidable. Noble metals, transition metal sulfur oxides, and carbon compounds are often used in PTT, while Phthalein, cyanogen, and porphyrins are commonly employed in PDT because they have good biocompatibility and can be used in cancer therapy.91–93 Gold-based NPs are in high demand in the PTT, but they are more expensive because of the time-consuming vital chemistry processes. Metal NPs were reported to have stayed in bodily tissue for a long time, and their potential toxicity made them unsuitable for the PTT. Studies conducted also state that the efficacy of core-shell NPS has been shown to be 20% higher than that of gold NRs.94–97
The nature and efficacy of the NPs, which can both absorb and convert energy into heat when stimulated by NIR, are the only factors that determine the success of PDT and PTT. The materials utilized in PDT and PTT will have an impact on the therapy’s efficacy. NIR, which has a wavelength range of 700–1100 nm, is utilized instead of visible light because photosensitizers can be triggered by it, and it can also penetrate deeply into malignant tissue and kill cancer cells. PTT uses localized photosensitizing chemicals that are activated by a specific band of light, whereas PDT uses singlet oxygen that causes irreversible free radical damage to the cancer tissue when activated by light.87,93,98
Magnetic NPs as photosensitizers/photothermal agents have also made significant progress. Magnetic nanomaterial-based PDT/PTT is an integrated platform and superior thermal therapeutic choice over conventional thermal therapies, demonstrating remarkable clinical outcomes through effective and numerous cytotoxic ways of killing various grades of cancer cells in future clinics.86,99
PDT, on the other hand, uses the NIR to detect photosensitive material (PS) that has accumulated in the tumor location. The catch is that when they come into contact with light, they release various ROS (reactive oxygen species), which effectively destroy cancer cells. Tochner employed Photofrin, one of the earliest photosensitizers created, to evaluate PDT for PM treatments in around 1985.100–102
Intracellular lipid peroxidation, DNA damage, and protein deterioration are the mechanisms of cancer cell death, but they do not cause any damage to nearby cells, resulting in direct cell necrosis, apoptosis, and indirect microvascular damage to cancer tissue. In the absence of light, the procedure entails the injection of a prodrug and its circulation throughout the body. When the light reaches the target site, it irradiates these particles. The tumor will shrink and eventually die out.103,104
The importance of PDT was initially demonstrated on rat glioma cells, followed by the first preclinical investigation on bladder carcinoma and the first clinical study on psoriasis, mycosis fungicides, and skin cancer. PDT has also been utilized in the treatment of tracheobronchial tree cancer, skin cancer, lung cancer, and esophageal cancer, according to various research publications from around 1982 to 1985.3,105
According to Mitton and Ackroyd (2008), PDT became popular in the 1970s as a cancer therapy technique after it was recognized that porphyrins become localized in tumors. These are used to treat a variety of cancers, most notably choroidal neovascularization (CNV). According to Hunt in 2002, this technology is particularly important because the PS exclusively targets undesirable tissue. Apoptosis is only limited to the areas where the specific wavelength of light is used. PDT can be used not only for cancer treatment but also for microbial treatment, which gained prominence in the mid-1990s. The NPs are supplied via oral, injectable, and intravenous routes, which most likely assist them to overcome protein interaction, phagocyte invasion, and cellular resistance, allowing them to be most effective in biomedical applications. The functions of IO-NPs NPs, as well as magnetic hyperthermia, PTT, and photodynamic therapy.106–108
Clinical Efficacy of IO-NPs
MNPs have piqued the interest of researchers all over the world due to their abundance, environmentally friendly features, and wide range of applications, which include catalysis, environmental cleanup, magnetic fluids, electronic communication, data storage, and biomedicine. The IO-NPs, which are well-known for their ease of production, modification, and low toxicity, are among the most well-known MNPs. According to recent studies, IO-NPs are employed for hyperthermia treatment more frequently than other MNPs. Because of their quick aggregation and partial oxidation, IONPs have a lower magnet, which hurts their ability to treat cancer. As a result, these IONPs are likely to be functionalized on the surface to prevent oxidation and maintain their treatment performance.109–112
The size, shape, and activity of NPs are revolutionizing nanomedicine, with applications ranging from molecular detection and sensing to gene delivery, DNA transfection, cell patterning, and imaging biological samples. Because of their unique physicochemical characteristics and mobility, NPs interact with a variety of cell types. IONPs can be synthesized in a variety of ways, including laser pyrolysis, solvothermal method, sonochemical approach, and others, all of which have their own set of benefits and drawbacks. Although significant progress has been achieved in the synthesis of NPs and their uses in biomedicine, they are still in their infancy, hampered by several significant difficulties. Because of their high superparamagnetic characteristics, magnetite, hematite, and maghemite are the most popular MNPs. Even though they have a strong aggregation property, they can achieve better colloidal stability, biocompatibility, and persistence. The presence of Fe2+ and Fe3+ iron species in magnetite gives it distinct properties from other IONPs.58,112–114
Hyperthermia Along with IO-NPs
Hyperthermia is the use of microwaves, NIR, and magnets to generate thermal energy to destroy cancer tissues. Because of their low toxicity in the body and direct usefulness in treating malignant tissues, these treatments have been applied all over the world. Because of their cellular architecture, cancer cells cannot endure heat and suffer severe damage, which can be employed to kill them. As the temperature rises from 42°C to 60°C, hyperthermia becomes more effective. Keeping all of these factors in mind, chemotherapy and radiotherapy are combined with hyperthermia to improve cancer treatment. In the case of MHT, IONPs are injected into the tumor and exposed to an external AMF, which generates heat, inducing cell death and little tissue damage. The combination of low iron concentration, laser irradiation, and a magnetic field resulted in total cancer ablation. When it comes to photodynamic therapy, photosensitizers are heated and ROS is produced, which aids in cancer ablation. The concept of hyperthermia is described as Figure 3.115–117
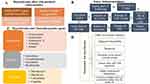 |
Figure 3 Clinical hyperthermia at 39–45 °C is a complex cancer therapy strategy. (A) Effects as a strong radiosensitizer. (B) An immunomodulator for tumors with the potential to act as an in-situ tumor vaccine. (C) Chemotherapeutic drugs act independently, furthermore, and synergistically. Future possibilities when magnetic NPs are used as a medium. (D) NPs based Hyperthermia advantages. Reprinted from Cancer Treatment Reviews, 41, Datta NR, Ordóñez SG, Gaipl US, et al. Local hyperthermia combined with radiotherapy and-/or chemotherapy: Recent advances and promises for the future, 742–753, Copyright 2015, with permission from Elsevier.117 |
Magnetic Hyperthermia with Iron Oxide Nanoparticle
MHT is a potent but experimental cancer treatment in which magnetic NPs convert electromagnetic energy to heat energy under the influence of an external AMF. A significant character has been achieved with magnetic NPs and alternating current hyperthermia for the treatment of cancer tissues. Radiotherapy and chemotherapy have been shown to have synergistic benefits when tumor cells are targeted with localized heating. The increase in temperature when the particles are heated improves cell oxygenation and therapeutic efficacy.118,119
IO-NP nanoformulations with sizes ranging from 10 to 100 nm in size are commonly employed in MHT for cancer treatment. When compared to other materials, this is due to their superior chemical stability, functionalization, and biocompatibility. The targeted heating of the MNPs that are directly injected into the tumor is where MHT’s effectiveness rests. This is where IO-NPs NPs come in help, as they have a high heat conversion efficiency when triggered with an alternating magnetic field. When the MHT is completed, another notable aspect is lysosomal damage. Magnetic hyperthermia as a treatment for brain tumors has received clinical approval in several European countries. However, this technique has not been widely used for the treatment of malignant cells, including in the United States, which may limit the growth of optimized magnetic IONPs. The main disadvantages are the high dose of magnetic IONPs and heterogeneous heating in the cellular environment. The concept of MHT of IONPs with hybrid NPs is given in Figure 4.120,121
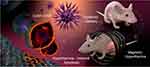 |
Figure 4 Hybrid NPs combined with IO-NPs nanoparticle for systemically delivered magnetic hyperthermia with improved heating efficiency. [Reprinted with permission from Albarqi HA, Wong LH, Schumann C, et al. Biocompatible nanoclusters with high heating efficiency for systemically delivered magnetic hyperthermia. ACS Nano. 2019;13(6):6383–6395. Copyright © 2019, American Chemical Society.121 |
Equipment for Measuring Magnetic Hyperthermia
By applying the characteristics of an alternating magnetic field that is induced in one of the following means, MIH involves heating of a localized tumor. Coil: The dimensions of the target tumor define the coil size. A coil diameter about 25 mm is adequate to produce a suitable magnetic field strength for tiny malignancies. Solenoid, birdcage, Helmholtz, and pancake coils are the four types of coils used to create magnetic hyperthermia (MH). When magnetic field strengths are more than 20 mT, solenoids must be cooled. When cooling rates are suboptimal (low or high), hyperthermia might be negatively impacted because of excessive heating or cooling, respectively. Operation at the ideal temperature, when the sample and coil should be in thermal equilibrium, is therefore required. Insulated coils and materials with low thermal conductivity, including expanded polystyrene, polyethylene, polyurethane, or a three-layer cork wall, are used to create this state.15,122,123
Thermocouple
Magnetic hyperthermic apparatus should have non-intrusive, precise, and sensitive thermometric components. The therapeutic impact may not be obtained if the temperature in the tumor tissue is less than that which was intended; on the other hand, a temperature that is too high may have negative effects on nearby healthy tissues. This issue is resolved by using highly developed thermometric systems, most often optical fiber thermometry, MRI, and IR thermography.122,124
The fundamental idea behind the magnetic hyperthermic approach is that raising temperatures to 42–46 °C, promotes “heat-controlled necrosis” by interfering with cancer cells’ enzymatic activities. Importantly, this only slightly harms the nearby tissues. Additionally, this method has several benefits over more common methods that cause hyperthermia, like radiofrequency and ultrasound radiation. For instance, the magnetic hyperthermic application is far more precise than these more traditional thermal approaches because it is not constrained by target depth, backscattering, or the heat-sink effects of big blood arteries. Using MNPs, which offer even temperature distributions in tumors, MH treatment can also be carried out at the cellular level. Additionally, compared to other modalities, this kind of intervention is less intrusive.125–127
Iron Oxide Nanoparticles in Photothermal Therapy
PTT generates heat for cancer treatment by using NIR radiation-absorbing NPs (ideally Au NPs). The light energy excites the NPs, causing them to get agitated, at which point they emit heat energy, killing the target cells. Under an AMF, magnetic IO-NPs NPs have been widely employed as an MRI contrast agent and MHT inducer. There are two approaches to using IO-NPs: one is to combine IO-NPs with any PA (ligand-Fe complexes), and the other is to simply use IO-NPs as PA.128 The ferromagnetic characteristic of iron causes IO-NPs with bare surfaces to agglomerate. They are avoided by coating them with appropriate materials that make them hydrophilic and biocompatible while also improving their targeting capabilities. In the case of hyperthermia, IO-NPs can be functionalized with medicines, proteins, enzymes, antibodies, and other molecules to assist target specific locations. The synthesis and surface modification of magnetic IO-NPs can be done in a variety of ways. Magnetic IO-NPs NPs can be precipitated in an aqueous solution or aqueous core of water in oil microemulsions to create IONPs with diameters ranging from 1 to 20 nm. Precipitation in the presence of polymers produces NPs with diameters of 20–50nm, while surface coating with surfactants or polymers produces NPs with diameters greater than 50nm. The size, solubility, and surface chemistry of MNPs vary based on their core properties. When iron NPs are submerged in biological fluids during the hyperthermia process, various surface coatings protect the cores from corrosion. Due to their superparamagnetic properties, magnetite and maghemite, or their combination as both, are commonly used for PTT. The temperature of the IO-NPs can be elevated to 42 ° C, resulting in cancer cell necrosis. Normally, IO-NPs NPs do not have a high conductivity by themselves, thus they are coated with photothermal agents (PA). The MNPs in combination with the PA itself produce excellent results in terms of targeting and NIR absorption, which aid in imaging and hyperthermia.129–132
Iron Oxide Nanoparticles in Photodynamic Therapy
The cancer cell ablation at the targeted tumor location is achieved by administering photosensitizer to cancer-infested sites under NIR irradiation, resulting in ROS production. The PDT’s key advantages are that they are specific to the tumoral spot and can be repeated, if necessary, in the interim. IONPs are an efficient material since they are affordable, less poisonous, and do not require surgery, but their lack of selectivity is a disadvantage. IONPs are used in conjunction with photosensitizers to overcome this limitation. Most notably, the presence of PDT accelerates the Fenton process and causes synergistic ROS production in macrophages by increasing oxidative stress. Combining these three components to provide high anticancer activity as a unit.37,133–135 The approach is explained in Figure 5.
 |
Figure 5 The Fenton reaction and oxidative stress on macrophages are further accelerated by the presence of PDT, leading to synergistic ROS generation via hybrid IONPs. Reprinted with permission from Liang X, Chen M, Bhattarai P, Hameed S, Tang Y, Dai Z. Complementing cancer photodynamic therapy with ferroptosis through iron oxide loaded porphyrin-grafted lipid nanoparticles. ACS Nano. 2021;15(12):20164–20180. Copyright © 2021, American Chemical Society.135 |
Hybrid Nanoparticles Formed with Iron Oxide Nanoparticles
There are two ways to use IO-NPs for PTT: (a) as a standalone PA; and (b) in combination with any other PA to produce hybrid NPs that display the key characteristics of magnetism and NIR absorption (for MRI, magnetic targeting, photoacoustic tomography, and PTT). The apparent photothermal performance of IO-NPs might be poor due to their low NIR molar absorption coefficient. Because of this, they are frequently blended with other PA to produce a hybrid nanocomposite. The possibility of using magnetic and PA-containing nanocomposites as theranostic agents in the biomedical sector has attracted a lot of interest. As theranostic agents for drug administration and MRI, NPs with magnetite content and polymeric encapsulation are applied in numerous uses. When paired with other imaging modalities, MRI can provide supplemental diagnostic information for more accurate tumor characteristic identification and the precise direction of anticancer therapy. MRI delivers a high-resolution image of body components. A magnetic field can functionalize and direct magnetic NPs. Advanced MRI-guided medication and gene delivery, MHT cancer therapy, cell tracking and bioseparation, regenerative medicine, and tissue engineering are all made possible by them. Using MRI, IO-NPs can be utilized to diagnose liver, inflammatory, and liver and vascular conditions. Additionally, they are employed in therapeutic procedures such as magnetic fluid hyperthermia, macrophage polarisation, magnetic medication targeting, and iron supplementation in anemia. These characteristics make them particularly valuable in theranostic applications.136–140 As previously mentioned, surface modification of NPs with a targeting ligand (eg, peptide) is used to direct the NPs to the tumor side. Other methods of targeting, specifically physical targeting, have been developed as an alternative to biological targeting. One of these is magnetic targeting, whereby a magnetic field from outside the body can affect how magnetic NPs move. This is possible because the magnetization and NIR absorption capacities of the produced NPs are combined into a single structure. They can also be utilized in MRI or thermal imaging to assess the efficacy of the therapy in real-time.20
Nanomaterials based on gold are one of the PA. Gold nanostructures have a significant capacity for absorbing cross-sectional energy, which can provide enough heat. Because of their peak visible light absorptions, spherical gold NPs have not been very useful in vivo. For instance, the LSPR wavelength of a spherical gold nanoparticle with a diameter of 10 nm is 520 nm as opposed to 580 nm for particles with a diameter of 100 nm. However, one may adjust the LSPR wavelength to the NIR area thanks to the hierarchical building of gold NPs. As a result of their LSPR being in the NIR region, nanoshells, gold nanorods, nanostars, and nanocages have been employed as PA for eradicating in vivo tumoral models. Gold supra shells are a type of self-assembled nanostructure produced from the aforementioned hybrid gold-IO-NPs. Surface plasmon resonances in gold supra shells span a wide range of vis-NIR wavelengths. Such supra shells produced heat that could be used to kill cancer cells after being excited by two continuous wave lasers that emitted light at either 514 or 785 nm.141,142 Au is one of the most alluring components when paired with SPIONs. The combination of magnetic NPs with au has opened new options, particularly in the treatment of oncological diseases, to achieve more effective and varied therapy for various tumor types. Briefly stated Au NPs can be used as therapeutic methods such as radiotherapy (RT), photodynamic treatment (PDT), chemotherapy, and hyperthermia. The use of Au-based nanomaterials in clinical tests is increasing because so many of these procedures are minimally invasive or non-invasive.37,143
Most of the time, the tumor is thermally abated applying a laser beam that has been heated up by Au NPs. This encourages several biological processes, such as apoptosis, protein denaturation, and necrosis. This causes the death of the tumor cells. This therapy approach’s key drawback is the poor amount of light penetration inside the tissues, which means it can only be used to treat surface malignancies. Due to the potential to get beyond the constraints of Au NPs noted above, the creation of coupled Au-SPION nanosystems has received a lot of interest recently. According to some scientists, Au-incorporated SPIONS having high NIR light absorption could be a contender for cancerous PTT.143–145
Nearly 50% of cancer patients are treated with RT, making it a vital therapeutic approach. Due to several benefits of gold nanostructures, such as high absorption as well as efficiency in creating secondary electrons upon irradiation of g-ray or X-ray, there has recently been a lot of interest in using AuMNPs as radiosensitizers for cancer treatment, with a focus on solid hypoxic tumor. It has been demonstrated that Au MNPs, MNPs of various shapes as well as sizes, can greatly enhance the efficacy of cancer therapy when mediated by RT and HT. According to the effectiveness of the results, the physical and chemical characteristics of nanocomposites have a significant role in determining how well they work in multimodal imaging and therapy.146,147
Due to some limitations in drug loading, release rates, and retention time in the bloodstream blood circulation, the application usage of bare MNPs often tends to be unsuccessful drug carrier formulations. This is widely documented among the huge volume of material addressing the use of MNPs in the biomedical sector. Additionally, it was noted that MNPs may aggregate once they are part of a biological system, which can harm their colloidal stability. Magnetic nanoparticle use has been constrained commercially due to these disadvantages. By mixing biocompatible components, such as lipid-based nanocarriers, with magnetic nanoformulations, these issues can be solved. The NPs can function as a drug delivery vehicle and carry out a variety of biological interactions with the cell when coated with polymer or liposomes. On the other hand, because they alter the tumor’s atomic makeup when introduced, magnetic NPs like IO could be used as radiosensitizers in radiation. Magnetic nanoparticle addition would alter the radiation dose and picture contrast at the tumour.37,148–150
In this regard, Lassalle’s research team has lately developed several preliminary experiments. To test if a lipidic-magnetic nanosystem could transport and distribute active ingredients like Diclofenac, nanostructured lipid carriers (NLCs) were coated with magnetic NPs. Diclofenac is a regularly used non-steroidal anti-inflammatory medicine (NSAID). The physical state of the lipids that make up NLCs differs from that of the solid lipidic NPs (SLNs) in which they are found. While NLCs are made up of combinations of solid and liquid lipids, SLNs are made up of solid lipids (oils). It is important to emphasize that, following the method described by Nicolas et al, the MNPs used in this procedure were made of magnetite as the core and chitosan as the coating.37,151
Linoleic acid hydroperoxide (LAHP) as well as catalytic iron (II) ions were used to create activatable singlet oxygen (1O2)-generating device for targeted cancer therapy in an acidic tumor pH environment. One of the main byproducts of lipid peroxidation is LAHP, which through the Russell mechanism decomposes into ROS and 1O2 in the presence of Fe2+ and is linked to several disorders. In this case, LAHP organic substances/polymers having a surface-anchoring group incorporated with IO NPs. H+ may pass through the polymeric brushes and separate Fe2+ from IO-LAHP NPs’ surface, causing ROS and 1O2 to develop and ultimately killing cancer cells (ie, ferroptosis).152,153
In this case, LAHP polymers with a surface-anchoring group were carried by IO NPs. H+ may pass through the polymer brushes and separate Fe2+ from IO-LAHP NPs’ surface, causing ROS and 1O2 to develop and ultimately killing cancer cells (ie, ferroptosis). To evaluate the effective production of 1O2 species by iron (II)-catalyzed breakdown of LAHP molecules, a UV-constructed singlet oxygen scavenger 9,10-diphenylanthracene (DPA)-derived biosensor and fluorescence (FL) singlet-oxygen sensor green (SOSG) was made. The good efficacy of ferroptosis-based cancer therapy is shown by the findings of confocal microscopy imaging, a cell viability study, a flow cytometry investigation, and tumor-growth-inhibition curves.152,153
Future Perspective
Furthermore, the grade of IO-NPs materials can be altered based on various preparation processes to fulfill future clinical requirements. However, more study is needed to improve and confirm the clinical feasibility of IO-NPs formulations for cancer therapy in terms of efficacy and patient safety. These particles are biocompatible and biodegradable. The present focus on PTT, PDT, MHT, and IO-NPs can aid in the detection and management of diseases in their early stages Although the majority of conventional NPs have drawbacks such as poor physiological solubility, unfavorable pharmacokinetics, and low tumor selectivity, IONPs are anticipated to partially address these issues. Particularly in the areas of illness detection, timely screening, intracellular imaging, molecular studies, and multifunctional therapies, the future of IONPs in biomedical applications is quite promising. Although IO-NPs that have been approved by the US FDA are utilized by consumers, more study needs to be done on these materials before they can be made available to the general public alongside other therapy modalities like PTT, PDT, and MHT.154,155
Conclusion
The clinical value of PTT, PDT, and MHT as non-invasive techniques is of tremendous importance in various types of cancer treatment. In recent years, IO-NPs based formulations have been widely explored as photothermal materials, photodynamic materials, and magnetic sensitizers. Using IO-NPs formulations, the cancer treatment efficacy of PTT and PDT, as well as the MHT process, might be improved. Near-infrared therapies have been enhanced, and they have a bright future in life science. Because of their many properties, IO-NPs based formulations have a significant impact on magnetic hyperthermia, PTT therapy. IO-NPs based formulations seem to be promising therapeutic agents for any cancer treatment. IO-NPs based formulations can be utilized independently or in conjunction with other agents. They are expected to offer several benefits, including outstanding magnetic characteristics, good compatibility, and non-toxicity.
Abbreviations
MHT, magnetic Hyperthermia; PTT, photothermal therapy; PDT, photodynamic therapy; IONPs, Iron oxide nanoparticles; NP, Nanoparticles; NIR, Near infrared; MNPs, Magnetic Nanoparticles; PA, Photothermal agents; ROS, Reactive oxygen species.
Funding
This research was financially supported by the Taiwan National Science and Technology Council (NSTC) (grant numbers: 111-2320-B-038-039, 111-2221-E-038-002,112-2221-E-038-002-MY3 and 112-2622-E-038-006-) and Taipei Medical University, Shuang Ho hospital grant number:112TMU-SHH-24.
Disclosure
The authors declare no conflicts of interest.
References
1. World Health organisation. Cancer; 2022. Available from: https://www.who.int/news-room/fact-sheets/detail/cancer.
2. National Cancer institute. Hyperthermia to Treat Cancer; 2021. Available from: https://www.cancer.gov/about-cancer/treatment/types/hyperthermia.
3. Pinto A, Pocard M. Photodynamic therapy and photothermal therapy for the treatment of peritoneal metastasis: a systematic review. Pleura Peritoneum. 2018;3(4):20180124.
4. Kim J, Lee N, Hyeon T. Recent development of nanoparticles for molecular imaging. Philos Trans Royal Soc A. 2017;375(2107):20170022.
5. Lavacchi D, Roviello G, D’Angelo A. Tumor-agnostic treatment for cancer: when how is better than where. Clin Drug Investig. 2020;40:519–527.
6. Kong F-Y, Zhang J-W, Li R-F, Wang Z-X, Wang W-J, Wang W. Unique roles of gold nanoparticles in drug delivery, targeting and imaging applications. Molecules. 2017;22(9):1445.
7. Siddique S, Chow JC. Application of nanomaterials in biomedical imaging and cancer therapy. Nanomaterials. 2020;10(9):1700.
8. Siddique S, Chow JC. Gold nanoparticles for drug delivery and cancer therapy. Appl Sci. 2020;10(11):3824.
9. Vines JB, Yoon JH, Ryu NE, Lim DJ, Park H. Gold nanoparticles for photothermal cancer therapy. Front Chem. 2019;7:167.
10. Verma J, Lal S, Van Noorden CJF. Nanoparticles for hyperthermic therapy: synthesis strategies and applications in glioblastoma. Int J Nanomedicine. 2014;9:2863–2877.
11. Chatterjee DK, Diagaradjane P, Krishnan S. Nanoparticle-mediated hyperthermia in cancer therapy. Ther Deliv. 2011;2(8):1001–1014.
12. Zhao S, Yu X, Qian Y, Chen W, Shen J. Multifunctional magnetic iron oxide nanoparticles: an advanced platform for cancer theranostics. Theranostics. 2020;10(14):6278–6309.
13. Wang P, Chen B, Zhan Y, et al. Enhancing the efficiency of mild-temperature photothermal therapy for cancer assisting with various strategies. Pharmaceutics. 2022;14(11):2279.
14. Kang H, Hu S, Cho MH, Hong SH, Choi Y, Choi HS. Theranostic nanosystems for targeted cancer therapy. Nano Today. 2018;23:59–72.
15. Jose J, Kumar R, Harilal S, et al. Magnetic nanoparticles for hyperthermia in cancer treatment: an emerging tool. Environ Sci Pollut Res. 2020;27(16):19214–19225.
16. Melamed JR, Edelstein RS, Day ES. Elucidating the fundamental mechanisms of cell death triggered by photothermal therapy. ACS nano. 2015;9(1):6–11.
17. Pérez-Hernández M, Del Pino P, Mitchell SG, et al. Dissecting the molecular mechanism of apoptosis during photothermal therapy using gold nanoprisms. ACS nano. 2015;9(1):52–61.
18. Zhou Z, Wang Y, Yan Y, Zhang Q, Cheng Y. Dendrimer-templated ultrasmall and multifunctional photothermal agents for efficient tumor ablation. ACS nano. 2016;10(4):4863–4872.
19. Jiang X, Zhang S, Ren F, et al. Ultrasmall magnetic CuFeSe2 ternary nanocrystals for multimodal imaging guided photothermal therapy of cancer. ACS nano. 2017;11(6):5633–5645.
20. Estelrich J, Busquets MA. Iron oxide nanoparticles in photothermal therapy. Molecules. 2018;23(7):1567.
21. Chaudhuri R, Paria S. Core/shell nanoparticles: classes, properties, synthesis mechanisms, characterization, and applications. Chem Rev. 2012;112(4):2373–2433.
22. Moore JA, Chow JCL. Recent progress and applications of gold nanotechnology in medical biophysics using artificial intelligence and mathematical modeling. Nano Exp. 2021;2(2):022001.
23. Luo S, Zhang E, Su Y, Cheng T, Shi C. A review of NIR dyes in cancer targeting and imaging. Biomaterials. 2011;32(29):7127–7138.
24. Pauli J, Vag T, Haag R, et al. An in vitro characterization study of new near infrared dyes for molecular imaging. Eur J Med Chem. 2009;44(9):3496–3503.
25. Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65(1–2):271–284.
26. Choi H, Liu W, Misra P, Tanaka E, Zimmer J, Frangioni J. Renal clearance of quantum dots. Nat Biotechnol. 2007;25(10):1165–1170.
27. Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm. 2008;5(4):505–515.
28. Wong C, Stylianopoulos T, Cui J, et al. Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc Natl Acad Sci. 2011;108(6):2426–2431.
29. Huang K, Ma H, Liu J, et al. Size-dependent localization and penetration of ultrasmall gold nanoparticles in cancer cells, multicellular spheroids, and tumors in vivo. ACS nano. 2012;6(5):4483–4493.
30. Chauhan VP, Jain RK. Strategies for advancing cancer nanomedicine. Nat Mater. 2013;12(11):958–962.
31. Sun Q, Sun X, Ma X, et al. Integration of nanoassembly functions for an effective delivery cascade for cancer drugs. Adv Mater. 2014;26(45):7615–7621.
32. Linsinger T, Roebben G, Solans C, Ramsch R. Reference materials for measuring the size of nanoparticles. TrAC Trends Anal Chem. 2011;30:18–27.
33. Patra JK, Das G, Fraceto LF, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology. 2018;16(1):71.
34. Ravindran Girija A, Balasubramanian S. Theragnostic potentials of core/shell mesoporous silica nanostructures. Nanotheranostics. 2019;3(1):1–40.
35. Armijo LM, Wawrzyniec SJ, Kopciuch M, et al. Antibacterial activity of iron oxide, iron nitride, and tobramycin conjugated nanoparticles against Pseudomonas aeruginosa biofilms. J Nanobiotechnology. 2020;18(1):35.
36. Piñeiro Y, Vargas Z, Rivas J, López-Quintela M. Iron oxide based nanoparticles for magnetic hyperthermia strategies in biological applications. Eur J Inorg Chem. 2015;2015:1.
37. Montiel Schneider MG, Martín MJ, Otarola J, et al. Biomedical applications of iron oxide nanoparticles: current insights progress and perspectives. Pharmaceutics. 2022;14:1.
38. Kumar CS, Mohammad F. Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Adv Drug Deliv Rev. 2011;63(9):789–808.
39. Yoffe S, Leshuk T, Everett P, Gu F. Superparamagnetic Iron Oxide Nanoparticles (SPIONs): synthesis and surface modification techniques for use with MRI and other biomedical applications. Curr Pharm Des. 2012;2012:19.
40. Wahajuddin N, Arora S. Superparamagnetic iron oxide nanoparticles: magnetic nanoplatforms as drug carriers. Int J Nanomedicine. 2012;7:3445–3471.
41. Duyn JH. High-field MRI of brain iron. Methods Mol Biol. 2011;711:239–249.
42. Crețu BE, Dodi G, Shavandi A, Gardikiotis I, Șerban IL, Balan V. Imaging Constructs: the Rise of Iron Oxide Nanoparticles. Molecules. 2021;26:11.
43. Lu Y, Xu Y-J, Zhang G-B, et al. Iron oxide nanoclusters for T 1 magnetic resonance imaging of non-human primates. Nat Biomed Eng. 2017;2017:1.
44. Estelrich J, Escribano E, Queralt J, Busquets MA. Iron oxide nanoparticles for magnetically-guided and magnetically-responsive drug delivery. Int J Mol Sci. 2015;16(4):8070–8101.
45. Estelrich J, Sánchez-Martín MJ, Busquets MA. Nanoparticles in magnetic resonance imaging: from simple to dual contrast agents. Int J Nanomedicine. 2015;10:1727.
46. Quiñones ED, Lu TY, Liu KT, Fan YJ, Chuang EY, Yu J. Glycol chitosan/iron oxide/polypyrrole nanoclusters for precise chemodynamic/photothermal synergistic therapy. Int J Biol Macromol. 2022;203:268–279.
47. Sun C, Du K, Fang C, et al. PEG-mediated synthesis of highly dispersive multifunctional superparamagnetic nanoparticles: their physicochemical properties and function in vivo. ACS Nano. 2010;4(4):2402–2410.
48. Sun C, Lee JSH, Zhang M. Magnetic nanoparticles in MR imaging and drug delivery. Adv Drug Del Rev. 2008;60(11):1252–1265.
49. Stephen ZR, Kievit FM, Zhang M. Magnetite nanoparticles for medical MR imaging. Mater Today. 2011;14(7):330–338.
50. Gossuin Y, Gillis P, Hocq A, Vuong QL, Roch A. Magnetic resonance relaxation properties of superparamagnetic particles. Wiley Interdiscip Rev Nanomed. 2009;1(3):299–310.
51. Revia RA, Zhang M. Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: recent advances. Mater Today. 2016;19(3):157–168.
52. Lu H, Zheng W, Jiang Q. Saturation magnetization of ferromagnetic and ferrimagnetic nanocrystals at room temperature. Appl Phys. 2007;40(2):320.
53. Cornell RM, Schwertmann U. The Iron Oxides: Structure, Properties, Reactions, Occurrences, and Uses. Vol. 664. Wiley-vch Weinheim; 2003.
54. Jayan JS, Rajan R, Appukuttan S, Joseph K. Multifunctional nanomaterials for medical applications. Nanomater Nanotechnol Med. 2022;:479–515.
55. Guo K, Liu Y, Tang L, Shubhra QT. Homotypic biomimetic coating synergizes chemo-photothermal combination therapy to treat breast cancer overcoming drug resistance. Chem Eng J. 2022;428:131120.
56. Fu Q, Feng H, Liu L, et al. Spatiotemporally controlled formation and rotation of magnetic nanochain in vivo for precise mechanotherapy of tumor. Angew Chem Int Ed. 2022;2022:1.
57. Jia C, Guo Y, Wu FG. Chemodynamic therapy via Fenton and Fenton‐like nanomaterials: strategies and recent advances. Small. 2022;18(6):2103868.
58. Elahi N, Rizwan M. Progress and prospects of magnetic iron oxide nanoparticles in biomedical applications: a review. Artif Organs. 2021;45(11):1272–1299.
59. Singh D, Kurmi BD, Sarma G, Bhattacharya S, Nagdev S. Iron oxide nano particles and its applications to cure her2-positive mediated breast cancer. Curr Nanomed. 2022;12(1):17–31.
60. Sachdeva V, Monga A, Vashisht R, Singh D, Singh A, Bedi N. Iron oxide nanoparticles: the precise strategy for targeted delivery of genes, oligonucleotides and peptides in cancer therapy. J Drug Deliv Sci Technol. 2022;2022:103585.
61. Kawassaki RK, Romano M, Dietrich N, Araki K. Titanium and iron oxide nanoparticles for cancer therapy: surface chemistry and biological implications. Front Nanotechnol. 2021;3:735434.
62. Ahmad F, Salem-Bekhit MM, Khan F, et al. Unique properties of surface-functionalized nanoparticles for bio-application: functionalization mechanisms and importance in application. Nanomaterials. 2022;12:8.
63. Jung JH, Cho M, Seo TS, Lee SY. Biosynthesis and applications of iron oxide nanocomposites synthesized by recombinant Escherichia coli. Appl Microbiol Biotechnol. 2022;106(3):1127–1137.
64. Rahman MM, Islam MR, Akash S, et al. Recent advancements of nanoparticles application in cancer and neurodegenerative disorders: at a glance. Biomed Pharmacother. 2022;153:113305.
65. Zhu N, Ji H, Yu P, et al. Surface modification of magnetic iron oxide nanoparticles. Nanomaterials. 2018;8(10):810.
66. Materón E, Miyazaki C, Carr O, et al. Magnetic nanoparticles in biomedical applications: a review. Appl Surf Sci. 2021;6:100163.
67. Morán D, Gutiérrez G, Mendoza R, Rayner M, Blanco-López C, Matos M. Synthesis of controlled-size starch nanoparticles and superparamagnetic starch nanocomposites by microemulsion method. Carbohydr Polym. 2023;299:120223.
68. Iliasov A, Nizamov T, Naumenko V, et al. Non-magnetic shell coating of magnetic nanoparticles as key factor of toxicity for cancer cells in a low frequency alternating magnetic field. Biointerfaces. 2021;206:111931.
69. Nabavinia M, Beltran-Huarac J. Recent progress in iron oxide nanoparticles as therapeutic magnetic agents for cancer treatment and tissue engineering. ACS Appl Bio Mater. 2020;3(12):8172–8187.
70. Iliasov AR, Nizamov TR, Naumenko VA, et al. Non-magnetic shell coating of magnetic nanoparticles as key factor of toxicity for cancer cells in a low frequency alternating magnetic field. Colloids Surf B Biointerfaces. 2021;206:111931.
71. Arias LS, Pessan JP, Vieira APM, TMTd L, Delbem ACB, Monteiro DR. Iron oxide nanoparticles for biomedical applications: a perspective on synthesis, drugs, antimicrobial activity, and toxicity. Antibiotics. 2018;7(2):46.
72. Manshian BB, Jiménez J, Himmelreich U, Soenen SJ. Personalized medicine and follow-up of therapeutic delivery through exploitation of quantum dot toxicity. Biomaterials. 2017;127:1–12.
73. Nedyalkova M, Donkova B, Romanova J, Tzvetkov G, Madurga S, Simeonov V. Iron oxide nanoparticles–in vivo/in vitro biomedical applications and in silico studies. Adv Colloid Interface Sci. 2017;249:192–212.
74. Su S, Recent PMK. Advances in nanocarrier-assisted therapeutics delivery systems. Pharmaceutics. 2020;12:9.
75. Bernstein EF, Sarkas HW, Boland P. Iron oxides in novel skin care formulations attenuate blue light for enhanced protection against skin damage. J Cosmet Dermatol. 2021;20(2):532–537.
76. Ribeiro CDL, Santos JG, Souza JR, Paterno LG. Highly sensitive determination of salicylic acid in skin care product by means of carbon nanotube/iron oxide nanoparticle voltammetric sensors. J Solid State Electrochem. 2019;23(3):783–793.
77. Shiji R, Joseph MM, Sen A, Unnikrishnan B, Sreelekha T. Galactomannan armed superparamagnetic iron oxide nanoparticles as a folate receptor targeted multi-functional theranostic agent in the management of cancer. Int J Biol Macromol. 2022;219:740–753.
78. Abo-Zeid Y, Ismail NSM, McLean GR, Hamdy NM. A molecular docking study repurposes FDA approved iron oxide nanoparticles to treat and control COVID-19 infection. Eur J Pharm Sci. 2020;153:105465.
79. Thakor AS, Jokerst JV, Ghanouni P, Campbell JL, Mittra E, Gambhir SS. Clinically approved nanoparticle imaging agents. J Nucl Med. 2016;57(12):1833–1837.
80. Freis B, Cotin G, Perton F, et al. The size, shape, and composition design of iron oxide nanoparticles to combine, MRI, magnetic hyperthermia, and photothermia. Magnet Nanopart Hum Health Med. 2021;2021:380–429.
81. Genicio N, Bañobre-López M, Gröhn O, Gallo J. Ratiometric magnetic resonance imaging: contrast agent design towards better specificity and quantification. Coord Chem Rev. 2021;447:214150.
82. Stueber DD, Villanova J, Aponte I, Xiao Z, Colvin VL. Magnetic nanoparticles in biology and medicine: past, present, and future trends. Pharmaceutics. 2021;13(7):943.
83. Liu X, Zhang Y, Wang Y, et al. Comprehensive understanding of magnetic hyperthermia for improving antitumor therapeutic efficacy. Theranostics. 2020;10(8):3793–3815.
84. Fatima H, Charinpanitkul T, Kim K-S. Fundamentals to apply magnetic nanoparticles for hyperthermia therapy. Nanomaterials. 2021;11:1203.
85. Pivetta TP, Botteon CEA, Ribeiro PA, Marcato PD, Raposo M. Nanoparticle systems for cancer phototherapy: an overview. Nanomaterials. 2021;11(11):3132.
86. Han HS, Choi KY. Advances in nanomaterial-mediated photothermal cancer therapies: toward clinical applications. Biomedicines. 2021;9(3):1.
87. Ostańska E, Aebisher D, Bartusik-Aebisher D. The potential of photodynamic therapy in current breast cancer treatment methodologies. Biomed Pharmacother. 2021;137:111302.
88. Xu Y, Li H, Fan L, et al. Development of photosensitizer-loaded lipid droplets for photothermal therapy based on thiophene analogs. J Adv Res. 2021;28:165–174.
89. Agostinis P, Berg K, Cengel KA, et al. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011;61(4):250–281.
90. Sarbadhikary P, George BP, Abrahamse H. Recent advances in photosensitizers as multifunctional theranostic agents for imaging-guided photodynamic therapy of cancer. Theranostics. 2021;11(18):9054–9088.
91. Yang Z, Sun Z, Ren Y, et al. Advances in nanomaterials for use in photothermal and photodynamic therapeutics (review). Mol Med Rep. 2019;20(1):5–15.
92. Bucharskaya AB, Khlebtsov NG, Khlebtsov BN, et al. Photothermal and photodynamic therapy of tumors with plasmonic nanoparticles: challenges and prospects. Materials. 2022;15(4):1.
93. Chen J, Ning C, Zhou Z, et al. Nanomaterials as photothermal therapeutic agents. Prog Mater Sci. 2019;99:1–26.
94. Yang W, Liang H, Ma S, Wang D, Huang J. Gold nanoparticle based photothermal therapy: development and application for effective cancer treatment. Sustain Mater Technol. 2019;22:e00109.
95. Ashraf S, Pelaz B, Del Pino P, et al. Gold-based nanomaterials for applications in nanomedicine. Top Curr Chem. 2016;370:169–202.
96. Długosz O, Szostak K, Staroń A, Pulit-Prociak J, Banach M. Methods for reducing the toxicity of metal and metal oxide NPs as biomedicine. Materials. 2020;13(2).
97. Steinbrück A, Stranik O, Csaki A, Fritzsche W. Sensoric potential of gold-silver core-shell nanoparticles. Anal Bioanal Chem. 2011;401:1241–1249.
98. Gunaydin G, Gedik ME, Ayan S. Photodynamic therapy-current limitations and novel approaches. Front Chem. 2021;9:691697.
99. Shi X, Tian Y, Liu Y, et al. Research progress of photothermal nanomaterials in multimodal tumor therapy. Front Oncol. 2022;12:939365.
100. Ratkaj I, Mušković M, Malatesti N. Targeting microenvironment of melanoma and head and neck cancers in photodynamic therapy. Curr Med Chem. 2022;29(18):3261–3299.
101. Ming L, Cheng K, Chen Y, Yang R, Chen D. Enhancement of tumor lethality of ROS in photodynamic therapy. Cancer Med. 2021;10(1):257–268.
102. Sheng T, Ong YH, Busch TM, Zhu TC. Reactive oxygen species explicit dosimetry to predict local tumor control for Photofrin-mediated photodynamic therapy. Proc SPIE Int Soc Opt Eng. 2019;2019:10860.
103. Zhang C, Zhu N, Li H, et al. New Dawn for cancer cell death: emerging role of lipid metabolism. Mol Metab. 2022;63:101529.
104. Chen K-J, Plaunt AJ, Leifer FG, Kang JY, Cipolla D. Recent advances in prodrug-based nanoparticle therapeutics. Eur J Pharm Biopharm. 2021;165:219–243.
105. Ożóg Ł, Domka W, Bartusik-Aebisher D, Aebisher D. Fluorescence diagnostics and photodynamic therapy in cancer. Drug Res. 2020;77:517–540.
106. Cabuy E. Photodynamic therapy in cancer treatment. Reliab Cancer Ther. 2012;3:1–54.
107. Nikolova MP, Chavali MS. Metal oxide nanoparticles as biomedical materials. Biomimetics. 2020;5(2):5.
108. Kailass K, Sadovski O, Zipfel WR, Beharry AA. Two-photon photodynamic therapy targeting cancers with low carboxylesterase 2 activity guided by ratiometric fluorescence. J Med Chem. 2022;65(13):8855–8868.
109. Vargas-Ortiz JR, Gonzalez C, Esquivel K. Magnetic iron nanoparticles: synthesis, surface enhancements, and biological challenges. Processes. 2022;10(11):2282.
110. Bustamante-Torres M, Romero-Fierro D, Estrella-Nuñez J, Arcentales-Vera B, Chichande-Proaño E, Bucio E. Polymeric composite of magnetite iron oxide nanoparticles and their application in biomedicine: a review. Polymers. 2022;14(4):752.
111. Perecin CJ, Sponchioni M, Auriemma R, Cerize NN, Moscatelli D, Varanda LC. Magnetite nanoparticles coated with biodegradable zwitterionic polymers as multifunctional nanocomposites for drug delivery and cancer treatment. ACS Appl Nano Mater. 2022;2022:3.
112. Gambhir RP, Rohiwal SS, Tiwari AP. Multifunctional surface functionalized magnetic iron oxide nanoparticles for biomedical applications: a review. Appl Surf Sci. 2022;11:100303.
113. Mohanapriya V, Sakthivel R, Pham NDK, Cheng CK, Le HS, Dong TMH. Nanotechnology-A ray of hope for heavy metals removal. Chemosphere. 2022;2022:136989.
114. Petrov KD, Chubarov AS. Magnetite nanoparticles for biomedical applications. Encyclopedia. 2022;2(4):1811–1828.
115. Choubdar N, Avizheh S, Karimifard SA. Recent advances in efficacy of using doxorubicin gold nanoparticles for chemo-, radio-, photothermal, and photodynamic therapy. Curr Drug Deliv. 2022;19(7):745–762.
116. Dewhirst MW, Oleson JR, Kirkpatrick J, Secomb TW. Accurate three-dimensional thermal dosimetry and assessment of physiologic response are essential for optimizing thermoradiotherapy. Cancers. 2022;14(7):1701.
117. Datta NR, Ordóñez SG, Gaipl US, et al. Local hyperthermia combined with radiotherapy and-/or chemotherapy: Recent advances and promises for the future. Cancer Treatment Reviews. 2015;41:742–753.
118. Gavilán H, Avugadda SK, Fernández-Cabada T, et al. Magnetic nanoparticles and clusters for magnetic hyperthermia: optimizing their heat performance and developing combinatorial therapies to tackle cancer. Chem Soc Rev. 2021;50(20):11614–11667.
119. Seyedjamali T, Farahzadi MK, Arabi H. Investigation of physical properties of Fe3O4/Au-Ag@ MoS2 nanoparticles on heat distribution in cancerous liver tissue. Mater Res Express. 2022;9(9):095002.
120. Theodosiou M, Sakellis E, Boukos N, Kusigerski V, Kalska-Szostko B, Efthimiadou E. Iron oxide nanoflowers encapsulated in thermosensitive fluorescent liposomes for hyperthermia treatment of lung adenocarcinoma. Sci Rep. 2022;12(1):1–15.
121. Albarqi HA, Wong LH, Schumann C, et al. Biocompatible nanoclusters with high heating efficiency for systemically delivered magnetic hyperthermia. ACS Nano. 2019;13(6):6383–6395.
122. Fratila RM, De La Fuente JM. Nanomaterials for Magnetic and Optical Hyperthermia Applications. Elsevier; 2018.
123. Mendo SG, Alves AF, Ferreira LP, et al. Hyperthermia studies of ferrite nanoparticles synthesized in the presence of cotton. New J Chem. 2015;39(9):7182–7193.
124. Saccomandi P, Schena E, Silvestri S. Techniques for temperature monitoring during laser-induced thermotherapy: an overview. Int J Hyperth. 2013;29(7):609–619.
125. Oliveira-Silva R, Pereira RA, Silva FM, et al. Temperature-responsive nanomagnetic logic gates for cellular hyperthermia. Mater Horiz. 2019;6(3):524–530.
126. Huilgol NG, Gupta S, Dixit R. Chemoradiation with hyperthermia in the treatment of head and neck cancer. Int J Hyperth. 2010;26(1):21–25.
127. Huilgol NG, Gupta S, Sridhar C. Hyperthermia with radiation in the treatment of locally advanced head and neck cancer: a report of randomized trial. J Cancer Res Ther. 2010;6(4):492.
128. Nene A, Yu X, Kaithal P, Luo H, Somani P, Ramakrishna S. Magnetic Iron Oxide Nanoparticle (IONP) synthesis to applications: present and future. Materials. 2020;13:4644.
129. Zhang Y, Li F, Ya S, et al. An iron oxide nanoparticle-based transdermal nanoplatform for dual-modal imaging-guided chemo-photothermal therapy of superficial tumors. Acta Biomater. 2021;130:473–484.
130. Liu Q, Liu L, Mo C, et al. Polyethylene glycol-coated ultrasmall superparamagnetic iron oxide nanoparticles-coupled sialyl Lewis X nanotheranostic platform for nasopharyngeal carcinoma imaging and photothermal therapy. J Nanobiotechnology. 2021;19(1):1–14.
131. Wang M, Li Y, Wang M, et al. Synergistic interventional photothermal therapy and immunotherapy using an iron oxide nanoplatform for the treatment of pancreatic cancer. Acta Biomater. 2022;138:453–462.
132. Palzer J, Eckstein L, Slabu I, Reisen O, Neumann UP, Roeth AA. Iron oxide nanoparticle-based hyperthermia as a treatment option in various gastrointestinal malignancies. Nanomaterials. 2021;11(11):3013.
133. Kadkhoda J, Tarighatnia A, Barar J, Aghanejad A, Davaran S. Recent advances and trends in nanoparticles based photothermal and photodynamic therapy. Photodiagnosis Photodyn Ther. 2021;2021:102697.
134. Mona LP, Songca SP, Ajibade PA. Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy. Nanotechnol Rev. 2022;11(1):176–190.
135. Liang X, Chen M, Bhattarai P, Hameed S, Tang Y, Dai Z. Complementing cancer photodynamic therapy with ferroptosis through iron oxide loaded porphyrin-grafted lipid nanoparticles. ACS Nano. 2021;15(12):20164–20180.
136. Perecin CJ, Gratens XPM, Chitta VA, et al. Synthesis and characterization of magnetic composite theragnostics by nano spray drying. Materials. 2022;15(5):1755.
137. Anani T, Rahmati S, Sultana N, David AE. MRI-traceable theranostic nanoparticles for targeted cancer treatment. Theranostics. 2021;11(2):579.
138. Morris BJ, Willcox DC, Donlon TA, Willcox BJ. FOXO3: a major gene for human longevity-a mini-review. Gerontology. 2015;61(6):515–525.
139. Dadfar SM, Roemhild K, Drude NI, et al. Iron oxide nanoparticles: diagnostic, therapeutic and theranostic applications. Adv Drug Del Rev. 2019;138:302–325.
140. Siddique S, Chow JC. Recent advances in functionalized nanoparticles in cancer theranostics. Nanomaterials. 2022;12(16):2826.
141. Ali Dheyab M, Aziz AA, Jameel MS. Recent advances in inorganic nanomaterials synthesis using sonochemistry: a comprehensive review on iron oxide, gold and iron oxide coated gold nanoparticles. Molecules. 2021;26(9):2453.
142. Paterson S, Thompson SA, Wark AW, de la Rica R. Gold suprashells: enhanced photothermal nanoheaters with multiple localized surface plasmon resonances for broadband surface-enhanced Raman scattering. J Phys Chem C. 2017;121(13):7404–7411.
143. Zhao J, Wallace M, Melancon MP. Cancer theranostics with gold nanoshells. Nanomedicine. 2014;9(13):2041–2057.
144. Sabale S, Kandesar P, Jadhav V, Komorek R, Motkuri RK, Yu X-Y. Recent developments in the synthesis, properties, and biomedical applications of core/shell superparamagnetic iron oxide nanoparticles with gold. Biomater Sci. 2017;5(11):2212–2225.
145. Eyvazzadeh N, Shakeri-Zadeh A, Fekrazad R, Amini E, Ghaznavi H, Kamran Kamrava S. Gold-coated magnetic nanoparticle as a nanotheranostic agent for magnetic resonance imaging and photothermal therapy of cancer. Lasers Med Sci. 2017;32(7):1469–1477.
146. Gul S, Khan SB, Rehman IU, Khan MA, Khan M. A comprehensive review of magnetic nanomaterials modern day theranostics. Front Mater. 2019;6:179.
147. Yang D, Yang G, Yang P, et al. Assembly of Au plasmonic photothermal agent and iron oxide nanoparticles on ultrathin black phosphorus for targeted photothermal and photodynamic cancer therapy. Adv Funct Mater. 2017;27(18):1700371.
148. Tietze R, Zaloga J, Unterweger H, et al. Magnetic nanoparticle-based drug delivery for cancer therapy. Biochem Biophys Res Commun. 2015;468(3):463–470.
149. Tombácz E, Turcu R, Socoliuc V, Vékás L. Magnetic iron oxide nanoparticles: recent trends in design and synthesis of magnetoresponsive nanosystems. Biochem Biophys Res Commun. 2015;468(3):442–453.
150. Chow JCL. 9 - Magnetic nanoparticles as contrast agents in magnetic resonance imaging and radiosensitizers in radiotherapy. In: Hussain CM, Patankar KK, editors. Fundamentals and Industrial Applications of Magnetic Nanoparticles. Woodhead Publishing; 2022:291–316.
151. Nicolás P, Saleta M, Troiani H, Zysler R, Lassalle V, Ferreira ML. Preparation of iron oxide nanoparticles stabilized with biomolecules: experimental and mechanistic issues. Acta Biomater. 2013;9(1):4754–4762.
152. Zhou Z, Song J, Tian R, et al. Activatable singlet oxygen generation from lipid hydroperoxide nanoparticles for cancer therapy. Angew Chem. 2017;129(23):6592–6596.
153. Shen Z, Song J, Yung BC, Zhou Z, Wu A, Chen X. Emerging strategies of cancer therapy based on ferroptosis. Adv Mater. 2018;30(12):1704007.
154. Lucky SS, Soo KC, Zhang Y. Nanoparticles in Photodynamic Therapy. Chem Rev. 2015;115(4):1990–2042.
155. Bechet D, Couleaud P, Frochot C, Viriot M-L, Guillemin F, Barberi-Heyob M. Nanoparticles as vehicles for delivery of photodynamic therapy agents. Trends Biotechnol. 2008;26(11):612–621.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
