Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 15
Food Pyramid for Subjects with Chronic Obstructive Pulmonary Diseases
Authors Rondanelli M, Faliva MA, Peroni G , Infantino V , Gasparri C, Iannello G, Perna S, Alalwan TA , Al-Thawadi S, Corsico AG
Received 29 November 2019
Accepted for publication 20 April 2020
Published 19 June 2020 Volume 2020:15 Pages 1435—1448
DOI https://doi.org/10.2147/COPD.S240561
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Richard Russell
Mariangela Rondanelli,1,2 Milena Anna Faliva,3 Gabriella Peroni,3 Vittoria Infantino,2 Clara Gasparri,3 Giancarlo Iannello,4 Simone Perna,5 Tariq AbdulKarim Alalwan,5 Salwa Al-Thawadi,5 Angelo Guido Corsico6,7
1IRCCS Mondino Foundation, Pavia 27100, Italy; 2Department of Public Health, Experimental and Forensic Medicine, Unit of Human and Clinical Nutrition, University of Pavia, Pavia 27100, Italy; 3Endocrinology and Nutrition Unit, Azienda di Servizi alla Persona “Istituto Santa Margherita”, University of Pavia, Pavia 27100, Italy; 4General Management, Azienda di Servizi alla Persona “Istituto Santa Margherita”, Pavia 27100, Italy; 5Department of Biology, College of Science, University of Bahrain, Sakhir 32038, Bahrain; 6Center for Diagnosis of Inherited Alpha 1-Antitrypsin Deficiency, Department of Internal Medicine and Therapeutics, University of Pavia, Pavia 27100, Italy; 7Division of Respiratory Diseases, IRCCS Policlinico San Matteo Foundation, Pavia 27100, Italy
Correspondence: Gabriella Peroni
Endocrinology and Nutrition Unit, Azienda di Servizi alla Persona “Istituto Santa Margherita”, University of Pavia, Pavia 27100, Italy
, Tel +39 0382381739
Fax +39 0382381218
Email [email protected]
Abstract: Nutritional problems are an important part of rehabilitation for chronic obstructive pulmonary disease (COPD) patients. COPD patients often present with malnutrition, sarcopenia, and osteoporosis with possible onset of cachexia, with an inadequate dietary intake and a poor quality of life. Moreover, diet plays a pivotal role in patients with COPD through three mechanisms: regulation of carbon dioxide produced/oxygen consumed, inflammation, and oxidative stress. A narrative review based on 99 eligible studies was performed to evaluate current evidence regarding optimum diet therapy for the management of COPD, and then a food pyramid was built accordingly. The food pyramid proposal will serve to guide energy and dietary intake in order to prevent and treat nutritionally related COPD complications and to manage progression and COPD-related symptoms. The nutrition pyramid described in our narrative review is hypothetical, even in light of several limitations of the present review; the main limitation is the fact that to date there are no randomized controlled trials in the literature clearly showing that improved nutrition, via the regulation of carbon dioxide produced/oxygen consumed, inflammation and oxidative stress, improves symptoms and/or progression of COPD. Even if this nutritional pyramid is hypothetical, we hope that it can serve the valuable purpose of helping researchers focus on the often-ignored possible connections between body composition, nutrition, and COPD.
Keywords: COPD, nutrients, inflammation, fat free mass, antioxidants, gas exchanges
Introduction
Nutritional problems are an important part of rehabilitation for all disabled subjects, especially in chronic obstructive pulmonary disease (COPD) patients. There are numerous mechanisms that interfere with the functioning of the respiratory system in these patients. Nutrition plays a pivotal role, for both the prevention of risk of COPD, and treatment of COPD. The literature has shown that there is an obvious link between some dietary models and the progression of this disease. Dietary patterns associated with benefits in prevention of the risk of respiratory diseases include those typical of the Mediterranean diet, while fast food intake and westernized eating habits have adverse associations.1–7 In particular, the excessive consumption of red meat, processed meat and sweetened drinks and the reduction of dairy product intake showed a worsening of lung function,8 while a diet rich in whole grains, vegetables, fruit and fish showed a lower risk of newly diagnosed COPD.5
COPD and BMI
Weight and body composition also have an impact on progression of COPD. It should be emphasized that most studies consider body mass index (BMI) rather than body composition.9 Several studies have reported that low BMI is an independent risk factor for mortality in subjects with COPD,10,11 with a inflection point for BMI equal to 21 Kg/m2 and a mortality increase below this value.9,12 The prevalence of underweight patients with COPD varies, ranging between 3% and 19% with BMI ≤ 18.5 kg/m210,13,14 and equal to 22% if a BMI is lower than 21 kg/m2 is considered.15 This prevalence also increases with the severity of the disease,16 and the association with BMI is stronger in subjects with severe COPD.9
A low fat free mass (FFM) should be considered a predictor of independent mortality,17 and not an adaptive mechanism to reduce the metabolic rate.18 This unintentional weight loss can reach 80% due to the non-satisfaction of energy and protein needs.19 A frequent and involuntary weight loss inevitably leads to malnutrition, which can be established regardless of weight, with incidence estimates reaching 75%.19 Very often, with the worsening of the disease, the condition of pulmonary cachexia is reached; the exact cause and mechanisms of the disease are still poorly understood, but potential factors include oxidative stress and inflammation.20
Since weight reduction or a sudden weight loss negatively correlates with the progression of the disease itself, a BMI indicative of overweight or obesity could be considered as protective against COPD. Several studies have evaluated the prevalence of obesity in patients with COPD with results ranging 7.2% of the Spanish population to 54% of the resident population in Northern California, passing 14% in Northern Europe, on 18% in the Netherlands, 20% in Slovenia, 23% in Latin America, 24.6% in Canada.10,13–15,21–23
In the Copenhagen City Heart Study, in a17-year of follow-up study that had a group of 2132 subjects with COPD, a low BMI value was predictive of a poor prognosis (thus greater chance of mortality). The association between BMI and survival differed significantly with the stage of COPD. In mild and moderate COPD, the lowest risk occurred in normal-weight/overweight subjects (with a U-shaped relationship), while in severe COPD mortality continued to decrease with increasing BMI. The same result was noted for deaths from COPD-related respiratory causes by Landbo et al.9
Another large cohort study, which lasted 12 years, involved over 1 million South Koreans aged between 30 and 95 and showed that a higher BMI (BMI> 25 up to 30 kg/m2) clearly reduces the risk of mortality from respiratory causes in patients with COPD.24 This result was confirmed in 2012, in a large meta-analysis conducted by Cao et al, which considered 21,150 participants with COPD, and the study asserted that overweight and obesity were associated with lower mortality.11
In obstructive pulmonary diseases, therefore, what is called the “obesity paradox” can be present, which is more evident for subjects with severe bronchial obstruction.25 More than obesity, perhaps being overweight should be highlighted, as some studies indicated that patients with COPD show a lower mortality risk in overweight patients.10,15 To confirm this data, Eisner and colleagues evaluated the impact of fat mass (FM) on functional limitation: a greater FM was associated with a decrease in the walk test in six minutes (from −13 meters per 1 kg of mass increase fat in men and −11 meters in women) and a poorer Short Physical Performance Battery (SPPB) summary performance score.13
Thus it appears that the increase in FM, and not simply the loss of FFM, is an important precursor for the development of functional limitation and that this process occurs at an early age in COPD compared to the general population.13 In addition, visceral fat has been associated with an increased cardiovascular risk in subjects with COPD.26 Accordingly, nutritional status is of great importance and is a determining factor for the outcome of COPD.17
Caloric Intake
There are several papers that over the years have tried to define the daily calorie expenditure in patients with COPD. In 1994 Ganzoni et al27 hypothesized a daily calorie intake equal to 45 Kcal/Kg/body weight, taken up in a work in 2019 by Collins et al28 which, however, does not seem to have a response in clinical practice, as overestimated. In 1997 Baarends et al effectively evaluated a total daily calorie intake equal to the Basal Energy Expenditure (BEE) multiplied by 1.7; this energy expenditure seems to be mainly due to the effort given by physical activity (intended as physical activity levels, PAL)29 and not by the BEE component as previously described by other authors.30,31 In 2010, some authors validated a specific predictive equation for the calculation of BEE in underweight patients, which however takes into account the value of the FFM, and therefore is not easily feasible or accessible to everyone.32 Lastly, in 2011, some authors evaluated the total energy expenditure (TEE) in this category of subjects, using 2 frequency questionnaires on the levels of physical activity, subsequently comparing them with two methods for calculating the TEE; in the first method, the daily caloric intake was considered equal to30 kcal/Kg/body weight, while in the second case the energy requirement was obtained by multiplying the BEE x 1.7. By comparing the results of the frequency questionnaires with the methods of calculating the TEE, it was more suitable to administer a total caloric quantity equal to 30 kcal/kg/body weight. However, the authors underline how this formula can be effective on the calculation of needs referred to the population and not to the individual subject. For this reason, it is essential to carry out a personalized and tailored nutritional evaluation.33
Sarcopenia and Osteoporosis
The increase in oxidative stress in patients with cachectic COPD is negatively associated with FFM and muscle strength,34 and the mediators of systemic inflammation, such as TNF-α and NF-kB, are implicated in the muscle wasting of COPD.35,36 The decrease in muscle mass and muscle strength are typical consequences of sarcopenia; it is estimated that the percentage of sarcopenic COPD patients ranges from 12 to 39%, based on the evaluation method, bioempedentiometry (BIA) or double-beam X densitometer (DXA, gold standard instrument for the evaluation of body composition),37–40 with a higher incidence in subjects with lower BMI,37 with a worse BODE score37 and with cachexia.40 It also appears to be associated with some inflammatory markers such as IL-6 and TNFα.39 Furthermore, in a 2007 study on the assessment of body composition in patients with COPD, a clear association was found between well-preserved muscle mass (investigated with BIA) and lower risk of functional limitation (mainly assessed with 6-minute walk test).13 Additionally, subjects with COPD are more likely to suffer from osteoporosis, and osteopenia is also higher in (26% and 54% respectively) than in control groups, with an increase in prevalence and severity with increasing intensity of COPD.41 The incidence of new cases of osteoporosis during the course of the disease should not be underestimated; one work assessed a 14% increase in cases after a three-year follow-up period, mainly due to new vertebral fracture findings.42
This could be due to the combination of factors, including smoking or previous smoking, the use of systemic corticosteroids, vitamin D deficiency and loss of lean mass, all of which are risk factors for the incidence of osteoporotic disease. It is not only important to evaluate the BMI but also and above to evaluate all the body composition by BIA or DXA, which indicate the quantity of muscle, as a gold standard assessment, present and bone mineralometry in order to verify the presence of sarcopenia or osteoporosis. In these situations, nutrition can be useful as prevention and treatment.
In conclusion, COPD patients often present with malnutrition, sarcopenia, osteoporosis with the possibility of the onset of cachexia, with an inadequate dietary intake and a poor quality of life.19 Thus, the state of nutrition and the body composition of COPD patients must be assessed in order to provide adequate nutritional counseling. In particular, attention must paid to the number of meals and their quality in order to assess the total energy intake and the contribution of macro and micronutrients, so as to set an adequate and personalized dietary plan to obtain an improvement in nutritional status.19,43,44 According to a recent official statement by the American Thoracic Society (ATS)/European Respiratory Society (ERS), in the context of non-pharmacological therapies, the objective assessment of nutritional status must be considered as an integral part of the management of the respiratory patient, with particular attention to the analysis of the muscular compartment, both respiratory and peripheral muscles.45
Given this background, the present review aimed to evaluate the existing evidence regarding optimum diet therapy for the management of inflammation, oxidative stress and respiratory gas exchanges in subjects with COPD.
Materials and Methods
The present narrative review was performed following the steps of Egger et al46 as follows:
1. Configuration of a working group: three operators skilled in clinical nutrition, of whom one acting as a methodological operator and two participating as clinical operators.
2. Formulation of the revision question on the basis of considerations made in the abstract:
the state of the art on dietary management of COPD patients in order to manage the carbon dioxide produced and oxygen consumed, other than management of inflammation and oxidative stress.
3. Identification of relevant studies: a research strategy was planned on PubMed, Public MedIine run by the National Center of Biotechnology Information (NCBI) of the National Library of Medicine of Bethesda (USA), as follows:
a) definition of the key words: COPD, foods, inflammation, oxidative stress, nutrients, respiratory gas exchanges allowing the definition of the interest field of the documents to be searched, grouped in inverted commas (“ … ”) and used separately or in combination;
b) use of: the Boolean (a data type with only two possible values: true or false) AND operator, which allows the establishments of logical relations among concepts;
c) research modalities: advanced search;
d) limits: time limits: papers published in the last 20 years; humans; languages: English;
e) manual search performed by senior researchers who are experienced in clinical nutrition through the revision of reviews and individual articles on the management of respiratory gas exchanges, inflammation and oxidative stress by dietary approach in COPD published in journals qualified in the Index Medicus.
Results
This review included 77 eligible studies and the dedicated flowchart is shown in Figure 1.
 |
Figure 1 Flow diagram of the study. |
Carbohydrates
This research was conducted based on the keywords: “chronic obstructive pulmonary disease “AND “inflammation” AND “carbohydrates” AND “diet” AND “gas exchanges”; four articles were sourced: all clinical studies.
Lipids (OMEGA-3)
This research was conducted based on the keywords: “chronic obstructive pulmonary disease “AND “inflammation” AND “lipids” AND “PUFA” AND “omega-3”; six articles were sourced: two cross-sectional investigation; one clinical study; one placebo controlled, randomized, double blind study; one systematic review and a meta-analysis; one observational study.
Protein
This research was conducted based on the keywords: “aging” AND “nutrition” AND “dietary protein” AND “ exercise” AND “physical function”; eleven articles were sourced: seven case-control study; one recommendations from the ESPEN Expert Group’, Clinical Nutrition; one position paper; one review; one single-blind randomized crossover design.
Fiber and Antioxidants
This research was conducted based on the keywords: “chronic obstructive pulmonary disease” AND “fruit” AND “inflammation” AND “oxidative stress” AND “vegetables”; eleven articles were sourced: three were randomized controlled trials; two prospective cohort studies; three cross‐sectional investigations; one multiple linear regression analysis; one clinical study; one case-control study.
Alcohol
This research was conducted based on the keywords: “alcohol consumption ”AND “chronic obstructive pulmonary disease”; three articles were sourced: one cross‐sectional investigation; two prospective cohort study.
Salt/Sodium
This research was carried out based on the keywords: “chronic bronchitis”, AND “chronic obstructive pulmonary disease”, AND “diet” AND “respiratory”. Eleven articles were sourced: three prospective cohort studies; three review; one clinical study; one retrospective study; one “in vitro” study”; ine report; one EFSA reference.
Vitamin D
This research was conducted based on the keywords: “”pulmonary function” AND “respiratory tract infection” AND “inflammation” AND “oxidative stress” AND “vitamin D” OR “25 hydroxyvitamin D” OR “25(OH)D” AND “vitamin D deficiency”; nine articles were sourced, as follows: three cross‐sectional investigations; four randomized clinical trial studies; one prospective cohort study; one clinical study.
Dietary Supplement or Foods for Specified Medical Purpose
This research was conducted based on the keywords: “dietary supplement” AND “chronic obstructive pulmonary disease ”AND “pulmonary rehabilitation”; four articles were sourced: two systematic reviews and meta-analysis; one prospective randomized and controlled study; one clinical study.
It was therefore decided to graphically represent in a simple and intuitive way what should be proper nutrition for the COPD patient, specifying the quality and amount of food, in order to counter the states of chronic inflammation and increased oxidative stress, along with the management of carbon dioxide produced and oxygen consumed.
This pyramid, presented in Figure 2, is divided into three parts as follows:
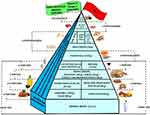 |
Figure 2 Food pyramid for the dietary management of COPD. |
(a) foods that should be consumed daily;
(b) foods that must be consumed 1, 2 or 4 times per week;
(c) foods to be eaten occasionally;
The food amounts are estimates based on nutritional and practical considerations.
The pennant at the top of the pyramid means that COPD subjects need a specific customized dietary supplementation with Vitamin D, n-3 fatty acids and antioxidants, such as Vitamin C.
Discussion
Carbohydrate
It is necessary to take into account the known respiratory difficulties in patients with COPD, and consequently evaluate the impact of nutrition on the production of carbon dioxide (V’CO2). High-calorie intakes, especially those with high carbohydrate content, increase V’CO2 and can precipitate respiratory failure in patients with severe lung disease.47 The respiratory quotient (RQ) is the ratio of CO2 production to O2 consumption. An RQ of 1 indicates 100% oxidation of carbohydrates, while the general value of 0.7 indicates the oxidation of 100% of lipids; this means that the production of CO2 is greater by carbohydrates than lipids. Increased CO2 production leads to an increase in alveolar ventilation with a greater workload from the respiratory system.47 Respiratory impairment, rather than guaranteeing oxygen intake, does not succeed in reducing, with an adequate exchange, the excessive concentration of carbon dioxide. Correct nutrition has a decisive role in these patients, both to rebalance nutritional status and to achieve that which is the priority objective in the presence of hypercapnia, ie the reduction of CO2 levels. This objective, which cannot be achieved by increasing the elimination of CO2, can be achieved by reducing the production of CO2 and optimizing the metabolic conditions of the patient. Respiratory impairment, rather than guaranteeing oxygen intake, does not succeed in reducing, with an adequate exchange, the excessive concentration of carbon dioxide. Several studies have proposed a lower percentage of carbohydrates in the diet in favor of a higher percentage of lipids; in fact, it seems that a diet rich in lipids is more advantageous for the patient with COPD than a diet with a high content of carbohydrates for ventilatory exchange,48 as a reduced calorie intake of carbohydrates reduces all unfavorable physiological anomalies in chronic respiratory failure.49 This data is also confirmed by other research in which a 22% improvement in forced vital capacity (CVF or FVC) and maximum expiratory volume in 1 second (FEV1) was obtained with a low carbohydrate diet, equal to 28%, with 55% of lipids.50 Another study found that with a carbohydrate intake not exceeding 200 g per day (with further improvement when the total of carbohydrates does not exceeded 50 g per day) resulted in a better general well-being of patients with chronic respiratory insufficiency.51
In conclusion, in COPD patients, it is useful to reduce the amount of carbohydrates to less than 200 grams of daily integral carbohydrates (equal to 1 portion of pasta or rice or other whole grains [80 g portion], 1 portion of whole meal bread [50 g portion] and 1 portion of whole grains for breakfast [30 g portion]52 in favor of lipids with percentages that can reach up to 55% depending on the severity of the disease. In the initial phase of the disease, it will be useful to increase the lipid part (30–35% of total calories) with a controlled amount of carbohydrates (45–50 %).
Lipids (OMEGA-3)
Increasing the daily lipid quota, it will be necessary to choose the quality. Although the topic is still being studied, some authors have positively evaluated the intake of polyunsaturated fatty acids (PUFA), given their anti-inflammatory action, and the establishment of a condition of chronic inflammation typical of the disease itself.53
When it comes to inflammation, some authors investigated for the first time in 2012 the existence of an association between consumption of omega-3 and omega-6 fatty acids using a food frequency questionnaire and some inflammatory blood markers in patients with COPD who are clinically stable. The results showed that a higher intake of ALA (α-linolenic acid, an essential fatty acid, founder of the omega-3, anti-inflammatory) was associated with lower levels of TNF-α, while higher assumptions of AA (arachidonic acid, pro-inflammatory omega-6) was correlated with higher concentrations of IL-6 and CRP.54 High levels of blood DHA (decosaesanoic acid, a semi-essential fatty acid) have been shown to be useful in reducing the risk of developing COPD, and therefore having a preventive role for chronic inflammatory conditions of the lung.55 More general intake of omega 3 fatty acids was associated with a higher FEV1,56 and thus also assuming a role in the treatment of the disease.
Furthermore, PUFAs (polyunsaturated fatty acids) have shown beneficial effects on the ability to respond to exercise in patients with COPD, during a targeted rehabilitation program57 and also specific improvement in the 6-minute walking test.58
In summary, lipids should be well represented and omega-3 fatty acids (α-linolenic acid, docosahexaenoic acid, eicosapentaenoic acid), with their anti-inflammatory action, are useful both in the prevention of the risk and treatment of the chronic inflammatory state that occurs in patients. Omega 3 has also proved useful in improving the response to exercise. So, in COPD patients, the diet should contain a higher percentage of lipids (50%, preferably omega-3: fish, 4 servings/week; seeds, nuts: 30 grams/day). The risks regarding dioxin and polychlorinated biphenyls (PCB) intake are small and the health benefits are greater than, or at least similar to, the health risks, as recently demonstrated in a study that evaluated quantitative benefit-risk assessment.59
Protein
Disturbances in intermediary protein metabolism have been demonstrated in patients with COPD. Yoneda T found in underweight COPD patients a decrease in plasma levels of branched-chain amino acids (BCAA) in relation to hypermetabolism, possibly resulting from the severity of COPD and respiratory muscle weakness, and various disturbances in plasma amino-acid levels.60
The results of the study by Yoneda T confirm previous studies.61,62
This low plasma BCAA levels in COPD are associated with disturbances in muscle energy metabolism during exercise,63 suggesting that preservation of BCAA levels is of importance in COPD. In contrast, COPD patients with preserved body weight have increased leucine turnover64 and preserved plasma leucine levels.62
Given this background, various studies assessed the effectiveness of multi-supplementation (milk protein, hydrolyzed casein, whey protein, soy protein, amino acids) on whole body protein anabolism in COPD patients, but conflicting results were found.65–67
However, although this topic has been studied extensively, there are no specific indications regarding amino acids/protein intake or supplementation in patients with COPD. So, considering that it is estimated that the percentage of sarcopenic COPD patients ranges from 12 to 39%, for the prevention of sarcopenia, a protein quota of 1–1.2 g/kg/body weight is recommended, while in the patient with overt sarcopenia the protein intake should be 1.5 g/kg of body weight, as reported in the guidelines of the European artificial nutrition society68 and in the position paper of the PROT-AGE study group.69
These indications are also reiterated in a recent review of 2019.28
So, in COPD patients, the diet should contain foods of animal origin (white meat: 3 portions/week, red meat: 1 portion/week, milk and yogurt: 1 portion/day, twice a week cheese, eggs: 2 portions/week, legumes: 2–3 portions/week, fish, 4 servings/week) to prevent or treat muscle loss.
Fiber and Antioxidants
In addition to polyunsaturated fatty acids, another important nutritional factor is the consumption of fruit and vegetables, as they are rich in fiber and antioxidants. While the increased intake of fruit and vegetables for a short time (12 weeks) did not lead to significant improvements in markers of systemic inflammation, airways and oxidative stress,70 long-term consumption of fruit, investigated with a food frequency questionnaire, was inversely associated with the incidence of COPD;71 in addition to fruit, fiber intake, through the consumption of whole grains, was associated with a lower incidence of new COPD diagnoses.5 The same conclusion emerged from the Morgen study, lasting 3 years, during which a questionnaire was administered to 13,651 subjects, with results showing an indirect beneficial association between the consumption of whole grains (> 45 g/day) and fruit (> 180 g/day) with the incidence of COPD.2 Lifestyle changes, with increased consumption of fruit and vegetables compared to the usual diet, improved lung function.72 Vice versa, a reduction in fruit consumption has proven to lead to a deterioration of FEV1.73 A strong inverse association between total fiber consumption and the incidence of COPD in smokers and ex-smokers was also observed.71 Vitamin C and Vitamin E have been studied as antioxidant factors. Blood levels of vitamin C and E were lower in patients with COPD.74 Vitamin C intake was positively correlated with FEV1,75 while vitamin E supplementation (400 IU per day) reduced blood markers of oxidative stress.76 Furthermore, a nutritional supplement characterized by the presence of both, with specific contributions equal to 180 mg/day of vitamin C and 30 mg day α-tocopherol, associated with zinc (15 mg/day) and selenium (50 μg/day) in patients with COPD undergoing pulmonary rehabilitation treatment showed significant improvements in muscle strength, suggesting a potential “add-on” effect.77 Other anti-oxidants, such as beta-cryptoxanthin, lutein zeaxanthin, retinol, beta-carotene and lycopene, both blood and food, were positively associated with FEV1%, supporting the hypothesis that an imbalance in the state antioxidant/oxidant is associated with chronic airflow limitation.78
In conclusion, it is favorably useful to modify the lifestyle by adding (a) large quantities of fruit, vegetables (5 portions per day: 3 of fruit and 2 of vegetables, 1 raw and cooked),52 (b) whole foods, to increase the fiber share,79 and (c) antioxidants in order to improve FEV1 and oxidative stress. Particular attention should be paid to the consumption of Vitamin C. The foods that contain the greatest quantities are citrus fruits, red fruits, kiwi, peppers, tomatoes, spinach, broccoli, lettuce.79 Another vitamin that is particularly required is Vitamin E, which is highly available when contained in extra virgin olive oil, wheat germ, dried fruit.79 Two minerals that are highly recommended by the examined studies are zinc, which is available in meat, fish, eggs, dairy products, seeds and legumes79 and selenium, which is present in foods of marine origin.79
Finally, it is important to note that these recommendations on vitamins were mainly derived from isolated studies, not from randomized-controlled studies, so such recommendations could be true for a minority of the patients, and should not be generalized.
Alcohol
Alcohol intake was also taken into consideration in the examined studied. Within the MORGEN Study, in addition to the intake of fruit and whole grains, the intake of alcohol in small quantities was positively correlated with COPD; in subjects who declared consumption between 1–30 g/day, a higher FEV1 is described and a lower prevalence of symptoms compared to those who did not consume alcohol or those who consumed more.2
Some authors demonstrated in 2012 a positive association between white wine intake and higher levels of FEV1 in the general population with a consequent lower risk of moderate airway obstruction.80
In another recent prospective cohort study carried out on more than 44,000 Swedish men, the lowest risk of COPD incidence was highlighted in subjects who declared, through a food frequency questionnaire, alcohol consumption between 18 and 23.9 g of ethanol per day.81
In conclusion, the protective role of moderate alcohol intake is confirmed, in the proportion of 125 mL of wine per day.52
Salt/Sodium
The quantity of sodium intake, as often happens in pathological conditions, must be kept under control.
Sodium is unfortunately well represented in the diet called “western diet” (mainly based on the consumption of fast foods, packaged foods, red meats and processed meats, sweets and carbonated and/or sweetened drinks) which, as already explained, are related to risk of COPD.3,5-7
Moreover, there is some evidence, even as the topic is under debate,82 that suggests a high sodium intake may accentuate airway reactivity and reduce flows,83 through potentiation of the electrogenic sodium pump in the membrane of the airway smooth muscle.84,85 Furthermore, the contractile response of airway smooth muscle cells to specific antigen has been demonstrated to be dependent on the level of hyperpolarization resulting from sodium influx.86 Therefore, a diet with a high salt content could predispose people toward the development of airway disease, particularly airway hyperreactivity.
Finally, a study was carried out on the Chinese population of Singapore, in which there was a 1.4-fold increase in the risk of cough with phlegm in subjects who had a meat-rich diet for a short period of time, or preserved or fried foods and noodles.87
An intake of about 2 g of sodium is the amount recommended by EFSA.88
Dietary Supplements
On top of the food pyramid for the dietary management of COPD, there is a pennant to draw attention to the fact that subjects with COPD require special dietary supplements: Vitamin D, n-3 fatty acids, antioxidants (Vitamin C and E, selenium, zinc).
Vitamin D
Several studies suggest that patients with COPD whose vitamin D (25-OH) values are lower than 20 ng/mL (deficient) may be at greater risk of exacerbations of the disease, worsening pulmonary function and decline in lung function over time.89–94 In subjects with severe deficiency (blood Vitamin D values <10 ng/mL) who received supplementation with Vitamin D at high doses (100,000 IU per4 weeks), a reduction in exacerbations was demonstrated93 and an improvement in FEV1 in patients with severe and very severe COPD was observed (100,000 IU once a month for 6 months).95 Daily Vitamin D supplementation has a significant effect in reducing the number of acute exacerbations when it is given for a prolonged period (2000 IU per day for 6 months).96
Foods that contain a good quantity of Vitamin D, such as fish, in particular fish oil and liver, are consumed less, while foods that are consumed daily, such as milk and eggs, are represented in smaller quantities.79 The impact of nutrition on the level of Vitamin D is therefore low and almost all of Vitamin D is synthesized in the skin through adequate sun exposure.
The calcium requirement in adults is 1000 mg per day while in menopausal women and in the elderly it is 1200 mg per day.
The vitamin D requirement in adults is 15 µg per day while in the elderly it is 20µg per day.97
To conclude, it is necessary for all patients with COPD to monitor blood levels of Vitamin D and provide adequate supplementation, and patients should be reminded that since Vitamin D is a fat-soluble vitamin, supplementation should be carried out during a meal in which there are lipids consumed.
Foods for Special Medical Purposes
To promote a significant increase in weight and muscle strength in malnourished patients, it is not enough to have a correct personalized dietary approach that brings adequate amounts of macro- and micronutrients; it is also important to get nutritional supplementation with foods for special medical purposes.43,44 In subjects who received a combination of nutritional supplementation with respiratory rehabilitation, improvements were noted, particularly in lean mass, compared to respiratory rehabilitation alone.98 Given the greater production of CO2 following carbohydrate intake compared to lipid intake, the ideal nutritional supplementation should mainly contain lipids. A drink rich in carbohydrates compared to a lipid-rich one leads to a significant increase in V’CO2 values with a worsening of performance in the 6-minute walk test with a greater risk of falling.47 It should be emphasized that nutritional supplements are effective when they are needed; normal-fed patients may not respond in the same way to nutritional supplementation.44
Conclusion
In conclusion, for the patient with COPD, it is necessary first of all to provide a nutritional and body composition assessment in order to estimate their needs and then build a personalized normocaloric dietary scheme if the patient is of normal-weight or overweight (BMI between 18 and 30 kg/m2), a high-calorie diet if the patient is malnourished (BMI <18 kg/m2), and a low-calorie diet if the patient is obese (BMI> 30 kg/m2). The diet should contain a higher percentage of lipids (up to about 50%), and a reduced amount of carbohydrates (about 30%) compared to that for healthy patient. The choice of fats should be directed towards polyunsaturated fats, preferably omega-3 (fish: 4 servings per week, seeds and nuts; 30 grams per day). The supply of antioxidants must be constant, especially with the use of extra virgin olive oil (2–3 servings per day of 10 mL) and nuts (rich in Vitamin E) in the portion of 30 grams per day, 5 portions in between fruits and vegetables every day, in particular citrus fruits, kiwis, red fruits, peppers, tomatoes, spinach, broccoli, lettuce due to the high Vitamin C content, and foods of animal origin such as meat (3 portions a week of white meat, 1 portion per week of red meat), milk (daily 1 portion of milk and 1 of yogurt, twice a week cheese), eggs (2 portions per week), legumes (2–3 portions per week) and fish (4 portions per week) for the high zinc and selenium content.
Also, the fiber must be well represented, with whole grains, in the quantity of at least 25 g per day.97
The protein content must be significantly present to prevent or treat muscle loss. In the former case, the protein intake must be 1–1.2 g/kg/lost body, while in the second case the proteins must be equal 1.5 g/kg/lost body with a specific supply of leucine equal to 2.5–2.8 g (meat, cheese, fish, eggs).
The calcium content will have to cover the estimated needs (1000 mg for the adult, 1200 mg for menopausal women), to prevent osteoporosis, through the daily consumption of water rich in calcium (2 liters), milk, yogurt and bi-weekly consumption of cheeses. It will also be necessary to evaluate the possibility of initiating specific integration with Vitamin D in the event of proven deficiency or insufficient levels.
The need to insert a food for special medical purposes specific for the disease with a quantity of lipids higher than that of carbohydrates will have to be assessed individually, through a personalized nutritional evaluation.
Two examples of diet (the first with 50% of lipids and 30% of carbohydrates, while in the second diet the percentages of lipids and carbohydrates are inverted.), with the related bromatological analysis, are presented in Tables 1 and 2.
 |
Table 1 Bromatological Analysis and Example of Diet with 50% of Lipids and 30% of Carbohydrates |
 |
Table 2 Bromatological Analysis and Example of Diet with 30% of Lipids and 50% of Carbohydrates |
In conclusion, the nutrition pyramid described in our narrative review is hypothetical, even in light of several limitations of the present review; the main limitation is the fact that to date there are no randomized controlled trials in the literature clearly showing that improved nutrition, via the regulation of carbon dioxide produced/oxygen consumed, inflammation and oxidative stress, improves symptoms and/or the progression of COPD. Further, to build the pyramid, we could only make a narrative review of the literature, not a meta-analysis.
Even if this nutritional pyramid is hypothetical, we hope that it can serve the valuable purpose of helping researchers focus on the often-ignored possible connections between nutrition and COPD. Further investigation is needed in the future and, specifically, more randomized clinical trials should be conducted that directly study nutrition and symptoms and/or progression of COPD in order to understand the specific mechanisms that interconnect the regulation of carbon dioxide produced/oxygen consumed, inflammation, oxidative stress and nutrition.
Abbreviation
AA, arachidonic acid; ALA, α-linolenic acid; ATS, American Thoracic Society; BCAA, branched-chain amino acids; BEE, Basal Energy Expenditure; BIA, bioempedentiometry; BMI, body mass index; BODE, Body-mass index, airflow Obstruction, Dyspnea, and Exercise; COPD, Chronic obstructive pulmonary disease; CRP, C-reactive protein; DHA, decosaesanoic acid; DXA, double-beam X densitometer; EFSA, European Food Safety Authority; ERS, European Respiratory Society; ESPEN, European Society for Clinical Nutrition and Metabolism; FEV1, maximum expiratory volume in 1 second; FFM, Fat Free Mass; FM, Fat Mass; FVC (or CVF), forced vital capacity; g, grams; kcal, kilocalories; Kg, kilograms; mg, milligrams; m, meter; NCBI, National Center of Biotechnology Information; NF-kB, Nuclear Factor kappa-light-chain-enhancer of activated B cells; PAL, Physical Activity Levels; PCB, polychlorinated biphenyls; PUFA, polyunsaturated fatty acids; RQ, respiratory quotient; SPPB, Short Physical Performance Battery; TEE, total energy expenditure; TNF-α, tumor necrosis factor α; V’CO2 - CO2, carbon dioxide; μg, micrograms.
Acknowledgments
The authors want to thank Dr. Antonella Riva and Dr. Giovanna Petrangolini, from INDENA SpA, as they have dealt with the revision for English language.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
All authors report no conflicts of interest in this work.
References
1. Willett WC, Sacks F, Trichopoulou A, et al. Mediterranean diet pyramid: a cultural model for healthy eating. Am J Clin Nutr. 1995;61(6):1402S–1406S. doi:10.1093/ajcn/61.6.1402S
2. Tabak C, Smit HA, Heederik D, Ocké MC, Kromhout D. Diet and chronic obstructive pulmonary disease: independent beneficial effects of fruits, whole grains, and alcohol (the MORGEN study). Clin Exp Allergy. 2001;31(5):747–755. doi:10.1046/j.1365-2222.2001.01064.x.
3. Varraso R, Fung TT, Barr RG, Hu FB, Willett W, Camargo CA. Prospective study of dietary patterns and chronic obstructive pulmonary disease among US women. Am J Clin Nutr. 2007;86(2):488–495. doi:10.1093/ajcn/86.2.488
4. Varraso R, Kauffmann F, Leynaert B, et al. Dietary patterns and asthma in the E3N study. Eur Respir J. 2009;33(1):33–41. doi:10.1183/09031936.00130807
5. Varraso R, Chiuve SE, Fung TT, et al. Alternate Healthy Eating Index 2010 and risk of chronic obstructive pulmonary disease among US women and men: prospective study. BMJ. 2015;350(feb03 7):h286–h286. doi:10.1136/bmj.h286
6. Wood LG, Garg ML, Gibson PG. A high-fat challenge increases airway inflammation and impairs bronchodilator recovery in asthma. J Allergy Clin Immunol. 2011;127(5):1133–1140. doi:10.1016/j.jaci.2011.01.036
7. Berthon BS, Wood LG. Nutrition and respiratory health—feature review. Nutrients. 2015;7(3):1618–1643. doi:10.3390/nu7031618
8. McKeever TM, Lewis SA, Cassano PA, et al. Patterns of dietary intake and relation to respiratory disease, forced expiratory volume in 1 s, and decline in 5-y forced expiratory volume. Am J Clin Nutr. 2010;92(2):408–415. doi:10.3945/ajcn.2009.29021
9. Landbo C, Prescott E, Lange P, Vestbo J, Almdal TP. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160(6):1856–1861. doi:10.1164/ajrccm.160.6.9902115
10. Hallin R, Gudmundsson G, Suppli Ulrik C, et al. Nutritional status and long-term mortality in hospitalised patients with chronic obstructive pulmonary disease (COPD). Respir Med. 2007;101(9):1954–1960. doi:10.1016/j.rmed.2007.04.009
11. Cao C, Wang R, Wang J, Bunjhoo H, Xu Y, Xiong W. Body mass index and mortality in chronic obstructive pulmonary disease: a meta-analysis. simpson C, ed. PLoS One. 2012;7(8):e43892. doi:10.1371/journal.pone.0043892
12. Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005–1012. doi:10.1056/NEJMoa021322
13. Eisner MD, Blanc PD, Sidney S, et al. Body composition and functional limitation in COPD. Respir Res. 2007;8(1):7. doi:10.1186/1465-9921-8-7
14. Montes de Oca M, Tálamo C, Perez-Padilla R, et al. Chronic obstructive pulmonary disease and body mass index in five Latin America cities: the PLATINO study. Respir Med. 2008;102(5):642–650. doi:10.1016/j.rmed.2007.12.025
15. Lainscak M, von Haehling S, Doehner W, et al. Body mass index and prognosis in patients hospitalized with acute exacerbation of chronic obstructive pulmonary disease. J Cachexia Sarcopenia Muscle. 2011;2(2):81–86. doi:10.1007/s13539-011-0023-9
16. Schols AM, Broekhuizen R, Weling-Scheepers CA, Wouters EF. Body composition and mortality in chronic obstructive pulmonary disease. Am J Clin Nutr. 2005;82(1):53–59. doi:10.1093/ajcn.82.1.53
17. Schols AM, Ferreira IM, Franssen FM, et al. Nutritional assessment and therapy in COPD: A European respiratory society statement. Eur Respir J. 2014;44(6):1504–1520. doi:10.1183/09031936.00070914
18. Filley GF, Beckwitt HJ, Reeves JT, Mitchell RS. Chronic obstructive bronchopulmonary disease. II. Oxygen transport in two clinical types. Am J Med. 1968;44(1):26–38. doi:10.1016/0002-9343(68)90234-9
19. Nguyen HT, Collins PF, Pavey TG, Nguyen NV, Pham TD, Gallegos DL. Nutritional status, dietary intake, and health-related quality of life in outpatients with COPD. Int J Chron Obstruct Pulmon Dis. 2019;14:215–226. doi:10.2147/COPD.S181322
20. Remels AHV, Gosker HR, Langen RCJ, Schols AMWJ. The mechanisms of cachexia underlying muscle dysfunction in COPD. J Appl Physiol. 2013;114(9):1253–1262. doi:10.1152/japplphysiol.00790.2012
21. Steuten LMG, Creutzberg EC, Vrijhoef HJM, Wouters EF. COPD as a multicomponent disease: inventory of dyspnoea, underweight, obesity and fat free mass depletion in primary care. Prim Care Respir J. 2006;15(2):84–91. doi:10.1016/j.pcrj.2005.09.001
22. Vozoris NT, O’Donnell DE. Prevalence, risk factors, activity limitation and health care utilization of an obese population-based sample with chronic obstructive pulmonary disease. Can Respir J. 2012;19(3):e18–e24. doi:10.1155/2012/732618
23. Zapatero A, Barba R, Ruiz J, et al. Malnutrition and obesity: influence in mortality and readmissions in chronic obstructive pulmonary disease patients. J Hum Nutr Diet. 2013;26(SUPPL.1):16–22. doi:10.1111/jhn.12088
24. Jee SH, Sull JW, Park J, et al. Body-mass index and mortality in Korean men and women. N Engl J Med. 2006;355(8):779–787. doi:10.1056/NEJMoa054017
25. Spelta F, Fratta Pasini AM, Cazzoletti L, Ferrari M. Body weight and mortality in COPD: focus on the obesity paradox. Eat Weight Disord Stud Anorexia Bulim Obes. 2018;23(1):15–22. doi:10.1007/s40519-017-0456-z
26. Van Borst B, Den, Gosker HR, Koster A, et al. The influence of abdominal visceral fat on inflammatory pathways and mortality risk in obstructive lung disease. Am J Clin Nutr. 2012;96(3):516–526. doi:10.3945/ajcn.112.040774
27. Ganzoni A, Heilig P, Schönenberger K, Hügli O, Fitting JW, Brändli O. High-caloric nutrition in chronic obstructive lung disease. Schweiz Rundsch Med Prax. 1994;83(1):13–16.
28. Collins PF, Yang IA, Chang Y-C, Vaughan A. Nutritional support in chronic obstructive pulmonary disease (COPD): an evidence update. J Thorac Dis. 2019;11(Suppl 17):S2230–S2237. doi:10.21037/jtd.2019.10.41
29. Baarends EM, Schols AMWJ, Pannemans DLE, Westerterp KR, Wouters EFM. Total free living energy expenditure in patients with severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1997;155(2):549–554. doi:10.1164/ajrccm.155.2.9032193
30. Wilson DO, Donahoe M, Rogers RM, Pennock BE. Metabolic rate and weight loss in chronic obstructive lung disease. J Parenter Enter Nutr. 1990;14(1):7–11. doi:10.1177/014860719001400107
31. Schols AMWJ, Fredrix EWHM, Soeters PB, Westerterp KR, Wouters EFM. Resting energy expenditure in patients with chronic obstructive pulmonary disease. Am J Clin Nutr. 1991;54(6):983–987. doi:10.1093/ajcn/54.6.983
32. Nordenson A, Grönberg AM, Hulthén L, Larsson S, Slinde F. A validated disease specific prediction equation for resting metabolic rate in underweight patients with COPD. Int J Chron Obstruct Pulmon Dis. 2010;5:271–276. doi:10.2147/copd.s12544
33. Slinde F, Grönberg AM, Svantesson U, Hulthén L, Larsson S. Energy expenditure in chronic obstructive pulmonary disease-evaluation of simple measures. Eur J Clin Nutr. 2011;65(12):1309–1313. doi:10.1038/ejcn.2011.117
34. Barreiro E, Rabinovich R, Marin-Corral J, Barberà JA, Gea J, Roca J. Chronic endurance exercise induces quadriceps nitrosative stress in patients with severe COPD. Thorax. 2009;64(1):13–19. doi:10.1136/thx.2008.105163
35. Sharma R, Anker SD. Cytokines, apoptosis and cachexia: the potential for TNF antagonism. Int J Cardiol. 2002;85(1):161–171. doi:10.1016/S0167-5273(02)00244-9.
36. Langen RCJ, Haegens A, Vernooy JHJ, et al. NF-κB activation is required for the transition of pulmonary inflammation to muscle atrophy. Am J Respir Cell Mol Biol. 2012;47(3):288–297. doi:10.1165/rcmb.2011-0119OC
37. Costa TM, Da RL, Costa FM, et al. Sarcopenia in COPD: relationship with COPD severity and prognosis. J Bras Pneumol. 2015;41(5):415–421. doi:10.1590/S1806-37132015000000040
38. Jones P, Dalziel, SR, Lamdin R, et al. Oral non-steroidal anti-inflammatory drugs versus other oral analgesic agents for acute soft tissue injury. Cochrane Database Syst Rev. 2015;2015(7). doi:10.1002/14651858.CD007789.pub2
39. Byun MK, Cho EN, Chang J, Ahn CM, Kim HJ. Sarcopenia correlates with systemic inflammation in COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:669–675. doi:10.2147/COPD.S130790
40. de Blasio F, Di Gregorio A, de Blasio F, Bianco A, Bellofiore B, Scalfi L. Malnutrition and sarcopenia assessment in patients with chronic obstructive pulmonary disease according to international diagnostic criteria, and evaluation of raw BIA variables. Respir Med. 2018;134:1–5. doi:10.1016/j.rmed.2017.11.006
41. EL-Gazzar AG, Abdalla ME, Almahdy MA. Study of Osteoporosis in chronic obstructive pulmonary disease. Egypt J Chest Dis Tuberc. 2013;62(1):91–95. doi:10.1016/J.EJCDT.2013.01.009
42. Graat-Verboom L, Smeenk FWJM, Van Den Borne BEEM, et al. Progression of osteoporosis in patients with COPD: a 3-year follow up study. Respir Med. 2012;106(6):861–870. doi:10.1016/j.rmed.2011.12.020
43. Collins PF, Stratton RJ, Elia M. Nutritional support in chronic obstructive pulmonary disease: a systematic review and meta-analysis. Am J Clin Nutr. 2012;95(6):1385–1395. doi:10.3945/ajcn.111.023499
44. Ferreira IM, Brooks D, White J, Goldstein R. Nutritional supplementation for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;12:CD000998. doi:10.1002/14651858.CD000998.pub3
45. Celli BR, Decramer M, Wedzicha JA, et al. An official american thoracic society/european respiratory society statement: research questions in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;191(7):e4–e27. doi:10.1164/rccm.201501-0044ST
46. Egger M, Smith GD, Altman DG. Systematic Reviews in Health Care: Meta-Analysis in Context. BMJ Books; 2001.
47. Efthimiou J, Mounsey PJ, Benson DN, Madgwick R, Coles SJ, Benson MK. Effect of carbohydrate rich versus fat rich loads on gas exchange and walking performance in patients with chronic obstructive lung disease. Thorax. 1992;47(6):451–456. doi:10.1136/thx.47.6.451
48. Kuo CD, Shiao GM, Lee JD. The effects of high-fat and high-carbohydrate diet loads on gas exchange and ventilation in COPD patients and normal subjects. Chest. 1993;104(1):189–196. doi:10.1378/chest.104.1.189
49. Tirlapur VG, Mir MA. Effect of low calorie intake on abnormal pulmonary physiology in patients with chronic hypercapneic respiratory failure. Am J Med. 1984;77(6):987–994. doi:10.1016/0002-9343(84)90177-3
50. Angelillo VA, Bedi S, Durfee D, Dahl J, Patterson AJ, O’Donohue WJ. Effects of low and high carbohydrate feedings in ambulatory patients with chronic obstructive pulmonary disease and chronic hypercapnia. Ann Intern Med. 1985;103(6 (Pt 1)):883–885. doi:10.7326/0003-4819-103-6-883
51. Kwan R, Mir MA. Beneficial effects of dietary carbohydrate restriction in chronic cor pulmonale. Am J Med. 1987;82(4):751–758. doi:10.1016/0002-9343(87)90011-8
52. SINU. Standard quantitativi delle porzioni. Available from: http://www.sinu.it/public/20141111_LARN_Porzioni.pdf.
53. Gan WQ, Man SFP, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax. 2004;59(7):574–580. doi:10.1136/thx.2003.019588
54. de Batlle J, Sauleda J, Balcells E, et al. Association between Ω3 and Ω6 fatty acid intakes and serum inflammatory markers in COPD. J Nutr Biochem. 2012;23(7):817–821. doi:10.1016/j.jnutbio.2011.04.005
55. Shahar E, Boland LL, Folsom AR, Tockman MS, McGovern PG, Eckfeldt JH. Docosahexaenoic acid and smoking-related chronic obstructive pulmonary disease. The atherosclerosis risk in communities study investigators. Am J Respir Crit Care Med. 1999;159(6):1780–1785. doi:10.1164/ajrccm.159.6.9810068
56. Garcia-Larsen V, Amigo H, Bustos P, Bakolis I, Rona RJ. Ventilatory function in young adults and dietary antioxidant intake. Nutrients. 2015;7(4):2879–2896. doi:10.3390/nu7042879
57. Broekhuizen R, Wouters EFM, Creutzberg EC, Weling-Scheepers CAPM, Schols AMWJ. Polyunsaturated fatty acids improve exercise capacity in chronic obstructive pulmonary disease. Thorax. 2005;60(5):376–382. doi:10.1136/thx.2004.030858
58. Matsuyama W, Mitsuyama H, Watanabe M, et al. Effects of omega-3 polyunsaturated fatty acids on inflammatory markers in COPD. Chest. 2005;128(6):3817–3827. doi:10.1378/chest.128.6.3817
59. Tuomisto JT, Asikainen A, Meriläinen P, Haapasaari P. Health effects of nutrients and environmental pollutants in Baltic herring and salmon: a quantitative benefit-risk assessment. BMC Public Health. 2020;20(1):64. doi:10.1186/s12889-019-8094-1
60. Yoneda T, Yoshikawa M, Fu A, Tsukaguchi K, Okamoto Y, Takenaka H. Plasma levels of amino acids and hypermetabolism in patients with chronic obstructive pulmonary disease. Nutrition. 2001;17(2):95–99. doi:10.1016/S0899-9007(00)00509-8
61. Pouw EM, Schols AM, Deutz NE, Wouters EF. Plasma and muscle amino acid levels in relation to resting energy expenditure and inflammation in stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;158(3):797–801. doi:10.1164/ajrccm.158.3.9708097
62. Engelen MP, Wouters EF, Deutz NE, Menheere PP, Schols AM. Factors contributing to alterations in skeletal muscle and plasma amino acid profiles in patients with chronic obstructive pulmonary disease. Am J Clin Nutr. 2000;72(6):1480–1487. doi:10.1093/ajcn/72.6.1480
63. Kutsuzawa T, Shioya S, Kurita D, Haida M. Plasma branched-chain amino acid levels and muscle energy metabolism in patients with chronic obstructive pulmonary disease. Clin Nutr. 2009;28(2):203–208. doi:10.1016/j.clnu.2009.01.019
64. Kao CC, Hsu JWC, Bandi V, Hanania NA, Kheradmand F, Jahoor F. Resting energy expenditure and protein turnover are increased in patients with severe chronic obstructive pulmonary disease. Metabolism. 2011;60(10):1449–1455. doi:10.1016/j.metabol.2011.02.013
65. Jonker R, Deutz NEP, Schols AMWJ, et al. Whole body protein anabolism in COPD patients and healthy older adults is not enhanced by adding either carbohydrates or leucine to a serving of protein. Clin Nutr. 2019;38(4):1684–1691. doi:10.1016/j.clnu.2018.08.006
66. Engelen MPKJ, De Castro CLN, Rutten EPA, Wouters EFM, Schols AMWJ, Deutz NEP. Enhanced anabolic response to milk protein sip feeding in elderly subjects with COPD is associated with a reduced splanchnic extraction of multiple amino acids. Clin Nutr. 2012;31(5):616–624. doi:10.1016/j.clnu.2012.04.006
67. Engelen MPKJ, Rutten EPA, De Castro CLN, Wouters EFM, Schols AMWJ, Deutz NEP. Supplementation of soy protein with branched-chain amino acids alters protein metabolism in healthy elderly and even more in patients with chronic obstructive pulmonary disease. Am J Clin Nutr. 2007;85(2):431–439. doi:10.1093/ajcn/85.2.431
68. Deutz NEP, Bauer JM, Barazzoni R, et al. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin Nutr. 2014;33(6):929–936. doi:10.1016/j.clnu.2014.04.007
69. Bauer J, Biolo G, Cederholm T, et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the prot-age study group. J Am Med Dir Assoc. 2013;14(8):542–559. doi:10.1016/j.jamda.2013.05.021
70. Baldrick FR, Elborn JS, Woodside JV, et al. Effect of fruit and vegetable intake on oxidative stress and inflammation in COPD: a randomised controlled trial. Eur Respir J. 2012;39(6):1377–1384. doi:10.1183/09031936.00086011
71. Kaluza J, Harris H, Wallin A, Linden A, Wolk A. Dietary fiber intake and risk of chronic obstructive pulmonary disease: a prospective cohort study of men. Epidemiology. 2018;29(2):254–260. doi:10.1097/EDE.0000000000000750
72. Keranis E, Makris D, Rodopoulou P, et al. Impact of dietary shift to higher-antioxidant foods in COPD: a randomised trial. Eur Respir J. 2010;36(4):774–780. doi:10.1183/09031936.00113809
73. Carey IM, Strachan DP, Cook DG. Effects of changes in fresh fruit consumption on ventilatory function in healthy British adults. Am J Respir Crit Care Med. 1998;158(3):728–733. doi:10.1164/ajrccm.158.3.9712065
74. Lin Y-C, Wu T-C, Chen P-Y, Hsieh L-Y, Yeh S-L. Comparison of plasma and intake levels of antioxidant nutrients in patients with chronic obstructive pulmonary disease and healthy people in Taiwan: a case-control study. Asia Pac J Clin Nutr. 2010;19(3):393–401.
75. Schwartz J, Weiss ST. Relationship between dietary vitamin C intake and pulmonary function in the first national health and nutrition examination survey (NHANES I). Am J Clin Nutr. 1994;59(1):110–114. doi:10.1093/ajcn/59.1.110
76. Daga MK, Chhabra R, Sharma B, Mishra TK. Effects of exogenous vitamin E supplementation on the levels of oxidants and antioxidants in chronic obstructive pulmonary disease. J Biosci. 2003;28(1):7–11. doi:10.1007/BF02970125.
77. Gouzi F, Maury J, Héraud N, et al. Additional effects of nutritional antioxidant supplementation on peripheral muscle during pulmonary rehabilitation in COPD patients: a randomized controlled trial. Oxid Med Cell Longev. 2019;2019:5496346. doi:10.1155/2019/5496346
78. Ochs-Balcom HM, Grant BJB, Muti P, et al. Antioxidants, oxidative stress, and pulmonary function in individuals diagnosed with asthma or COPD. Eur J Clin Nutr. 2006;60(8):991–999. doi:10.1038/sj.ejcn.1602410
79. BDA IEO. Banca Dati di Composizione degli Alimenti per studi epidemiologici in Italia; 2015. Available from: http://www.bda-ieo.it.
80. Siedlinski M, Boer JMA, Smit HA, Postma DS, Boezen HM. Dietary factors and lung function in the general population: wine and resveratrol intake. Eur Respir J. 2012;39(2):385–391. doi:10.1183/09031936.00184110
81. Kaluza J, Harris HR, Linden A, Alcohol Consumption WA. Risk of chronic obstructive pulmonary disease: a prospective cohort study of men. Am J Epidemiol. 2019;188(5):907–916. doi:10.1093/aje/kwz020
82. Britton J, Pavord I, Richards K, et al. Dietary sodium intake and the risk of airway hyperreactivity in a random adult population. Thorax. 1994;49(9):875–880. doi:10.1136/thx.49.9.875
83. Romieu I, Trenga C. Diet and obstructive lung diseases. Epidemiol Rev. 2001;23(2):268–287. doi:10.1093/oxfordjournals.epirev.a000806
84. Burney P. A Diet rich in sodium may potentiate asthma. Chest. 1987;91(6):143S–148S. doi:10.1378/chest.91.6_supplement.143s
85. Knox AJ, Ajao P, Britton JR, Tattersfield AE. Effect of sodium-transport inhibitors on airway smooth muscle contractility in vitro. Clin Sci. 1990;79(4):315–323. doi:10.1042/cs0790315
86. Monteleone CA, Sherman AR. Nutrition and asthma. Arch Intern Med. 1997;157(1):23–34. doi:10.1001/archinte.1997.00440220027005.
87. Butler LM, Koh WP, Lee HP, Tseng M, Yu MC, London SJ. Prospective study of dietary patterns and persistent cough with phlegm among Chinese Singaporeans. Am J Respir Crit Care Med. 2006;173(3):264–270. doi:10.1164/rccm.200506-901OC
88. EFSA. Tenori di sodio e cloruro di riferimento per l’alimentazione umanaitle. Available from: https://www.efsa.europa.eu/it/press/news/190403.
89. Black PN, Scragg R. Relationship between serum 25-hydroxyvitamin d and pulmonary function in the third national health and nutrition examination survey. Chest. 2005;128(6):3792–3798. doi:10.1378/chest.128.6.3792
90. Ginde AA, Mansbach JM, Camargo CA. Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the third national health and nutrition examination survey. Arch Intern Med. 2009;169(4):384–390. doi:10.1001/archinternmed.2008.560
91. Shaheen SO, Jameson KA, Robinson SM, et al. Relationship of vitamin D status to adult lung function and COPD. Thorax. 2011;66(8):692–698. doi:10.1136/thx.2010.155234
92. Lange NE, Sparrow D, Vokonas P, Litonjua AA. Vitamin D deficiency, smoking, and lung function in the normative aging study. Am J Respir Crit Care Med. 2012;186(7):616–621. doi:10.1164/rccm.201110-1868OC
93. Lehouck A, Mathieu C, Carremans C, et al. High doses of vitamin D to reduce exacerbations in chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2012;156(2):105–114. doi:10.7326/0003-4819-156-2-201201170-00004
94. Puhan MA, Siebeling L, Frei A, Zoller M, Bischoff-Ferrari H, Ter Riet G. No association of 25-hydroxyvitamin D with exacerbations in primary care patients with COPD. Chest. 2014;145(1):37–43. doi:10.1378/chest.13-1296
95. Zendedel A, Gholami M, Anbari K, Ghanadi K, Bachari EC, Azargon A. Effects of vitamin D intake on FEV1 and COPD exacerbation: a randomized clinical trial study. Glob J Health Sci. 2015;7(4):243–248. doi:10.5539/gjhs.v7n4p243
96. Khan DM, Ullah A, Randhawa FA, Iqtadar S, Butt NF, Waheed K. Role of Vitamin D in reducing number of acute exacerbations in chronic obstructive pulmonary disease (COPD) patients. Pakistan J Med Sci. 2017;33(3):610–614. doi:10.12669/pjms.333.12397
97. SINU. Tabelle Larn. Available from: http://www.sinu.it/html/pag/tabelle_larn_2014_rev.asp.
98. Gurgun A, Deniz S, Argın M, Karapolat H. Effects of nutritional supplementation combined with conventional pulmonary rehabilitation in muscle-wasted chronic obstructive pulmonary disease: a prospective, randomized and controlled study. Respirology. 2013;18(3):495–500. doi:10.1111/resp.12019
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
