Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 14
Forced expiratory volumes in 3 s is a sensitive clinical measure for assessment of bronchodilator reversibility in elderly Chinese with severe lung function impairment
Received 9 December 2018
Accepted for publication 17 May 2019
Published 7 August 2019 Volume 2019:14 Pages 1803—1811
DOI https://doi.org/10.2147/COPD.S197552
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Chunxue Bai
Mingming Pan, Hongsheng Zhang, Tieying Sun
Department of Respiratory and Critical Care Medicine, Beijing Hospital, National Center of Gerontology, Beijing, People’s Republic of China
Purpose: Sensitively assessing bronchial reversibility by spirometry is difficult in patients with serious airflow limitation and the elderly. Some patients cannot exhale for ≥6 s to achieve FVC testing criteria. The aim of this study was to assess if FEV3 could be a more sensitive and an acceptable surrogate for evaluating bronchial reversibility in such patients.
Patients and methods: Subjects who had undergone pulmonary function examination in Beijing hospital from July 2003 to April 2015 were included in the study. Patients with FEV1<50% of the predicted value were classified as the severely lung function–impaired group. Correlation between the severity of lung function impairment and changes in FEV1, FEV3 and FVC in response to a bronchodilator was estimated.
Results: A total of 7745 tests on elderly subjects with a median age of 71 years were reviewed. The severely lung function–impaired group of 1728 accounted for 22.3% of the total number of subjects. There were significantly more patients in the severely lung function–impaired group who exhibited positive response in FEV3 or FVC and negative response in FEV1 after bronchodilator test (FEV1 negative response but FVC positive response, χ2=626.97, P<0.001; FEV1 negative response but FEV3 positive response, χ2=372.83, P<0.001). With the progressive increase in lung function impairment, ΔFEV1 increased and then declined, while ΔFVC and ΔFEV3 increased progressively. Changes in FEV3 or FVC significantly exceeded the change in FEV1 in the severely lung function–impaired groups (P<0.001).
Conclusion: In elderly subjects, especially those with severe lung function impairment, FEV3 combined with FVC is a more effective and sensitive primary clinical outcome measure to detect bronchial reversibility. In subjects who cannot complete ≥6 s forced expiration and whose FVC is unreliable, FEV3 combined with FEV1 might be clinically more valuable in detecting bronchial reversibility.
Keywords: airway obstruction, FEV3, forced expiratory volume in 3 second, bronchodilator responsiveness, lung function tests, elderly patients
Introduction
Therapeutic effectiveness of inhaled drugs in patients with chronic airway inflammatory diseases such as COPD and asthma is usually assessed by the changes in pulmonary function. FEV1 is the most commonly used indicator of pulmonary function. Clinical trials have revealed that changes in FEV1 before and after treatment are not sensitive enough to reflect the effect of bronchodilators in patients with serious airflow limitation, especially the elderly.1 FVC was reported to be more sensitive than FEV1 in detecting bronchial reversibility in COPD patients.2 Patients who did not show bronchial responsiveness in FEV1 might show changes in lung volume measurements called “volume response”.3
According to the American Thoracic Society/European Respiratory Society (ATS/ERS) criteria, FVC testing requires a forced expiratory time of 6 s or a plateau in the volume–time curve.4 This is often a relatively long time to exhale, especially for patients with severe airway limitation, aged people and patients with diseases such as severe cough and heart failure.5,6 In a study on elderly population, 25% of the subjects could complete FEV3 but not FVC.7 Another study demonstrated that among 2,928 lung function tests only 47% could exhale for <4 s.6
The FEV3 is the rapidly exhaled volume during the first 3 s of a forced expiratory maneuver, which starts at the level of total lung capacity. It is reproducible, needs shorter expiratory effort and provides an exact outcome.8 Studies have reported that FEV3 could be considered as a possible surrogate for FVC9 as well as an alternative to FEV1.10 However, whether FEV3 can detect bronchodilator responsiveness as sensitively as FVC in the elderly population remains controversial.
In the present study, we studied a large unselected Chinese elderly population presenting to our hospital defined by lung function characteristics in relation to bronchodilator response. We attempted to evaluate the changes in FEV1, FVC and FEV3 in response to a bronchodilator and reveal if changes in FEV3 post-bronchodilator test could be an acceptable and sensitive indicator to evaluate bronchodilator response in the elderly patients with severe airflow limitation. In addition, we attempted to find some sensitive indicators that would reflect the improvement in lung function accurately in patients who in spite of their best ability could not breathe continuously for 6 s – a problem that needs clinical resolution.
Materials and methods
Elderly subjects who had undergone pulmonary function examinations in Beijing hospital from July 2003 to April 2015 were analyzed with the following characteristics: age 60 years or older, completion of post-bronchodilator spirometry that included FEV3 in lung function test.
Measurement of pulmonary function: All subjects underwent pulmonary function tests in Beijing Hospital on a Vmax 622 pulmonary function instrument (Sensormedics, USA) and plethysmograph (Sensormedics, USA) according to pulmonary function test guidelines.5 Each lung function test was repeated 3 times and the best value was considered for analysis. All subjects underwent a bronchodilator test which required the administration of 400 μg of salbutamol via metered dose inhaler. Spirometry was repeated 15 mins after short-acting β2-agonist administration. Data from patients were collected anonymously. The enrollees were divided into 6 groups according to ERS criteria for categorizing the severity of lung function impairment based on FEV1% predicted value:4 Group A, FEV1≥80% predicted value; Group B, 70% predicted value ≤ FEV1<80% predicted value; Group C, 60% predicted value ≤ FEV1<70% predicted value; Group D, 50% predicted value ≤FEV1<60% predicted value; Group E, 35% predicted value ≤FEV1<50% predicted value and Group F, FEV1<35% predicted value.4 Changes (Δ) in FEV1, FEV3 and FVC after the bronchodilator test were expressed in milliliters, percentage of predicted value or percentage of baseline. To minimize any bias due to age, height and weight, ΔFEV1, ΔFEV3 and ΔFVC were also z score normalized. Correlation between lung function impairment and changes in FEV1, FEV3 and FVC after bronchodilator responsiveness test was estimated. The study protocol was approved by the Beijing Hospital ethics committee.
Data was analyzed using the SPSS 17.0 software. Normally distributed data was expressed as  , and non-normally distributed data was expressed as M (Q1, Q3). Chi-square test was used to compare the relative frequencies of patients among the groups. Mann–Whitney U test was used for comparing data that was not normally distributed. P<0.05 was regarded as statistically significant.
, and non-normally distributed data was expressed as M (Q1, Q3). Chi-square test was used to compare the relative frequencies of patients among the groups. Mann–Whitney U test was used for comparing data that was not normally distributed. P<0.05 was regarded as statistically significant.
Ethics approval and informed consent
The study was conducted in accordance to the Declaration of Helsinki and approved by the ethics committee of Beijing hospital. Informed consents were waived due to the retrospective nature of the study, and all patient data was anonymized.
Results
In the present study, a total of 7745 tests in subjects aged 60 years or older with a median age of 71 years were reviewed. Out of the total, 61.7% were males (4781) and 38.3% were females (2964). Out of the 7745 patients, 2985 (38.5%) were in Group A, 1129 (14.6%) were in Group B, 1066 (13.8%) were in Group C, 837 (10.8%) were in Group D, 1183 (15.3%) were in Group E and 545 were in Group F (7%). There was no significant difference in age and gender distribution between the groups (P>0.05) (Table 1). In order to facilitate statistical analysis, Groups E and F were classified as severely lung function–impaired groups and Groups A–D were classified as not severely lung function–impaired groups.
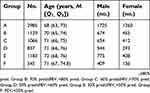 |
Table 1 Baseline characteristics of the study population in subgroups stratified by severity of lung function impairment |
According to FVC criteria in evaluating positive bronchodilator response (a change post-bronchodilator test in FVC ≥12% of baseline and ≥200 mL), the cutoff value of FEV3 in evaluating positive bronchodilator test has been counted. A ROC curve was used to determine the best corresponding cutoff for FEV3 (Figure 1). The area under the ROC curve was 0.969 (95% CI: 0.965–0.973, P<0.001). Post-bronchodilator test a change in FEV3≥10% of baseline and ≥165 mL had the best sensitivity (92.5%) and specificity (90.4%), and there was an excellent agreement between the two diagnostic cutoffs (κ=0.737; P<0.001).
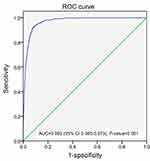 |
Figure 1 Receiver operating characteristic (ROC) curve showing the performance of FEV3 in evaluating bronchodilator test based on FVC evaluation criteria. Abbreviation: AUC, area under the curve. |
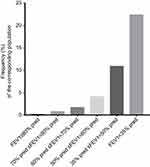
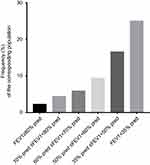
Post-bronchodilator FEV1 increased by ≥12% of baseline in 21% (1627) of subjects, but the percentage was reduced to 14.6% (1131) according to the criteria that both FEV1 changed ≥12% of baseline and ≥200 mL. Similar results were observed with FEV3 and FVC (Table 2). Out of the 496 subjects with post-bronchodilator increase in FEV1 by ≥12% of baseline but <200 mL, 389 were in the severely lung function–impaired group (213 cases, 18% in Group E; 176 cases, 32.3% in Group F). There was a significant difference in the frequency of patients between the severely lung function–impaired group and the not severely lung function–impaired group (χ2=962.77, P<0.001) (Table 3, Figure 2).
 |
Table 2 Characteristics of bronchodilator response in the study population |
 |
Table 3 Characteristics of post-bronchodilator response in subgroups stratified by level of lung function impairment |
Significant bronchodilation is regarded as a change in post-bronchodilator FEV1 or FVC ≥12% of baseline and ≥200 mL according to the ATS/ERS guidelines.4 There were 314 subjects – mainly in the severely lung function–impaired groups (129 cases, 10.9% in Group E; 122 cases, 22.4% in Group F) – whose bronchodilator test was negative when evaluated with FEV1, but became positive with FVC. There was a significant difference in the frequency of patients between the severely lung function–impaired group and not-severely lung function–impaired group (χ2=626.97, P<0.001) (Table 3, Figure 3). There were 593 subjects, mainly in the severely lung function–impaired groups, whose bronchodilator test was negative when evaluated with FEV1 but was positive when evaluated with FEV3 (196 cases, 16.6% in Group E; 136 cases, 25% in Group F). There was a significant difference in the frequency of patients between severely lung function–impaired group and not severely lung function impaired (χ2=420.14, P<0.001). (Table 3, Figure 4).
The post-bronchodilator changes in the z score of FEV1, FVC and FEV3 were related to the severity of lung function impairment (Table 4, Figure 5). As the severity increased, ∆zFEV1 after the bronchodilator test increased gradually and peaked in Groups D and E, but decreased in Group F. In contrast, ∆zFVC continued to increase with the increase in the severity of ventilatory impairment, which peaked in Group F. Post-bronchodilator changes in zFVC were less than zFEV1 in Groups A, B and C (Group A z= –3.16, Group B z= –2.86, Group C z= –2.86, all P<0.05) and similar to ΔzFEV1 in Group D (z= –0.27, P>0.05). But in Groups E and F, ΔzFVC significantly exceeded ΔzFEV1 (Group E z= – 4.15, Group F z= –6.96, all P<0.001). Changes in zFEV3 exhibited similar trends. Post-bronchodilator ∆zFEV3 was less than or equal to the change in zFEV1 for subjects in Groups A to D (Group A z= –2.03, P<0.05, Group B z= –1.51, Group C z= –0.98, Group D z= –0.68, P>0.05), but in Groups E and F, ΔzFEV3 significantly exceeded ΔzFEV1 (Group E z= –3.16, P<0.05, Group F z= –4.41, P<0.001). Similar results were obtained after subgroup analysis of male and female subjects (Tables 4–6).
 |
Table 4 Spirometric Indexes expressed as change post-bronchodilator and z scores in subgroups stratified by level of lung function impairment |
 |
Table 5 Spirometric Indexes in male subjects expressed as change in post-bronchodilator and z scores in subgroups stratified by level of lung function impairment |
 |
Table 6 Spirometric Indexes in female subjects expressed as change in post-bronchodilator and z-scores in subgroups stratified by level of lung function impairment |
Discussion
Elderly subjects – who are also the main population of COPD – are more prone to severe and fixed airways resistance. Increased airway resistance increases intrathoracic gas volume and results in diminished lung elastic recoil pressure.11 After treatment with a bronchodilator, elderly patients – especially those with serious airflow limitation – report improvement in symptoms and exercise tolerance with little change in FEV1.12 Although FEV1 is a widely used efficacy end point, it cannot sensitively reflect the real change in lung function due to the improvement in airway obstruction.13
The bronchodilator test is a better guide to disease progression and can identify patients who might benefit from treatment with a bronchodilator.14 In this study, we evaluated changes in lung function parameters before and after bronchodilator treatment to find appropriate indicators for evaluating lung funtion improvement in the elderly. The findings showed that there were notably more subjects in the severely lung function–impaired group exhibiting significant improvement in FEV3 or FVC with less change in FEV1 in response to the bronchodilator test (FEV1 less than 50% predicted value). To minimize any bias due to age, height and weight, we also expressed values or changes as z scores. The z scores indicate the standard deviation of a measurement that differed from, the average predicted value, which was free of any bias. Hence, this result showed that an increase in airflow obstruction increased ΔFEV1 followed by a decline, while ΔFVC and ΔFEV3 increased progressively. In subjects with mild respiratory defect, ΔFEV3 or ΔFVC was less than or equal to ΔFEV1, but ΔFEV3 or ΔFVC exceeded the relative change in FEV1 in severely lung function–impaired group. Results also indicated that FEV3 and FVC are sensitive indicators of bronchodilation in severe airway obstruction, whereas FEV1 is more sensitive in assessing bronchodilation in mild ventilatory dysfunction.
It has been reported that acute FVC response to bronchodilators was significantly more correlated with health-related quality of life than FEV1 response.15 Using the criterion of “clinically significant” to describe an increase in FVC, FEV1 (≥12% of pre-bronchodilator and ≥0.2 L ) or FEV3 (≥10% of pre-bronchodilator and ≥0.165 L ), we observed that FVC and FEV3 were better measurements for assessing reversibility than FEV1, especially in patients with severely impaired lung function. Also, there was a strong correlation between FEV3 and FVC in assessing the responsiveness to bronchodilator. Repeated FVC can be stressful in aged patients with severe obstruction.5 Thus, it seems that when FVC is not reliable, FEV3 might have special clinical application value in detecting bronchial reversibility.
Our study presents new data on FEV3 implying its clinical applicability for better interpretation of reversibility monitoring, particularly in severely impaired patients who cannot blow for ≥6 s even after their best attempts. Larger FEV3 and FVC responses can provide useful information regarding reduction of hyperinflation with beneficial effects on dyspnea, exercise tolerance and function of small airways,16 which has important clinical application value.
FEV6 has been proposed as an alternative to FVC. Our previous research17 found that FEV6 strongly correlated with FVC which did not vary with the forced expiratory time and it could be used as a valid alternative to FVC in diagnosing airflow obstruction in elderly males. FEV6 is closer to FVC in terms of expiratory time, whereas FEV3 is between FEV1 and FEV6 in expiratory time. FEV3 also makes spirometry easier, faster and safer than FVC measurement and does not correlate with forced expiratory time. In addition, FEV3 has unique application advantages in patients who cannot exhale for 6 s. In such subjects, addition of FEV3 in the daily practice of pulmonary medicine will help physicians to find clinically important relief for hyperinflation.
One of the strengths of our study is that we discussed unselected data on bronchodilator response across various lung function impairment in an aging population. Also, the subjects involved in this study were not limited to any disease or a diagnosis of airflow obstruction. The result reflected the characteristics of common clinical practices in the real world, thus highlighting their clinical usefulness. However, the present study also has some limitations. Being a retrospective study, it failed to provide information about clinical symptoms and disease severity of the subjects. It also lacked information about the use of medication before spirometry. The lung function tests were part of the routine clinical maneuvers, and some subjects might not have withheld medications before testing which may have led to an underestimation of bronchodilator responsiveness. Another significant caveat is the lack of specific diagnosis-related subgroup analyses, which requires further study in the future.
Currently, FEV3 can be recorded with many spirometers. Thus, according to the result of our study, we encourage to report and analyze FEV3 for better assessment of spirometry. Compared with the score of the clinical symptom and life quality, parameters of spirometry such as FEV3 are more objective. Sensitive assessment of improvement in pulmonary function is helpful for disease evaluation and treatment options. However, correlations between the characteristic changes in FEV3 and subsequent clinical manifestations remains to be further studied.
In summary, this population-based retrospective study showed that in elderly subjects, especially those with severe lung function impairment, FEV3 combined with FVC can be a clinically effective and sensitive outcome measure to detect bronchial reversibility. In those elderly subjects who cannot complete ≥6 s of forced expiration and whose FVC is not reliable, FEV3 combined with FEV1 can be clinically valuable in detecting bronchial reversibility.
Acknowledgments
National Key Research and Development Program of China, key special projects of major chronic non-communicable disease prevention and control (2016YFC1304600); Beijing Hospital research fund (BJ-2015-028).
Disclosure
The authors report no conflict of interest in this work.
References
1. Cazzola M, MacNee W, Martinez FJ, et al. Outcomes for COPD pharmacological trials: from lung function to biomarkers. Eur Respir J. 2008;31(2):416-469. doi:10.1183/09031936.00099306
2. Quanjer PH, Ruppel GL, Langhammer A, et al. Bronchodilator response in FVC is larger and more relevant than in FEV1 in severe airflow obstruction. Chest. 2016;151(5):1088–1098. doi:10.1016/j.chest.2016.12.017
3. Cerveri I, Pellegrino R, Dore R, et al. Mechanisms for isolated volume response to a bronchodilator in patients with COPD. J Appl Physiol. 2000;88(6):1989–1995.
4. Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–968. doi:10.1183/09031936.05.00035205
5. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi:10.1183/09031936.05.00034805
6. Eaton T, Withy S, Garrett JE, Mercer J, Whitlock RM, Rea HH. Spirometry in primary care practice: the importance of quality assurance and the impact of spirometry workshops. Chest. 1999;116(2):416–423. doi:10.1378/chest.116.2.416
7. Allen S, Yeung P, Janczewski M, Siddique N. Predicting inadequate spirometry technique and the use of FEV1/FEV3 as an alternative to FEV1/FVC for patients with mild cognitive impairment. Clin Respir J. 2008;2(4):208–213. doi:10.1111/j.1752-699X.2008.00063.x
8. Ioachimescu OC, Venkateshiah SB, Kavuru MS, McCarthy K, Stoller JK. Estimating FVC from FEV2 and FEV3: assessment of a surrogate spirometric parameter. Chest. 2005;128(3):1274–1281. doi:10.1378/chest.128.3.1274
9. Lam DC, Fong DY, Yu WC, et al. FEV3, FEV6 and their derivatives for detecting airflow obstruction in adult Chinese. Int J Tuberc Lung Dis. 2012;16(5):681–686. doi:10.5588/ijtld.11.0044-e
10. Sharafkhaneh A, Babb TG, Officer TM, Hanania NA, Sharafkhaneh H, Boriek AM. The confounding effects of thoracic gas compression on measurement of acute bronchodilator response. Am J Respir Crit Care Med. 2007;175(4):330–335. doi:10.1164/rccm.200701-094ED
11. Riccardo P, Emanuele C, Alessandro G, et al. Severity grading of chronic obstructive pulmonary disease: the confounding effect of phenotype and thoracic gas compression. J Appl Physiol. 2015;118(7):796–802. doi:10.1152/japplphysiol.00801.2014
12. O’Donnell DE, Forkert L, Webb KA. Evaluation of bronchodilator responses in patients with “irreversible” emphysema. Eur Respir J. 2001;18(6):914–920. doi: 10.1183/09031936.01.00216501
13. Rodriguez-Carballeira M, Heredia JL, Rue M, Quintana S, Almagro P. The bronchodilator test in chronic obstructive pulmonary disease: interpretation methods. Respir Med. 2007;101(1):34–42. doi:10.1016/j.rmed.2006.04.018
14. Omata M, Wakabayashi R, Kudoh S, Kida K. Correlation between bronchodilator responsiveness and quality of life in chronic obstructive pulmonary disease. Allergol Int. 2007;56(1):15–22. doi:10.2332/allergolint.O-06-431
15. Hankinson JL, Crapo RO, Jensen RL. Spirometric reference values for the 6-s FVC maneuver. Chest. 2003;124(5):1805–1811. doi:10.1378/chest.124.5.1805
16. O’Donnell DE. Is sustained pharmacologic lung volume reduction now possible in COPD? Chest. 2006;129(3):501–503. doi:10.1378/chest.129.3.501
17. Pan MM, Zhang HS, Sun TY. Value of forced expiratory volume in 6 seconds (FEV6) in the evaluation of pulmonary function in Chinese elderly males. Zhonghua Yi Xue Za Zhi. 2017;97(20):1556–1561. doi:10.3760/cma.j.issn.0376-2491.2017.23.012
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.