Back to Journals » OncoTargets and Therapy » Volume 11
Forecast of actin-binding proteins as the oncotarget in osteosarcoma – a review of mechanism, diagnosis and therapy
Authors Fu Y, Yu W, Cai H, Lu A
Received 14 December 2017
Accepted for publication 7 February 2018
Published 20 March 2018 Volume 2018:11 Pages 1553—1561
DOI https://doi.org/10.2147/OTT.S159894
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Tohru Yamada
Yucheng Fu,1 Wei Yu,2 Hongliu Cai,1 Anwei Lu1
1Department of Surgical Intensive Care Unit, The First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, Zhejiang Province, People’s Republic of China; 2Department of Orthopedics, Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, Zhejiang Province, People’s Republic of China
Abstract: Osteosarcoma (OS) is the most common bone malignant tumor with a high rate of lung metastasis and principally emerges in children and adolescents. Although neoadjuvant chemotherapy is widely used around the world, a high rate of chemoresistance occurs and frequently generates a poor prognosis. Therefore, finding a new appropriate prognostic marker for OS is a valuable research direction, which will give patients a better chance to receive proper therapy. Actin-binding proteins (ABPs) are a group of proteins that interact with actin cytoskeleton and play a crucial role in the regulation of the cell motility and morphology in eukaryotes. Meanwhile, ABPs also act as a bridge between the cytomembrane and nucleus, which transmit the outside-in and inside-out signals in cytoplasm. Furthermore, ABPs alter the dynamic structure of actin and regulate the invasion and metastasis of cancer. Hence, ABPs have a wide application in predicting the prognosis, and may be new targets, in tumor therapy. This review focuses on a series of ABPs and discusses their modulatory functions. It provides a new insight into the classification of ABPs’ functions in the process of invasion and metastasis in OS and illuminates the potential ability in predicting the prognosis of OS patients.
Keywords: actin-binding proteins, osteosarcoma, oncotarget, tumor invasion and metastasis, oncotherapy
Introduction
Osteosarcoma (OS), the most common primary sarcoma of bone in humans, has two peak incidences at different ages.1,2 The most important peak age is early adolescent, which accounts for 2.4% of all malignancies in pediatric patients.3 The second peak occurs in elderly people, which occupies only ~13%–30% of all OS patients.2 With the development of treatment strategy, especially the surgery methods and neoadjuvant chemotherapy, the 5-year survival rate of OS patients has been raised to 70%–80%. However, a large number of patients still face frustrated outcome since resistance of chemotherapy.4,5 Moreover, the conventional chemotherapeutic methods are also confronted with life-threatening side effects such as cardiotoxicity, myelosuppression and gastrointestinal, hepatic and renal dysfunctions. On the other hand, chemotherapy resistance is also a huge problem that threatens the long-term survival of OS patients.6,7
Now researchers are paying more attention to studying the diagnosis and prognosis of OS. Recent research reveals that PTEN, an inhibitor of tumor cells, is decreased in OS cells and upregulation of PTEN blocks the processes of adhesion migration and invasion in OS.8 This is consistent with the result that PTEN suppresses the activation of AKT pathway induced by inhibition of PIP3 in tumor cells.9 Yin et al indicated that E-cadherin presents a repressive effect in patients with OS. Down-expression of E-cadherin is significantly related to metastasis induced by Twist.10 Although multiple biomarkers have been studied in OS, it is still a huge challenge to find an effective marker to predict the metastasis and prognosis of OS.
Actin and actin-binding proteins (ABPs)
Actin, one of the most crucial dynamic structures in eukaryotic cells, has two main states, filamentous actin (F-actin) and globular actin (G-actin).11,12 As a component of cytoskeleton, actin plays a major role in maintaining the morphology of cells. With regulation of multiple proteins, actin filaments assemble and crosslink to bundles. Then, plasma membrane protrudes and pseudopods are formed.13 It is also found that actin interplays with focal adhesions and promotes inside-out and outside-in signals that dynamically regulate the cell adhesion between cell–cell and cell–matrix.14 Moreover, with polymerization and depolymerization of fibrils, actin provides the force to promote the cell motility.15,16 Likewise, actin filaments participate in the formation of ATP-dependent contractile structures together with myosin filaments in muscle cells.11,17 Now, it has been proved that actin promotes invasion and metastasis of cancer cells with abnormal regulation.18–20
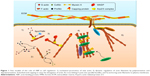
To achieve complex functions of actin cytoskeleton, a process of ABPs’ precise regulation is extremely required. Until now, dozens of ABPs have been found in various actin regulatory processes, and their functions are diverse from each other.21 Totally, they can be divided into five aspects (Figure 1):
- Acting as a start of G-actin nucleation and promoting the formation of new actin filaments: the assembly of actin is the beginning of cell motion and morphology alteration. Actin-related protein 2/3 (Arp2/3) complex is the most important regulator of the initial process. With the activating of WASP family proteins, Arp2/3 complex promotes the nucleation of new actin filaments in the branch of the original one and then dendritic actin networks are formed.22 In addition, formin (FMN) and Spir are another couple of actin nucleation proteins in cells. On a molecular level, single Spir cannot recruit and assemble activated actin, while Spir/FMN complex starts the nucleation and primarily forms unbranched actin filaments such as actin cables, filopodia, stress fibers, cell adhesions, and cytokinetic actin rings (CARs).23,24
- Inducing the polymerization and depolymerization of actin filaments: in the dynamic process of actin, profilin plays a vital role in regulating actin polymerization. Via connecting with actin monomers, profilin participates in both Arp2/3- and FMN-dependent actin nucleation. Once new actin filaments are formed by Spir/FMN complex, profilin/actin complex elongates filaments at a rapid rate, which is 10-fold faster than the rate of free barbed ends assembled by the Arp2/3 complex.23,25 However, it is also found that Arp2/3 complex-mediated branch formation can also be suppressed by high concentrations of profilin induced by WASP.26 This suggests that profilin regulates the assembly of actin in a concentration-dependent pathway. Profilin inhibits the polymerization of actin at high concentrations, whereas it enhances the polymerization at low concentrations. Another actin formation regulator is actin-depolymerizing factor (ADF)/cofilin. It promotes the actin depolymerizing in a pH-dependent manner.27 However, it also presents an inhibiting effect of actin depolymerization after phosphorylation by LIM kinase.28,29 Thus, this indicates that cofilin plays a key role in dynamic alteration of structure of actin filaments.
- Controlling the actin filaments’ elongation by regulation of capping barbed end: in case of unlimited elongation, actin needs a stop signal, which is important for the stabilization of cell morphology. The most typical protein is cap protein (CP), which maintains the stability of actin filaments and prevents the addition or loss of actin monomers at the end, inducing cell motility, morphogenesis and endocytosis.30 On the contrary, Ena/WASP has an opposite function that inhibits the capping effect of CP and promotes the formation of longer and unbranched actin filaments at the leading edge.31 Villin, one of the proteins from the gelsolin family, also participates in the severing and capping of actin filament in a Ca2+-dependent manner.32
- Organizing and cross-linking F-actin into parallel bundles: to support the mechanical strength and stabilization, actin is cross-linked into numerous parallel bundles, which requires the mediator to accomplish this process. Fascin-induced production of actin bundles is the main process in the formation of cell protrusions.33 Fascin-1 promotes the cross-linking of F-actin in Rac and Cdc42 pathway with stimulation of outside signals such as integrin, syndecan-1 and insulin-like growth factor receptor (IGFR). Furthermore, as the motor of actin filaments, the processivity of myosin X is another promoter in the process of actin cross-linking. With the assistance of myosin X, elongated parallel bundles are achieved in filopodia, which play a role in regulating cell motility and migration.33,34 In addition, α-actinin also acts as an actin filament cross-linking protein, and is abundant in muscle and nonmuscle cells. On the other hand, some studies also prove that α-actinin binds to three different β subunits of integrins, participating in the modulation of cell–matrix’s connection.35,36
- Anchoring actin filaments to plasma membrane and regulating the cell junction, shape and motility: these proteins mediate the connection of actin and cytomembrane. They also conduct the signals from member to nucleus. Talin has a globular N-terminal head region that contains an FERM domain binding to the first NPxY motif on the integrin β subunit. It is a 220-kDa rod domain that can multiply and connect with actin and vinculin.37,38 Kindlin, another ABP, also has an FERM domain that binds to the second NPxY motif on the integrin β subunit.38 Both of them induce the inside-out and outside-in signals in the regulation of integrin activation.39,40 What is more, ERM proteins, including band 4.1, ezrin, radixin and moesin, also contain an FERM domain that induces the cross-linking of actin bundles in binding of membrane and cytoskeleton.41,42 Then, ERM proteins regulate the cell adhesion and participate in the formation of filopodia, microspikes and microvilli.43–45 Tensin is a bridge between integrins and actin cytoskeleton. However, tensin appears later in focal adhesions and enriches in fibrillar adhesions, while it disappears in nascent adhesions.14 Meanwhile, some documents also prove that tensin has the ability to cap the barbed end of actin filaments.46,47
To achieve the precise activation and regulation of ABPs, multiple signal pathways are involved in this process. In these signal pathways, the Rho-family small GTPases are the most classic one, which mainly contains Rho, Rac, Cdc42, etc.48 Rho can be recruited together with profilin and pl40mDia that stimulate actin polymerization. Rac can increase the number and motor activity of actomyosin II ATPase by enhancing the phosphorylation of serine-19 of myosin II regulatory light chain (MLC).49 At the same time, Rac participates in the phosphorylation of uniform cofilin, which requires the uniform basal Rac signaling and alternates during the course of migration.50 Cdc42 induces the formation of filopodia with the generation of WASP, profilin and Arp2/3 complex alone with phosphatidylinositol 4,5-bisphosphate (PIP2) in many cell types.51 Membrane phosphatidylinositol (PI) is also a key regulator in alternative organization and dynamics of actin cytoskeleton. Most of the ABPs contain the PI-binding domain. The PI and phosphoinositides act as the secondary signal in ABP-induced signal transmission. This regulation results in the alternation of cell behavior, including cell–cell signal transition, cytoskeleton reassembly and cell apoptosis.11
ABPs and OS
The invasion and metastasis of OS is an extremely complicated process, which contains numerous mutations in modulatory processes of ABPs. With the abnormal expression of ABPs, actin filaments elongate without correct regulation. Thus, this leads to the expansion of filopodia in OS, and further invasion and metastasis occur.52,53 On the other hand, focal adhesion loses its function because of the absence of ABPs’ anchoring effect. Therefore, cancer cells separate themselves from the original location and promote the distant metastasis.54,55 Moreover, abnormal regulation of ABPs in the F-actin assembly prevents apoptosis of OS.56,57 The alteration of integrin-mediated cell adhesion signals leads to the inadequate or inappropriate cell–matrix connection in normal cells. The apoptosis that caused by abnormal adhesion calls cell anoikis. With the abnormal expression of ABPs, cancer cells lose these signals and avoid anoikis, which leads to long-term survival.58,59 In summary, a malfunction in any of these three steps can induce the invasion and metastasis of cancer cells. This also suggests that tumorigenesis may not occur by means of mutative expression of single proteins but may be affected by multiple abnormal regulations of ABPs, which offers a potential research option to seek the relationship between synthetical ABPs’ expression and tumor prognosis.60 Here are some relationships between different ABPs and OS as well as other tumors (Table 1).
  | Table 1 Summary of relevant ABPs in OS and other tumors |
Arp2/3 complex
Multiple research has revealed that Arp2/3 complex is a positive factor that accelerates the invasion and metastasis of cancer cells. Arp2/3 complex contains seven subunit proteins, and all of these subunits can enhance the migratory capacity of cells. It has been reported that silencing Arp2/3 complex inhibits cell migration and invasion in head and neck cancer, pancreatic cancer and glioma.61–63 The study of Bernardini et al64 has revealed that the dephosphorylation of ARPC5L, a subunit of Arp2/3 complex, suppresses the migration and adhesion of OS induced by SI-83 in vitro. Nevertheless, the expression level of Arp2/3 complex in OS tissue remains unknown.
Profilin
With the extracellular regulation, profilin is involved in the dynamics of actin assembly. Profilin has been extensively studied in breast cancer. Many studies prove that profilin suppresses the invasion and metastasis of cancer cells and silencing profilin results in the oncogenic properties of breast cancer, consistently.65–67 However, the study of Ding et al68 reveals a reverse conclusion that loss of profilin 1 dramatically inhibits the metastatic outgrowth of disseminated breast cancer, which is relevant to the anoikis or the transformation of gene expression pattern. Meanwhile, profilin is also down-expressed in bladder cancer and pancreatic cancer.69,70 In conclusion, the downregulation of profilin is a disadvantage to survival rate and promotes the metastasis in multiple cancers. However, there are no relevant studies that reveal the specific function of profilin to OS yet.
Villin
Villin is a member of actin filament capping proteins, which are related to the regulation of calcium. The expression of villin is varied in tumor cells that can make differential diagnosis and predict prognosis. It is revealed that villin is a sensitive biomarker to distinguish between intrahepatic cholangiocarcinoma and breast cancer in liver metastasis.71 In the study of colon cancer, it is also proved that villin acts as a cancer suppressor and higher expression of villin results in a better survival rate.72 However, a high expression of villin is also found in esophageal tumors including Barrett’s adenocarcinoma, which indicates that villin may be a novel biomarker in the diagnosis and prognosis of esophageal tumors.73 However, the regulation of villin is still controversial and requires further research. The relation between OS and villin is still unclear.
Fascin
Fascin has been widely researched in different tumor cells. It promotes the activity of NF-κB induced by p53 pathway and facilitates the invasion of colorectal cancer.74 Meanwhile, the interaction between fascin and transforming growth factor (TGF) has been illuminated in gastric cancer. The TGF-β enhances the expression of fascin, which is identical with the increase of Smad3.75 This indicates that the upregulation of fascin induced by TGF-β is important to the pathway of Smad3 in tumor cells. In addition, the abnormal expression of fascin in non-small cell lung cancer, ovarian cancer and pancreatic cancer has also been reported.76–78 In childhood, the elevated level of fascin in solid tumors including OS indicates poor histopathological subtype with expression of TGF-β receptor, which contributes to a high risk of metastasis.79 As a result, fascin is a critical marker in tumorigenesis and is related to the survival time of patients.
Talin
The focal adhesion protein talin functions as a bridge between integrin and actin. It is a downstream target of activated Rap1 and participates in the formation of integrin activation complex together with RIAM and Rap1.80 Talin has been identified as a poor prognosis biomarker in various tumors. It promotes the development of tumorigenesis in nasopharyngeal cancer, colon cancer, prostate cancer and glioma.81–84 No specific documents have elaborated the expression of talin in OS yet. However, an in vitro study implied that talin may increase the cell motility in OS by interacting with vinculin, which is regulated by the activity of nonlocalized Rac1 in one of the OS cell lines (U2OS).85 Since the importance of integrin in lung metastasis has been found, talin may also have a high possibility of regulating the integrin-induced metastasis of OS cells.86
Kindlin
Kindlin, a member of fermitin family, consists of three main homologs; kindlin1, kindlin2 and kindlin3. As the integrin linker, kindlin connects to the NPxY motif of β1 or β3 integrin cytoplasmic domain, which forms the integrin activative complex together with talin.87 Unlike talin, the expression of kindlin is controversial in various cancers. Some research identifies kindlin as an inhibitor in ovarian and colorectal cancers, while opposite results come out in gastric and pancreatic cancers.88–91 This opposite conclusion may result from two aspects: 1) the diversity of tissues leads to the different expression of kindlin, and 2) kindlin may have various functions in different stages of cell activities. It might promote the invasion of tumor cells, while it inhibits the proteolytic degradation of the extracellular matrix (ECM) and suppresses the metastasis. Similar to talin, the β-integrin regulation of kindlin in OS is only illuminated in the cell line. It implies that the tissue distinction exists in various kinds of tumors, which indicates the urgency to study kindlin in OS.92
ERM family proteins
With phospholipids and kinases-mediated phosphorylation, the ERM family regulates the cell structure, such as microvilli, ruffling membranes and adhesion of cells.93 It has been reported that the level and phosphorylation of ERM family proteins are related to tumorigenesis, which has been confirmed in various cancers such as prostate, breast and stomach.94–97 Among the ERM family, ezrin plays the most significant role in tumor metastasis. Different from other ABPs, the function of ezrin in OS has been researched in depth. Among this family, according to the study by Khanna et al, ezrin is a significant poor prognosis biomarker that is associated with OS metastasis. This may be due to the fact that ezrin promotes the activation of PI3K-Akt and MAPK pathway.98 It also implies that CD44, the hyaluronic acid receptor that is also responsible for cancer metastasis, is induced by ezrin.99 Besides cell migration, ezrin also coordinates HSP70 to modulate cell apoptosis of human OS, especially early apoptosis.100 However, the specific theories of ezrin in tumor-promoting effects remain unidentified and require further research.
ABPs and oncotherapy in OS
The actin cytoskeleton is an important structure that regulates the cancer apoptosis and is involved in the formation of chemoresistance in oncotherapy. With the abnormal regulation of ABPs, actin activates the caspase-3 pathway, which feeds back to the further interaction with actin and leads to cell death.18,101,102 However, two aspects of villin’s regulation in the apoptosis of gastrointestinal epithelium have been reported, which include maintaining morphology and homeostasis.103 These processes may also be responsible for chemoresistance in OS therapy, which has been proved in ovarian and head-and-neck cancers.104,105 Meanwhile, in breast cancer, it has been indicated that fascin has the capability to enhance the resistance of chemotherapy and makes for a poor prognosis in patients. It is induced by increasing PI3K/Akt activation, which enhances anti-apoptotic genes and reduces proapoptotic ones.106 Likewise, talin plays an essential role in the regulation of cisplatin resistance within the microenvironment of the carcinoma matrix via the pathway of NF-kB in oral squamous cell carcinoma.107 As the cross-linker of actin filament, the expression of L-plastin and the phosphorylation of Ser5 cause alterative sensitivity to TNF-α and lead to the resistance of breast cancer to TNF-α.108,109
However, the precise molecular mechanism regarding the multidrug resistance induced by ABPs remains to be addressed in OS cells. α-Actinin is important in keeping morphology of cells. Research has revealed that rapid downregulation of α-actinin exists in drug-treated OS cells, which implies the regulative role of α-actinin in drug-induced apoptosis.110 It has been proven that interaction of ezrin with P-glycoprotein (Pgp) is important in the establishment of Pgp-mediated multidrug resistance in human OS cells.111 At the same time, ezrin is also involved in the inhibition of OS metastatic behavior induced by rapamycin, which relates to ezrin-associated phosphorylation of S6K1 and 4E-BP1 in the mTOR signal pathway.112 The research by Kim et al113 indicates that the chemoresponsive patients who are ezrin positive have a poor outcome with early metastasis and need more active tumor surveillance, including images of the lungs and bones, after the curative surgery. These findings provide us with a new concept to the chemosensitivity of ABPs and other therapy in OS.
Conclusion and prospects
Much research has proven the important role of multiple ABPs in the regulation of proliferation, adhesion, invasion, metastasis, apoptosis and angiogenesis in the progress of cancer cells. Multiple functions of ABPs in different cancer cells also make it necessary to analyze various ABPs by an integrated method. Meanwhile, ABPs also present a potential role in chemotherapy such as inducing chemoresistance. At the same time, the importance of diverse regulation of ABPs is revealed in OS research. ABPs may act as favorable biomarkers in predicting the prognosis of OS and altering the treatment of OS patients individually. However, carcinogenesis in OS is an intricate process that requires further study in the future. Concerning different tumor tissues and microenvironments, intensive studies about interaction between ABPs and OS are still very important and in great demand.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Renard AJ, Veth RP, Schreuder HW, et al. Osteosarcoma: oncologic and functional results. A single institutional report covering 22 years. J Surg Oncol. 1999;72(3):124–129. | ||
Joo MW, Shin SH, Kang YK, et al. Osteosarcoma in Asian populations over the age of 40 years: a multicenter study. Ann Surg Oncol. 2015;22(11):3557–3564. | ||
Zhang J, Yan YG, Wang C, Zhang SJ, Yu XH, Wang WJ. MicroRNAs in osteosarcoma. Clin Chim Acta. 2015;444:9–17. | ||
Aponte-Tinao L, Ayerza MA, Muscolo DL, Farfalli GL. Survival, recurrence, and function after epiphyseal preservation and allograft reconstruction in osteosarcoma of the knee. Clin Orthop Relat Res. 2015;473(5):1789–1796. | ||
Anderson ME. Update on survival in osteosarcoma. Orthop Clin North Am. 2016;47(1):283–292. | ||
Jaffe N. Historical perspective on the introduction and use of chemotherapy for the treatment of osteosarcoma. Adv Exp Med Biol. 2014;804:1–30. | ||
Luetke A, Meyers PA, Lewis I, Juergens H. Osteosarcoma treatment – where do we stand? A state of the art review. Cancer Treat Rev. 2014;40(4):523–532. | ||
Hu Y, Xu S, Jin W, Yi Q, Wei W. Effect of the PTEN gene on adhesion, invasion and metastasis of osteosarcoma cells. Oncol Rep. 2014;32(4):1741–1747. | ||
Yang JM, Nguyen HN, Sesaki H, Devreotes PN, Iijima M. Engineering PTEN function: membrane association and activity. Methods. 2015;7(7–78):119–124. | ||
Yin K, Liao Q, He H, Zhong D. Prognostic value of Twist and E-cadherin in patients with osteosarcoma. Med Oncol. 2012;29(5):3449–3455. | ||
Saarikangas J, Zhao H, Lappalainen P. Regulation of the actin cytoskeleton-plasma membrane interplay by phosphoinositides. Physiol Rev. 2010;90(1):259–289. | ||
Rubenstein PA, Wen KK. Insights into the effects of disease-causing mutations in human actins. Cytoskeleton (Hoboken). 2014;71(4):211–229. | ||
Lee CW, Vitriol EA, Shim S, Wise AL, Velayutham RP, Zheng JQ. Dynamic localization of G-actin during membrane protrusion in neuronal motility. Curr Biol. 2013;23(12):1046–1056. | ||
Ciobanasu C, Faivre B, Le Clainche C. Integrating actin dynamics, mechanotransduction and integrin activation: the multiple functions of actin binding proteins in focal adhesions. Eur J Cell Biol. 2013;92(10–11):339–348. | ||
Pantaloni D, Le Clainche C, Carlier MF. Mechanism of actin-based motility. Science. 2001;292(5521):1502–1506. | ||
Artman L, Dormoy-Raclet V, von Roretz C, Gallouzi IE. Planning your every move: the role of beta-actin and its post-transcriptional regulation in cell motility. Semin Cell Dev Biol. 2014;34:33–43. | ||
Ono S. Regulation of structure and function of sarcomeric actin filaments in striated muscle of the nematode Caenorhabditis elegans. Anat Rec (Hoboken). 2014;297(9):1548–1559. | ||
Gross SR. Actin binding proteins: their ups and downs in metastatic life. Cell Adh Migr. 2013;7(2):199–213. | ||
Yamaguchi H. Pathological roles of invadopodia in cancer invasion and metastasis. Eur J Cell Biol. 2012;91(11–12):902–907. | ||
Nurnberg A, Kollmannsperger A, Grosse R. Pharmacological inhibition of actin assembly to target tumor cell motility. Rev Physiol Biochem Pharmacol. 2014;166:23–42. | ||
dos Remedios CG, Chhabra D, Kekic M, et al. Actin binding proteins: regulation of cytoskeletal microfilaments. Physiol Rev. 2003;83(2):433–473. | ||
Goley ED, Welch MD. The ARP2/3 complex: an actin nucleator comes of age. Nat Rev Mol Cell Biol. 2006;7(10):713–726. | ||
Tittel J, Welz T, Czogalla A, et al. Membrane targeting of the Spir formin actin nucleator complex requires a sequential handshake of polar interactions. J Biol Chem. 2015;290(10):6428–6444. | ||
Goode BL, Eck MJ. Mechanism and function of formins in the control of actin assembly. Annu Rev Biochem. 2007;76:593–627. | ||
Suarez C, Carroll RT, Burke TA, et al. Profilin regulates F-actin network homeostasis by favoring formin over Arp2/3 complex. Dev Cell. 2015;32(1):43–53. | ||
Rodal AA, Manning AL, Goode BL, Drubin DG. Negative regulation of yeast WASP by two SH3 domain-containing proteins. Curr Biol. 2003;13(12):1000–1008. | ||
Grintsevich EE, Reisler E. Drebrin inhibits cofilin-induced severing of F-actin. Cytoskeleton (Hoboken). 2014;71(8):472–483. | ||
Maekawa M, Ishizaki T, Boku S, et al. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 1999;285(5429):895–898. | ||
Wolf M, Zimmermann AM, Gorlich A, et al. ADF/cofilin controls synaptic actin dynamics and regulates synaptic vesicle mobilization and exocytosis. Cereb Cortex. 2015;25(9):2863–2875. | ||
Cooper JA, Sept D. New insights into mechanism and regulation of actin capping protein. Int Rev Cell Mol Biol. 2008;267:183–206. | ||
Mejillano MR, Kojima S, Applewhite DA, Gertler FB, Svitkina TM, Borisy GG. Lamellipodial versus filopodial mode of the actin nanomachinery: pivotal role of the filament barbed end. Cell. 2004;118(3):363–373. | ||
Friederich E, Vancompernolle K, Louvard D, Vandekerckhove J. Villin function in the organization of the actin cytoskeleton. Correlation of in vivo effects to its biochemical activities in vitro. J Biol Chem. 1999;274(38):26751–26760. | ||
Hashimoto Y, Kim DJ, Adams JC. The roles of fascins in health and disease. J Pathol. 2011;224(3):289–300. | ||
Ricca BL, Rock RS. The stepping pattern of myosin X is adapted for processive motility on bundled actin. Biophys J. 2010;99(6):1818–1826. | ||
Edlund M, Lotano MA, Otey CA. Dynamics of alpha-actinin in focal adhesions and stress fibers visualized with alpha-actinin-green fluorescent protein. Cell Motil Cytoskeleton. 2001;48(3):190–200. | ||
Gluck U, Ben-Ze’ev A. Modulation of alpha-actinin levels affects cell motility and confers tumorigenicity on 3T3 cells. J Cell Sci. 1994;107(pt 7):1773–1782. | ||
Critchley DR, Gingras AR. Talin at a glance. J Cell Sci. 2008;121(pt 9):1345–1347. | ||
Ye F, Snider AK, Ginsberg MH. Talin and kindlin: the one-two punch in integrin activation. Front Med. 2014;8(1):6–16. | ||
Petrich BG. Talin-dependent integrin signalling in vivo. Thromb Haemost. 2009;101(6):1020–1024. | ||
Xu Z, Gao J, Hong J, Ma YQ. Integrity of kindlin-2 FERM subdomains is required for supporting integrin activation. Biochem Biophys Res Commun. 2013;434(2):382–387. | ||
Yang Q, Onuki R, Nakai C, Sugiyama Y. Ezrin and radixin both regulate the apical membrane localization of ABCC2 (MRP2) in human intestinal epithelial Caco-2 cells. Exp Cell Res. 2007;313(16):3517–3525. | ||
Barreiro O, Yanez-Mo M, Serrador JM, et al. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J Cell Biol. 2002;157(7):1233–1245. | ||
Gandy KA, Canals D, Adada M, et al. Sphingosine 1-phosphate induces filopodia formation through S1PR2 activation of ERM proteins. Biochem J. 2013;449(3):661–672. | ||
Sala-Valdes M, Ursa A, Charrin S, et al. EWI-2 and EWI-F link the tetraspanin web to the actin cytoskeleton through their direct association with ezrin-radixin-moesin proteins. J Biol Chem. 2006;281(28):19665–19675. | ||
Ikenouchi J, Hirata M, Yonemura S, Umeda M. Sphingomyelin clustering is essential for the formation of microvilli. J Cell Sci. 2013;126(pt 16):3585–3592. | ||
Chuang JZ, Lin DC, Lin S. Molecular cloning, expression, and mapping of the high affinity actin-capping domain of chicken cardiac tensin. J Cell Biol. 1995;128(6):1095–1109. | ||
Lo SH, Janmey PA, Hartwig JH, Chen LB. Interactions of tensin with actin and identification of its three distinct actin-binding domains. J Cell Biol. 1994;125(5):1067–1075. | ||
Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420(6916):629–635. | ||
Brzeska H, Szczepanowska J, Matsumura F, Korn ED. Rac-induced increase of phosphorylation of myosin regulatory light chain in HeLa cells. Cell Motil Cytoskeleton. 2004;58(3):186–199. | ||
Zhang L, Luo J, Wan P, Wu J, Laski F, Chen J. Regulation of cofilin phosphorylation and asymmetry in collective cell migration during morphogenesis. Development. 2011;138(3):455–464. | ||
Gauthier-Campbell C, Bredt DS, Murphy TH, El-Husseini Ael D. Regulation of dendritic branching and filopodia formation in hippocampal neurons by specific acylated protein motifs. Mol Biol Cell. 2004;15(5):2205–2217. | ||
Bao J, Wang S, Gunther LK, Kitajiri S, Li C, Sakamoto T. The actin-bundling protein TRIOBP-4 and -5 promotes the motility of pancreatic cancer cells. Cancer Lett. 2015;356(2 pt B):367–373. | ||
Lorente G, Syriani E, Morales M. Actin filaments at the leading edge of cancer cells are characterized by a high mobile fraction and turnover regulation by profilin I. PLoS One. 2014;9(1):e85817. | ||
Fukumoto M, Kurisu S, Yamada T, Takenawa T. alpha-Actinin-4 enhances colorectal cancer cell invasion by suppressing focal adhesion maturation. PLoS One. 2015;10(4):e0120616. | ||
Blackstone BN, Li R, Ackerman WET, Ghadiali SN, Powell HM, Kniss DA. Myoferlin depletion elevates focal adhesion kinase and paxillin phosphorylation and enhances cell-matrix adhesion in breast cancer cells. Am J Physiol Cell Physiol. 2015;308(8):C642–C649. | ||
Wang Z, Zhang J, Ye M, et al. Tumor suppressor role of protein 4.1B/DAL-1. Cell Mol Life Sci. 2014;71(24):4815–4830. | ||
Lee HR, Kim J, Park J, Ahn S, Jeong E, Park H. FERM domain promotes resveratrol-induced apoptosis in endothelial cells via inhibition of NO production. Biochem Biophys Res Commun. 2013;441(4):891–896. | ||
Kanda Y, Kawaguchi T, Kuramitsu Y, et al. Fascin regulates chronic inflammation-related human colon carcinogenesis by inhibiting cell anoikis. Proteomics. 2014;14(9):1031–1041. | ||
Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol. 2001;13(5):555–562. | ||
Peng ZM, Yu W, Xie Y, et al. A four actin-binding protein signature model for poor prognosis of patients with esophageal squamous cell carcinoma. Int J Clin Exp Pathol. 2014;7(9):5950–5959. | ||
Kinoshita T, Nohata N, Watanabe-Takano H, et al. Actin-related protein 2/3 complex subunit 5 (ARPC5) contributes to cell migration and invasion and is directly regulated by tumor-suppressive microRNA-133a in head and neck squamous cell carcinoma. Int J Oncol. 2012;40(6):1770–1778. | ||
Liu Z, Yang X, Chen C, et al. Expression of the Arp2/3 complex in human gliomas and its role in the migration and invasion of glioma cells. Oncol Rep. 2013;30(5):2127–2136. | ||
Rauhala HE, Teppo S, Niemela S, Kallioniemi A. Silencing of the ARP2/3 complex disturbs pancreatic cancer cell migration. Anticancer Res. 2013;33(1):45–52. | ||
Bernardini G, Laschi M, Serchi T, et al. Proteomics and phosphoproteomics provide insights into the mechanism of action of a novel pyrazolo[3,4-d]pyrimidine Src inhibitor in human osteosarcoma. Mol Biosyst. 2014;10(6):1305–1312. | ||
Rizwani W, Fasim A, Sharma D, Reddy DJ, Bin Omar NA, Singh SS. S137 phosphorylation of profilin 1 is an important signaling event in breast cancer progression. PLoS One. 2014;9(8):e103868. | ||
Joy ME, Vollmer LL, Hulkower K, et al. A high-content, multiplexed screen in human breast cancer cells identifies profilin-1 inducers with anti-migratory activities. PLoS One. 2014;9(2):e88350. | ||
Zou L, Ding Z, Roy P. Profilin-1 overexpression inhibits proliferation of MDA-MB-231 breast cancer cells partly through p27kip1 upregulation. J Cell Physiol. 2010;223(3):623–629. | ||
Ding Z, Joy M, Bhargava R, et al. Profilin-1 downregulation has contrasting effects on early vs late steps of breast cancer metastasis. Oncogene. 2014;33(16):2065–2074. | ||
Zoidakis J, Makridakis M, Zerefos PG, et al. Profilin 1 is a potential biomarker for bladder cancer aggressiveness. Mol Cell Proteomics. 2012;11(4):M111009449. | ||
Yao W, Ji S, Qin Y, et al. Profilin-1 suppresses tumorigenicity in pancreatic cancer through regulation of the SIRT3-HIF1alpha axis. Mol Cancer. 2014;13:187. | ||
Yang Z. The utility of villin and mammaglobin in the differential diagnosis between intrahepatic cholangiocarcinoma and breast cancer. Appl Immunohistochem Mol Morphol. 2015;23(1):19–25. | ||
Al-Maghrabi J, Gomaa W, Buhmeida A, Al-Qahtani M, Al-Ahwal M. Loss of villin immunoexpression in colorectal carcinoma is associated with poor differentiation and survival. ISRN Gastroenterol. 2013;2013:679724. | ||
Regalado SP, Nambu Y, Iannettoni MD, Orringer MB, Beer DG. Abundant expression of the intestinal protein villin in Barrett’s metaplasia and esophageal adenocarcinomas. Mol Carcinog. 1998;22(3):182–189. | ||
Sui X, Zhu J, Tang H, et al. p53 controls colorectal cancer cell invasion by inhibiting the NF-kappaB-mediated activation of Fascin. Oncotarget. 2015;6(26):22869–22879. | ||
Li L, Cao F, Liu B, Luo X, Ma X, Hu Z. TGF-beta induces fascin expression in gastric cancer via phosphorylation of smad3 linker area. Am J Cancer Res. 2015;5(6):1890–1896. | ||
Alici O, Kefeli M, Yildiz L, Baris S, Karagoz F, Kandemir B. Fascin and EMMPRIN expression in primary mucinous tumors of ovary: a tissue microarray study. Pathol Res Pract. 2014;210(12):934–938. | ||
Ling XL, Zhang T, Hou XM, Zhao D. Clinicopathological significance of fascin-1 expression in patients with non-small cell lung cancer. Onco Targets Ther. 2015;8:1589–1595. | ||
Li A, Morton JP, Ma Y, et al. Fascin is regulated by slug, promotes progression of pancreatic cancer in mice, and is associated with patient outcomes. Gastroenterology. 2014;146(5):1386–1396.e1–e17. | ||
Tanyildiz HG, Kaygusuz G, Unal E, Tacyildiz N, Dincaslan H, Yavuz G. The prognostic importance of TGF-beta, TGF-beta receptor, and fascin in childhood solid tumors. Pediatr Hematol Oncol. 2017;34(4):238–253. | ||
Han J, Lim CJ, Watanabe N, et al. Reconstructing and deconstructing agonist-induced activation of integrin alphaIIbbeta3. Curr Biol. 2006;16(18):1796–1806. | ||
Sen S, Ng WP, Kumar S. Contributions of talin-1 to glioma cell-matrix tensional homeostasis. J R Soc Interface. 2012;9(71):1311–1317. | ||
Xu YF, Ren XY, Li YQ, et al. High expression of Talin-1 is associated with poor prognosis in patients with nasopharyngeal carcinoma. BMC Cancer. 2015;15:332. | ||
Bostanci O, Kemik O, Kemik A, et al. A novel screening test for colon cancer: Talin-1. Eur Rev Med Pharmacol Sci. 2014;18(17):2533–2537. | ||
Zhang W, Mao YQ, Wang H, Yin WJ, Zhu SX, Wang WC. MiR-124 suppresses cell motility and adhesion by targeting Talin 1 in prostate cancer cells. Cancer Cell Int. 2015;15:49. | ||
Carisey A, Tsang R, Greiner AM, et al. Vinculin regulates the recruitment and release of core focal adhesion proteins in a force-dependent manner. Curr Biol. 2013;23(4):271–281. | ||
Gvozdenovic A, Boro A, Meier D, et al. Targeting alphavbeta3 and alphavbeta5 integrins inhibits pulmonary metastasis in an intratibial xenograft osteosarcoma mouse model. Oncotarget. 2016;7(34). | ||
Calderwood DA, Campbell ID, Critchley DR. Talins and kindlins: partners in integrin-mediated adhesion. Nat Rev Mol Cell Biol. 2013;14(8):503–517. | ||
Ren C, Du J, Xi C, et al. Kindlin-2 inhibits serous epithelial ovarian cancer peritoneal dissemination and predicts patient outcomes. Biochem Biophys Res Commun. 2014;446(1):187–194. | ||
Ren Y, Jin H, Xue Z, et al. Kindlin-2 inhibited the growth and migration of colorectal cancer cells. Tumour Biol. 2015;36(6):4107–4114. | ||
Shen Z, Ye Y, Kauttu T, et al. Novel focal adhesion protein kindlin-2 promotes the invasion of gastric cancer cells through phosphorylation of integrin beta1 and beta3. J Surg Oncol. 2013;108(2):106–112. | ||
Mahawithitwong P, Ohuchida K, Ikenaga N, et al. Kindlin-1 expression is involved in migration and invasion of pancreatic cancer. Int J Oncol. 2013;42(4):1360–1366. | ||
Dong JM, Tay FP, Swa HL, et al. Proximity biotinylation provides insight into the molecular composition of focal adhesions at the nanometer scale. Sci Signal. 2016;9(432):rs4. | ||
Bonilha VL. Focus on molecules: ezrin. Exp Eye Res. 2007;84(4):613–614. | ||
Arpin M, Chirivino D, Naba A, Zwaenepoel I. Emerging role for ERM proteins in cell adhesion and migration. Cell Adh Migr. 2011;5(2):199–206. | ||
Ghaffari A, Hoskin V, Szeto A, et al. A novel role for ezrin in breast cancer angio/lymphangiogenesis. Breast Cancer Res. 2014;16(5):438. | ||
Li L, Wang YY, Zhao ZS, Ma J. Ezrin is associated with gastric cancer progression and prognosis. Pathol Oncol Res. 2011;17(4):909–915. | ||
Valdman A, Fang X, Pang ST, Nilsson B, Ekman P, Egevad L. Ezrin expression in prostate cancer and benign prostatic tissue. Eur Urol. 2005;48(5):852–857. | ||
Khanna C, Wan X, Bose S, et al. The membrane-cytoskeleton linker ezrin is necessary for osteosarcoma metastasis. Nat Med. 2004;10(2):182–186. | ||
Martin TA, Harrison G, Mansel RE, Jiang WG. The role of the CD44/ezrin complex in cancer metastasis. Crit Rev Oncol Hematol. 2003;46(2):165–186. | ||
Yao Q, Zhao HY, Xie BZ. Effects of ezrin and heat shock protein 70 on apoptosis and proliferation of human osteosarcoma cells. Orthop Surg. 2015;7(3):273–280. | ||
Posey SC, Bierer BE. Actin stabilization by jasplakinolide enhances apoptosis induced by cytokine deprivation. J Biol Chem. 1999;274(7):4259–4265. | ||
Odaka C, Sanders ML, Crews P. Jasplakinolide induces apoptosis in various transformed cell lines by a caspase-3-like protease-dependent pathway. Clin Diagn Lab Immunol. 2000;7(6):947–952. | ||
Wang Y, George SP, Roy S, Pham E, Esmaeilniakooshkghazi A, Khurana S. Both the anti- and pro-apoptotic functions of villin regulate cell turnover and intestinal homeostasis. Sci Rep. 2016;6:35491. | ||
Li M, Yin J, Mao N, Pan L. Upregulation of phosphorylated cofilin 1 correlates with taxol resistance in human ovarian cancer in vitro and in vivo. Oncol Rep. 2013;29(1):58–66. | ||
Wang PW, Abedini MR, Yang LX, et al. Gelsolin regulates cisplatin sensitivity in human head-and-neck cancer. Int J Cancer. 2014;135(12):2760–2769. | ||
Ghebeh H, Al-Khaldi S, Olabi S, et al. Fascin is involved in the chemotherapeutic resistance of breast cancer cells predominantly via the PI3K/Akt pathway. Br J Cancer. 2014;111(8):1552–1561. | ||
Eberle KE, Sansing HA, Szaniszlo P, Resto VA, Berrier AL. Carcinoma matrix controls resistance to cisplatin through talin regulation of NF-kB. PLoS One. 2011;6(6):e21496. | ||
Janji B, Vallar L, Al Tanoury Z, et al. The actin filament cross-linker L-plastin confers resistance to TNF-alpha in MCF-7 breast cancer cells in a phosphorylation-dependent manner. J Cell Mol Med. 2010;14(6A):1264–1275. | ||
Janji B, Giganti A, De Corte V, et al. Phosphorylation on Ser5 increases the F-actin-binding activity of L-plastin and promotes its targeting to sites of actin assembly in cells. J Cell Sci. 2006;119(pt 9):1947–1960. | ||
Fellenberg J, Dechant MJ, Ewerbeck V, Mau H. Identification of drug-regulated genes in osteosarcoma cells. Int J Cancer. 2003;105(5):636–643. | ||
Brambilla D, Zamboni S, Federici C, et al. P-glycoprotein binds to ezrin at amino acid residues 149-242 in the FERM domain and plays a key role in the multidrug resistance of human osteosarcoma. Int J Cancer. 2012;130(12):2824–2834. | ||
Wan X, Mendoza A, Khanna C, Helman LJ. Rapamycin inhibits ezrin-mediated metastatic behavior in a murine model of osteosarcoma. Cancer Res. 2005;65(6):2406–2411. | ||
Kim C, Shin E, Hong S, et al. Clinical value of ezrin expression in primary osteosarcoma. Cancer Res Treat. 2009;41(3):138–144. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.