Back to Journals » Journal of Asthma and Allergy » Volume 15
Food Allergy-Induced Autism-Like Behavior is Associated with Gut Microbiota and Brain mTOR Signaling
Authors Cao LH, He HJ, Zhao YY, Wang ZZ, Jia XY, Srivastava K , Miao MS, Li XM
Received 22 November 2021
Accepted for publication 30 March 2022
Published 16 May 2022 Volume 2022:15 Pages 645—664
DOI https://doi.org/10.2147/JAA.S348609
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Luis Garcia-Marcos
Li-Hua Cao,1 Hong-Juan He,1 Yuan-Yuan Zhao,2 Zhen-Zhen Wang,1 Xing-Yuan Jia,3 Kamal Srivastava,4,5 Ming-San Miao,1 Xiu-Min Li4,6
1Academy of Chinese Medical Sciences, Henan University of Chinese Medicine, Zhengzhou, 450046, Henan Province, People’s Republic of China; 2School of Pharmacy, Henan University of Chinese Medicine, Zhengzhou, 450046, Henan Province, People’s Republic of China; 3Department of Pharmacy, Henan Province Hospital of Traditional Chinese Medicine, Zhengzhou, 450046, Henan Province, People’s Republic of China; 4Department of Pathology, Microbiology and Immunology, New York Medical College, Valhalla, NY, 10595, USA; 5General Nutraceutical Technology, Elmsford, NY, 10523, USA; 6Department of Otolaryngology, New York Medical College, Valhalla, NY, 10595, USA
Correspondence: Xiu-Min Li; Ming-San Miao, Tel +1 914-594-4197, Fax +1 371-65962546, Email [email protected]; [email protected]
Purpose: Food allergy-induced autism-like behavior has been increasing for decades, but the causal drivers of this association are unclear. We sought to test the association of gut microbiota and mammalian/mechanistic target of rapamycin (mTOR) signaling with cow’s milk allergy (CMA)-induced autism pathogenesis.
Methods: Mice were sensitized intragastrically with whey protein containing cholera toxin before sensitization on intraperitoneal injection with whey-containing alum, followed by intragastric allergen challenge to induce experimental CMA. The food allergic immune responses, ASD-like behavioral tests and changes in the mTOR signaling pathway and gut microbial community structure were performed.
Results: CMA mice showed autism-like behavioral abnormalities and several distinct biomarkers. These include increased levels of 5-hydroxymethylcytosine (5-hmC) in the hypothalamus; c-Fos were predominantly located in the region of the lateral orbital prefrontal cortex (PFC), but not ventral; decreased serotonin 1A in amygdala and PFC. CMA mice exhibited a specific microbiota signature characterized by coordinate changes in the abundance of taxa of several bacterial genera, including the Lactobacillus. Interestingly, the changes were accompanied by promoted mTOR signaling in the brain of CMA mice.
Conclusion: We found that disease-associated microbiota and mTOR activation may thus play a pathogenic role in the intestinal, immunological, and psychiatric Autism Spectrum Disorder (ASD)-like symptoms seen in CAM associated autism. However, this is only a preliminary study, and their mechanisms require further investigation.
Keywords: cow’s milk allergy, autism-like behavior, gut microbiota, mTOR signaling pathway
Introduction
Over the last few decades, food-mediated allergic reactions have become a growing public health concern in the United States, with approximately 10% of the population having been documented.1–3 Food allergies seriously endanger the health of adults and children, causing serious adverse reactions and even death. It has a high incidence among infants and young children, adversely affecting their physical and mental health, and their families experience decreased quality of life. Cow's mike allergy (CMA) is the most common food allergy in children, affecting up to 2–3%.4,5 The clinical symptoms of food allergy, include urticaria, eczema, respiratory tract and gastrointestinal symptoms, and even potentially life-threatening anaphylaxis.6 With CMA, like other allergies, there are no satisfactory therapies available yet, apart from strict avoidance of the allergenic foods.7 There are also challenges in finding suitable substitutes to replace nutrients provided by milk products.8
Accumulating evidence points to the association of food allergy/hypersensitivity with abnormal psychosocial behavior such as Autism Spectrum Disorder (ASD), anxiety, depression, obsessive-compulsive disorder and attention deficit hyperactivity disorder (ADHD).9–14 Nonetheless, the underlying mechanisms of behavioral dysfunction caused by allergic reactions are yet to be determined due to inconsistent results across studies.
ASD, also called autism or autism spectrum disorder, is a complex neurodevelopmental disorder characterized by impaired social communication and interaction, and by repetitive, stereotyped behaviors. Studies have demonstrated pervasive gut dysbiosis in ASD, ie, disturbance in communication between the gut and the brain (referred to as “gut-brain axis”).15–17 The mammalian microbiome can affect behavior and several symbionts even produce neurotransmitters.18 For example, transplantation of gut microbiota from human donors with ASD into germ-free mice resulted in mice displaying autism-like behavior.19 The intestinal bacterial communities between ASD individuals and the normal control group are different, as is the case in ASD mice model.20–22 Interestingly, growing evidence points to the gut microbiota having an important role in the development of food allergy.23–26 It has been suggested the gut microbiota is a target for innovative strategies against food allergy.27 Azza Abdel-Gadir et al underscore the potential for microbial therapies in treating this disorder.28 Such findings could have radical implications for the treatment of ASD and/or food allergy, and the results of the studies are encouraging. However, few studies have focused on the relationship between microbiota signature and allergy-induced autism.
Mammalian/mechanistic target of rapamycin (mTOR) signaling pathway plays a pivotal role in protein synthesis and regulation of brain homeostasis in autism patients.29,30 The mTOR pathway may be a pivotal link between the immune disturbances and behavioral deficits observed in autism.31,32 As a greater body of evidence points toward elevated inflammatory states and immune dysfunction as key characteristics of ASD pathophysiology. There are differences between the immune cytokine profiles such as interleutin (IL)-2, IL-4, IL-5, IL-6, IL-10, tumor necrosis factor (TNF)-α, transforming growth factor (TGF)-β, and interferon (IFN)-γ of individuals with ASD and healthy controls.33,34 IL-33 also plays an important role in allergic diseases35 and it might be useful in treating food-induced anaphylaxis in patients with ASD.36 Yet, their direct role in food allergy associated autism like behavioral abnormality remains to be established.
We hypothesize that perturbation of the gut microbiota leads to changes in the immune system and the gut-brain axis, which may affect the occurrence of autism (allergy-induced autism). In order to explore the contribution of the microbiota to allergy-induced autism-like behavioral etiology, we established a novel model of cow’s mike allergy-induced autism. We investigated the mechanism of action involving the microbial metabolites in the gut that affect brain function and autism-like behavior in association mTOR signaling and CMA reactions.
Materials and Methods
Mice
Three-week-old specific pathogen-free male C3H/He N mice were purchased from the Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China) and maintained under barrier conditions in filter-topped microloan cages with wood-chip bedding. Mice were fed a cow’s milk-free diet (River Laboratory Animal Technology Co., Ltd. Beijing, China). Animals were single-housed under standard laboratory conditions of food and water ad libitum, 22 ± 2°C, a 12 h light–dark cycle (lights on at 08:00) and relative humidity 50–60% unless otherwise specified. The animal study was reviewed and approved by Animal Care and Use Committee of Henan University of Chinese Medicine. All experiments were conducted in accordance with the guidelines of the Animal Care and Use Committee of Henan University of Chinese Medicine. Every effort was made to minimize the number and suffering of animals used.
Whey Sensitization/Challenge Protocol and Assessment of Anaphylaxis
The model of cow’s mike allergy-induced autism was established as described by de Theije’s et al, with modifications.37 Whey is a milk protein fraction composed of several milk allergens (β-lactoglobulin, α-lactalbumin, bovine lactoferrin, bovine serum albumin, and bovine immunoglobulins), some of which are major allergens, and used to induce our food allergy model.38 After 1 week acclimation, mice were sensitized intragastrically (i.g) with 20 mg whey (Hefei Bomei Biotechnology Co., LTD, China) in 0.5 mL of PBS plus 10 μg cholera toxin (CT, List Biological Laboratories, Campbell, CA, USA) as an adjuvant from week 1 through week 5 and oral 100 mg whey containing 10 μg CT from week 6 through week 7. Mice were then sensitized via intraperitoneal injection (i.p.) with 500 μg whey in 0.2 mL of PBS containing 2 mg Alum (ThermoFisher, USA) from week 8 through week 9. Naive mice received PBS alone. Sham mice received CT and alum alone. A schematic for the sensitization and challenge timeline was depicted in Figure 1.
 |
Figure 1 Schematic representation of experimental design (A) and overview of groups (B). |
To further exploit behavior and avoid multiple behavioral testing on 1 day, self-grooming and T maze spontaneous alternation were assessed one and two days after the last sensitization, respectively. One week after the last sensitization, mice were challenged i.g. with 50 mg whey in 0.5 mL of PBS. The day after challenge, mice were exposed to a social behavior test as described below. Anaphylactic symptoms and body temperature were evaluated approximately 30 minutes after the challenge, as described previously:39,40 0, no symptoms; 1-mild reaction, scratching and rubbing around the snout and head; 2-moderate reactions, puffiness around the eyes and snout, diarrhea, pilar erection, reduced activity, and/or decreased activity with increased respiratory rate; 3-severe reactions, wheezing, labored respiration, cyanosis around the mouth and the tail; 4-near fatal, no activity after prodding, or tremor and convulsion; and 5, death.
Blood was collected after cardiac puncture. Serum was prepared by centrifuging at 9168 g for 15 min at 4°C after standing for 30 min at room temperature. Plasma was harvested by centrifuging at 2292 g for 15 min at 4°C within 20 minutes after blood collection. After sacrifice, serum, plasma, brain and intestinal tissues were immediately isolated and stored at −80°C until further use.
Behavioral Tests
Self-Grooming
After the last sensitization, mice were scored for spontaneous grooming behaviors as described below.37,41 Self-grooming behavior was scored by placing each mouse in an empty cage with the dimensions of 33 cm length × 21.5 cm wide × 20 cm high, without bedding where each mouse was allowed to habituate for 5 minutes. Video recordings were used for behavioral scorings of frequency and cumulative time spent grooming all body regions. Each mouse was scored for 5 min by two independent researchers who were blinded for treatment schedule. To eliminate possible bias due to odors left by previous mice the cage was cleaned with water followed by 70% ethanol after each mouse was tested.
T-Maze Spontaneous Alternation
One day after the self-grooming test, mice were scored for T-maze alternation as described earlier with minor modifications.37 The T-maze with the dimensions of 36.4 cm long × 7.6 cm wide × 15 cm high and two lateral arms was 26.6 cm long × 7.6 cm wide × 15 cm high. After the animals had been released from the start arm, they were free to choose between both lateral arms. As soon as the animal had entered one lateral arm, it was confined in the goal arm by lowering the door for 30 seconds. A time interval of 2 min, and a trial consisted of 2 runs. A total of 5 trials were performed. The T-maze was cleaned with 70% ethanol after each mice was tested to eliminate possible bias due to odors left by previous mice. The alternation ratio was defined as the number of trials in which an animal alternated divided by the total number of trials.
Social Interaction Test (SIT)
The morning after the whey challenge, mice were exposed to a SIT as described below.41,42 In brief, Mice were placed in a 45 × 45 cm open field, with an empty wire cage located against one wall. After a 5 min habituation phase (no target), an age- and gender-matched unfamiliar target mouse was placed into the wire cage for an additional 5 min (target). By using tracking software, an interaction zone around the wire cage was digitally determined. Total distance moved, time spent in the interaction zone and latency in the interaction zone were recorded.
Measurements of Serum Whey-Specific Immunoglobulins
Serum samples were collected 30 min after oral challenge. Whey-IgE, whey-IgG2a, whey-IgG1 levels in serum were measured by enzyme linked immunosorbent assay (Elisa) as reported previously.40,43 Briefly, microtiter plates (Corning Costar, Lowell, MA, USA) were coated with 100 μL whey (3000 μg/well, sample wells), 100 μL anti-mouse IgE (2 μg/mL, #553413, BD Biosciences, USA, for IgE reference wells), or DNP-HSA (Sigma-Aldrich for IgG2a and IgG1 reference wells) and incubate for overnight at 4°C.
After washings with PBST (0.05% Tween 20 in PBS), plates were blocked with 2% BSA-PBS. Washed plates were incubated with 50 μL diluted serum samples (sample dilution recommended 1:20, sample wells), 50 μL mouse IgE (Starting concentration 100ng/mL followed by 7 serial dilutions in 2% BSA-PBS, #557079, BD Biosciences, USA, reference wells), anti-DNP–IgG2a (Starting concentration 100ng/mL followed by 7 serial dilutions in 1% HSA-PBST, YULMADNP205, Accurate Antibodies, USA, reference wells), or anti-DNP-IgG1 (Starting concentration 100ng/mL followed by 7 serial dilutions in 1% HSA-PBST, YULMADNP105, Accurate Antibodies, USA, reference wells) overnight at 4°C.
After washings with PBST, plates were subsequently incubated with 100 μL/well biotinylated anti-IgE (2 μg/mL in 2% BSA-PBS, #553419, BD Biosciences, USA), IgG2a (1 μg/mL in 1% HAS-PBST, # 553388, BD biosciences, USA) or IgG1 (1 μg/mL in 1% HAS-PBST, # 553441, BD biosciences, USA)_ detection antibodies, and developed using AVP-Avidin peroxidase (1:2000 in 2% BSA-PBS or 1:2000 in 1% HSA-PBST, MFCD00130587, Sigma Aldrich, USA) and ABTS substrate (5120-0043, KPL, USA).
Color was allowed to develop for at least 30–60 minutes and absorbance was measured at 405 nm on a microplate reader (FilterMAX F5, Molecular Devices, USA).
Measurements of Serum mMCP-1, IL-6, IL-10, and Plasma Histamine Levels
Mast cell protease-1 (mMCP-1) levels in serum samples collected 30 min after oral challenge were measured using Mouse MCPT-1/mMCP-1 Elisa Kit (MultiSciences, Hangzhou, China). IL-6 and IL-10 levels were determined by Mouse IL-6 or IL-10 Elisa development Kit (Mabtech, Nacka, Sweden) and histamine levels were determined by HIS (Histamine) Elisa Kit (Multi sciences, China), according to the protocol of the manufacturer.
Histopathological Examination, Immunohistochemistry and Toluidine Blue Staining
Tissue processing and embedding: After ear, jejunum and brains were fixed with 10% formalin for 24 h. The samples were dehydrated by incubations in different concentration of alcohol and then, cleared with xylene. Afterwards, samples were embedded in paraffin at 56°C in a hot air oven for 24 h. Coronal brain sections were processed for paraffin embedding and 4-μm sections were prepared.44
Histopathological Examination: Jejunum sections were stained with hematoxylin and eosin (HE), and then examined pathological change under a light microscope (Olympus BX61, Tokyo, Japan), and photographed at 400 × magnification, as described previously.44
Immunohistochemistry: 4 brain areas prefrontal cortex (PFC), amygdala, hippocampus and hypothalamic were selected for study of potential changes. serotonin 1A (5-HT1A), c-Fos and 5-hydroxymethylcytosine (5-hmC) were measured respectively in brain by immunohistochemistry. Brain sections were incubated with a blocking buffer for 1 h. After washing with PBS, the sections were immersed in 0.3% PBST for 10 min. The sections were blocked and incubated with anti- 5-HT1A (ab85615, Abcam, USA), anti- c-Fos (ab222699, Abcam, USA) or anti- 5-hmC antibodies (ab214728, Abcam, USA) overnight. Afterwards, the sections were washed with PBS and then incubated with a secondary antibody (BS13278, Bioworld, USA) for 1 h at room temperature. After washing, sections were then stained with DBA (ThermoFisher, USA) and examined under a light microscope (Olympus BX61, Tokyo, Japan) to determine the expression of 5-HT1A, c-Fos, 5-hmC, and photographed at 400 × magnification. Positive staining shows different degrees of yellow or pale brown, and the area percentage (Area%) was measured by Image J software.
Toluidine blue staining: Mast cells were stained according to the toluidine blue staining method described previously.10 The number of mast cells in each group were observed under a light microscope (Olympus BX61, Tokyo, Japan).
Western Blotting
After sacrificing, brain tissues were immediately isolated from mice and snap frozen in dry ice, stored at −80°C until analysis. Coronal slices of 500 mm were sectioned. Then, bilateral brain regions (hippocampus, hypothalamus, PFC, amygdala) were isolated from the coronal slices using a scalpel. The levels of the phospho-mTOR (p-mTOR), p-AKT, mTOR, AKT, p70s6k proteins were analyzed using the Western blot methodology, as described previously.45,46 The primary antibodies against p-mTOR (ab109268), p-AKT (ab81283), mTOR (ab32028), AKT (ab227385), p70s6k (ab186753) were from Abcam, Cambridge, MA, USA. The primary antibody against GAPDH (P04406) was from Abways Technology, Shanghai, China. The secondary antibody anti-Rabbit IgG (H&L)-HRP (BS13278) was from Bioworld Technology, Beijing, China. The gray values of the protein bands were calculated using Image J software.
mRNA Expression Analysis
Total RNA of the brain (hippocampus) was isolated by TRIzol Reagent (Ambion, USA). The PrimeScriptTM RT reagent kit with gDNA Eraser (TAKARA, Japan) was used for reverse transcription, and the cDNA was stored at −20°C. RT-PCR was performed in Applied Biosystems 7500 Fast Real-Time PCR System (ThermoFisher Scientific, USA), using SYBR Premix Ex TaqTM II kit (TAKARA, Japan). Relative gene expression in relation to reference gene (GAPDH) was calculated using the 2−∆∆CT method. Primers for IL-4, IL-18, IL-33, IL-1β, TGF-β and GAPDH were commercially purchased from Sangon Biotech (Shanghai, China).
Bacterial DNA Isolation and 16S rRNA Gene Sequence Analysis
Stool samples were snap-frozen in liquid nitrogen before storage at −80°C. Total community genomic DNA isolation was performed using a fecal DNA isolation kit (MoBio Laboratories, USA), following the manufacturer’s instructions. Our target was the V3–V4 hypervariable region of the bacterial 16S rRNA gene. Sequencing was performed using the Illumina MiSeq system (Illumina MiSeq, USA), according to the manufacturer’s instructions. Alpha diversity analysis, including Shannon, ACE, Chao1, Simpson index, was calculated using Mothur. PCOA analysis based on unweighted unifrac distance matrix using R studio. STAMP software (version 2.1.3) and Welch’s t-test were used to compare the abundance of bacterial. Heat maps were constructed based on the abundance of bacterial genus levels or OTUS using GraphPad Prism 8 software (version 8, GraphPad Software, Inc., San Diego, CA, USA). A detailed procedure is described in Supplementary Methods.
Western Blotting
Experimental results are expressed as mean ± standard error of mean (SEM). All data were analyzed with GraphPad Prism 8 software (version 8, GraphPad Software, Inc., San Diego, CA, USA), except for the 16S rRNA gene sequence analyses. Statistical significance was determined by using the Student t test or one-way ANOVA. Results were considered statistically significant when P<0.05.
Results
Severe Anaphylactic Reactions Occurred and the Levels of Serum Whey-Specific Immunoglobulins and IL-6 and IL-10 Increased in CMA Mice
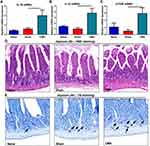
Food-induced allergic responses were quantified by using a clinical allergy score, body temperature, plasma histamine and serum mMCP-1 levels. Compared to control sham-sensitized mice, severe allergic reactions occurred in the allergic group mice (Figure 2B), and allergic mice body temperatures (Figure 2A) were decreased, while the plasma histamine (Figure 2C) and serum mMCP-1 (Figure 2D) concentrations were increased in allergic mice.
Increased levels of IgE are characteristic signs of food allergy. Serum samples from the animals were analyzed for whey-specific IgE, IgG1 and IgG2a levels using Elisa. Based on the data shown in Figure 2, mice exposed to CT plus whey displayed a significant increase in whey-specific IgE (Figure 2E), IgG1 (Figure 2F) and IgG2a (Figure 2G) after 9 weeks, compared with sham mice. Here, the higher levels of this immunoglobulin attested to the development of allergy to the whey. Allergic mice showed significantly increased serum levels of IL-6 (Figure 2H) and IL-10 (Figure 2I).
The CMA Mice Exhibited Severe Autism-Like Behaviors
To assess the effect of the allergic response on social behavior, mice were exposed to a social interaction test the day after challenge with whey (Figure 3A). Compared with sham mice, the allergic mice spent the same time in the interaction zone as the sham-sensitized control mice in the absence of a social target (Figure 3C). However, compared with the sham group, the allergic mice spent significantly less time in the interaction zone in the presence of a social target (Figure 3C). In addition, latency of first approach to the social target was significantly increased in allergic mice compared to sham mice (Figure 3B), while in the absence of social goals, there was no significant difference in the latency between the two groups of mice (Figure 3B). Compared with the sham group, the moved distance of allergic mice did not change, regardless of whether social targets existed or not (Figure 3D).
To further study the behavior of allergic mice sensitized by whey, self-grooming and T-maze were used as measures of repetitive behavior. A significant difference in time spent grooming in the novel environment was observed between the allergic and sham mice (Figure 3E). T-mazes can be used in a variety of ways to assess the cognitive ability of an animal, such as spatial memory and/or to strengthen repetitive behavior in food allergic mice.47,48 The mice in the control group tend to choose the other arm after continuously entering the T-maze. In contrast, the alternation ratio of food allergic mice is significantly lower (Figure 3F).
Intestinal Anaphylaxis in CMA Mice is Associated with Mast Cell Accumulation and Elevation of IL-18, IL-33, mTOR mRNA
To investigate the pathogenesis and changes of intestinal inflammatory factors in allergic mice, we performed RT-PCR to analyze gene expression levels of IL-18, IL-33 and m-TOR. Compared with the sham group, the inflammatory factors IL-18 (Figure 4A), IL-33 (Figure 4B) and m-TOR (Figure 4C) mRNA in the small intestine of allergic mice were over expressed. However, there was no significant change in IL-4, IL-1β and TGF-β (data not shown).
The H&E section of the lower part of the jejunum showed that the intestinal mucosa of the mice in the naive and sham group was clearly structured, the intestinal villi were arranged neatly, and there was no obvious damage to the villi and no inflammatory cell infiltration. Compared with the sham group, the intestinal villi of the allergic group mice were arranged irregularly and injured and the accumulation of mast cells in the small intestinal lamina propria (Figure 4D and E). This shows that whey allergen caused intestinal mucosal lesions in the allergic mice.
Neuronal Activation and DNA Metabolites Were Increased and Reduced in the Monoamines in the Brain of CMA Mice
The recent discovery of the highest concentration of 5-hmC in the brain adds a new dimension to the epigenetic regulation of neurogenesis and the development of complex behavior disorders.49 DNA methylation plays an important role in the pathogenesis of ASD.50 5-hmC is an important indicator reflecting the degree of DNA methylation and a stable epigenetic marker.51 In order to examine whether allergen-mediated peripheral inflammation occurring in the intestine could lead to epigenetic modification of gene expression. Here, we hypothesized that CMA would lead to the expression of 5-hmC. To test this idea, brain sections (coronal) from naive, sham, allergic mice were immunostained for 5-hmC. Here, allergic mice showed significantly higher expression of 5-hmC in hypothalamus (Figure 5A and E).
The expression of c-Fos in the brain can be used as a sign of neuron activation. Compared to sham mice, robust c-Fos induction was observed in the lateral orbital (LO) area of the PFC of allergic mice (Figure 5B and F). But the induction was limited to this area because there was no difference in the ventral orbital (VO) area of the PFC (representative photomicrographs and data not shown).
5-HT1A is a subtype of 5-HT receptor, which is the most widely distributed in the brain, and has become an important target for clinical drug treatment of depression and anxiety disorder. Altered 5-HT1A functionality in hippocampus could contribute to the relatively low sociability of BTBR mice (a potentially useful tool for autism research).52 To investigate whether change in 5-HT1A was involved in CMA mice, the levels of 5-HT1A have been analyzed by immunohistochemistry. The allergic mice showed significantly lower expression of 5-HT1A in amygdala (Figure 5C and G) and PFC (Figure 5D and H), compared with sham mice.
Considerable Differences in Gut Microbiota of CMA Mice
A number of studies have reported that gut microbiota have been demonstrated to play significant roles in allergy and ASD.6,53 In order to assess the difference in bacterial diversity between the three groups, the sequences were aligned to estimate the alpha diversity and beta diversity. There were no significant differences in the Shannon, ACE, Chao1 and Simpson indices between the naive, sham, and allergic groups (Figure 6A). Of note, the unweighted PCoA plots revealed a distinct clustering of microbiota composition for naive, sham, and allergic groups (Figure 6B). These results suggest that the diversity of gut microbes in whey allergic mice did not change significantly, but their abundances were different. Figure 6 Continue.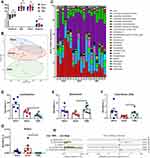
16S rRNA amplicon sequencing analyses confirmed that CMA were associated with pronounced changes in gut microbiota composition. We have observed considerable differences in gut microbiota of each group samples in genus levels (Figure 6C). In order to determine the changes in the fecal microflora of allergic mice, Student’s t test was performed for bacteria genus level. At the genus level (Figure 6H), Barnesiella (Figure 6E) and Clostridium_XIVa (Figure 6F) were significantly more abundant in the allergic group than in the sham group; however, Lactobacillus (Figure 6D) and Parvibacter (data not shown) was significantly reduced in the allergic group. Furthermore, we observed that Bosea was not present in allergic group (Figure 6G). According to the relative abundance data of the samples, we screened out 72 OTUs with higher relative abundance. The relative abundance of these 72 OTUs was further analyzed by clustering analysis represented by a heat map (Figure 6I). These data suggest that the abundance of OTU1, OTU215, OTU49, OTU 41 and OTU23 in the allergic group has a significant change, compared with the sham group.
m-TOR Signaling Pathway Were Enhanced in the Brain of CMA Mice, Allergic Reactions and Behavioral Dysfunction Severity is Correlated with Gut Microbiota and mTOR Signaling
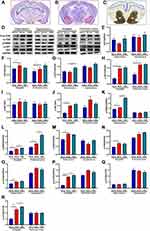
Research suggests that abnormal mTOR activation in ASD patients,29 ASD-related behavioral deficits and several neurological impairments can be rescued by inhibiting the mTOR signaling pathway.54 To elucidate the changes in the mTOR signaling pathway in the brains of allergic mice, the relative expression of mTOR, AKT, p70S6K, p-AKT, p-mTOR in hypothalamus (Figure 7A), hippocampus (Figure 7A), amygdala (Figure 7B) and PFC (Figure 7C) were detected by WB.
Based on the data shown in Figure 7, p70S6K expression was enhanced in PFC, amygdala and hippocampus of allergic mice (Figure 7D, K and L), except hypothalamus. However, the expression of AKT (Figure 7D–F), p-AKT/AKT (Figure 7D, I and J) and mTOR (Figure 7D, M and N) was not significantly changed in any area of brain. Furthermore, the expression of p-AKT (Figure 7D and G) was induced in hippocampus, compared with sham, but not in the hypothalamus, amygdala or PFC (Figure 7D, G and H). We observed that p-mTOR and p-mTOR/mTOR was induced in amygdala of allergic mice (Figure 7D, P and R). But this increase was limited to this area, because there was no difference in the hypothalamus, hippocampus and PFC (Figure 7D and O–R). Pearson’s correlation analysis showed that the levels of mTOR signaling proteins negatively correlated with Lactobacillus abundance (Figure 8). However, abundance of Clostredium_XIVa was shown to positively correlate with p70S6K levels in the hippocampus and PFC (Figure 8). Notably, both the degree of autism-like behavior and level of mMCP-1 were positively associated with mTOR signaling pathway proteins, while negatively correlated with the relative abundance of Lactobacillus (Figure 8). Otherwise, mTOR signaling pathway proteins, autism-like behavior, histamine, or mMCP-1 were not correlated with Bosea and Barnesiella abundance (Figure 8).
Discussion
In the present study, we describe the systemic allergic reactions in the setting of CMA, including immunoglobulins, cytokine and mast cell dysregulation. And then, we provide several conceptual advances about mechanism of behavioral dysfunction caused by allergic reactions. First, we demonstrate that CMA mice showed autism-like behavioral abnormalities, including reduced social interaction and increased repetitive self-grooming behavior. Meanwhile, enhanced mTOR signaling pathway was found in the brain of CMA mice, especially amygdala. Second, allergy-associated autism mice exhibited a specific microbiota signature characterized by coordinate changes in the abundance of taxa of several bacterial genus, including the Lactobacillus, Barnesiella, Clostridium_XIVa and Bosea. Third and finally, allergic reactions and behavioral dysfunction severity are negatively correlated with the abundance of Lactobacillus, but positively correlated with mTOR signaling. Furthermore, mTOR signaling proteins levels were negatively correlated with Lactobacillus abundance, while positively correlated with Clostridium_XIVa abundance.
Approximately 60% of CMA patients have an IgE-dependent disease, although estimates vary depending on the study population and age.8 Mice challenged with whey showed severe systemic allergic reactions, decreased rectal temperature, increased levels of mMCP-1 and IgG1 in the blood, and the immune system shifted toward TH2.55 mMCP-1 is a factor in allergic reactions caused by degranulation and release of active mediators of mast cells, which may be involved in the occurrence of food allergy.56 Some studies reported that serum IL-6 and IL-10 levels may be useful markers for follow-up in food allergy, especially among IgE-mediated food allergic patients.57,58 Another study suggests that people with ASD have higher levels of IL-6, IL-10 and immunoglobulins IgG compared with controls.59,60 IL-6 is known to be involved in pro-inflammatory signaling and has been shown to be permeable to the blood–brain barrier and potentially interact with the hypothalamus to induce subjective disease perception. Since IL-6 is thought to be a molecular signal of disease when it interacts with the brain, this mechanism may contribute to the cognitive and behavioral deficits observed in ASD.33 Changes in IL-1ß/IL-10 ratios along with changes in miRNA expression may serve as biomarkers for immune-mediated inflammation in ASD.61 In our study, we found that the levels of serum IL-6 and IL-10 in the allergic group were higher than the sham group. The results suggest that IL-6 and IL-10 levels may play an important role in the development of IgE-mediated food allergy. Furthermore, the small intestines of the allergic mice showed severe lesions. Intestinal villi were injured, and mast cells had accumulated. Salmond et al found that IL-33-induced lung inflammation in mice by activating mTOR through P110 δ phosphoinositide 3 kinase.62 Jia et al demonstrated that rapamycin protects mice against LPS-induced acute lung injury partly by inhibiting the production and secretion of IL-18.63 Moreover, recent studies, separately, showed that alarmins like IL-33 and IL-18 could play a key role in the pathogenesis of mental disorders.64,65 Interestingly, we found the expression of IL-33 and IL-18 mRNA in the intestines had increased, as well as mTOR mRNA in CMA mice.
Growing evidence has strengthened the possible relation of autism to food allergy.66 The results of several studies are consistent with our finding. In the current study, we also showed that a type I hypersensitivity reaction of mice, established shortly after weaning, is associated with autism-like behavior. In addition to impaired social interactions, compared with sham mice, food allergic mice showed augmented self-grooming, which indicates increased repetitive behaviors. The decrease in changes in the T-maze is not only a test of spatial memory but also involves the willingness to explore new environmental stimuli and repetitive behaviors. Here, we demonstrated that the curiosity of allergic mice (the “willingness” to explore the other place) in response to the new place was decreased. Previous studies showed that indicate that male mice are more susceptible to whey-sensitization and the effect of the antigen manifests as a decrease in their stereotypical burrowing behavior.10 However, it is not clear why sex dichotomy in the susceptibility to allergy and behavioral disorders, which require further investigation in the future.
Disrupted brain 5-HT homeostasis and dysfunction of the amygdala and PFC are linked to ASD.67,68 The 5-HT1A receptor is the most widely distributed 5-HT receptor subtype in the brain, and plays an important role in the regulation of serotonergic neurotransmission. It is abundantly present in the frontal cortex and hippocampus, and has been implicated in the pathogenesis of anxiety, depression, and autism, suggesting it is an important target for drug therapy. In the valproate (VPA)-induced rat model of ASD, treatment with a 5-HT1A receptor agonist, increased social interaction and improved fear memory extinction in the VPA-exposed offspring.67 Genome-wide 5-hmC analysis shows that the distribution of 5-hmC in the brain is highly variable. It has been demonstrated that there is an enrichment of cerebellar 5-hmC in ASD when compared with controls.69 It has been discovered that neural factors were also involved in the effector phase of food allergy (pathophysiological response after activation of mast cells). The evidence is that the increased expression of c-Fos in the intestinal muscularis neurons and brain after the intestinal exposure to antigens.70 With a whey-induced allergic model, we investigated the possible role of amygdala 5-HT1A, 5-hmC and c-Fos in allergy-induced autism-like behavioral. Here, these were detected using immunohistochemistry. We found a significantly lower 5-HT1A expression in the amygdala and PFC, higher 5-hmC in the hypothalamus, and increased c-Fos levels in LO, but not VO. The results provide evidence that 5-hmC, 5-HT1A and c-Fos may contribute to allergy-induced autism-like behavior.
Links between food allergy and autism are still unclear, but gut microbiota play an important role in pathophysiology of both disorders.71,72 Children with autism often have gastrointestinal problems related to the severity of autism problem, so-called “brain-gut-microbiome axis”.15,73–77 A clinical trial evaluated the impact of Microbiota Transfer Therapy on gastrointestinal and ASD symptoms of 18 ASD-diagnosed children. The results showed approximately 80% reduction of GI symptoms at the end of treatment, and significantly improved behavioral ASD symptoms that remained improved 8 weeks after treatment ended.22 Some studies found that Bacterial taxa within Clostridia and Firmicutes were associated with the disappearance of milk allergy symptoms,43 and germ-free mice are more likely to be allergic to milk protein and ovalbumin.78 In ASD patients, the abundance of harmful bacteria such as Clostridium and Barnesiella is higher, compared with the control group.53,79 However, the results of beneficial bacteria such as Bifidobacterium and Lactobacillus in different studies are not consistent.80,81 Although the current study results are different, it still emphasizes that microbiota changes play an important role in the pathogenesis of autistic and food allergic patients.19,82 In order to clarify how the food allergy immune response affects the autistic behavior of mice, gut microbial community structure were assessed by using 16S rRNA sequencing. In this model, our current study also found that Barnesiella and Clostridium_XIVa were significantly more abundant in the allergic group than in the sham mice. However, Lactobacillus was significantly reduced in the allergic mice. Furthermore, we observed that Bosea was not present in allergic mice. Our results demonstrate that the autism-like behavior in mice were related to changes of microbiota, but we do not conclude that bacteria are entirely causal for the symptoms. Factors such as allergic reactions, coupled with altered microbiota, may together influence the etiology of autism-like behavior. However, these hypotheses need more data to prove in future research.
Akt (Protein Kinase B, PKB/AKT), a serine/threonine protein kinase, is a direct downstream effector of phosphoinositide 3-kinase (PI3K) and a key component of the PI3K/mTOR/Akt signaling network. mTOR associates with several protein components to form two functionally distinct complexes: mTORC1 and mTORC2. Activation of mTORC1 follows that of PI3K, which, through distinct interactions with pyruvate dehydrogenase kinase 1 (PDK1), can phosphorylate and partially activate Akt at threonine (Thr)-308. Additional evidence suggests that Akt can indirectly promote the activation of ribosomal S6 kinase (S6K) and eukaryotic translation initiation factor 4E (eIF4E)-binding protein 1 (4E-BP1) by directly phosphorylating mTOR. Studies have shown that the mTOR signaling pathway plays a significant role in the pathogenesis of ASD.30 It is a central point in the gut-immune-brain axis.83 The excessive activation of PI3K/AKT/mTOR pathway in allergic mice with ASD-like behaviors was detected in our study. Rapamycin is an effective and specific mTOR inhibitor, which has been shown to improve cognitive impairment in mice,29 indicating that the disorder of the PI3K/AKT/mTOR pathway may contribute to autism pathophysiology. Interestingly, mTOR signaling is also involved in various immune response and inflammatory processes,79 mouse food allergy was attenuated by rapamycin through an immunosuppressive effect and inhibition of intestinal mast cell hyperplasia.84 Another data suggest that basophil stimulation is associated with modulation of mTOR effector phosphoproteins.85 The over-activation of mTOR signaling pathway both in the brain and in the intestine of allergic mice may point to an important link between food allergy and ASD.41 Emerging evidence suggests that rapamycin and other mTOR inhibitors might also be good candidates for therapeutic drugs for food allergy.84 Meanwhile, rapamycin could inhibit the signaling to improve the allergic immune responses and the allergic-induced autism-like behavioral symptoms.41
Furthermore, any mTOR inhibitors can alter gut microbiota. Metformin, an mTORC1 inhibitor, could promoted the abundance of mucin-degrading bacterium Akkermansia muciniphila. This abundance was correlated with a reduction of inflammation symptoms.86 Resveratrol, an activator of mTORC2, but inhibitor of mTORC1, suppresses the growth of bacterial strains, including Lactococcus and Clostridium XI.87 Several groups have identified the metabolites secreted by microbiota that are links between mTOR signaling and microbiota.88 For instance, butyrate, induces autophagy via the mTOR pathway.
Autism is well established to be a developmental syndrome of hippocampal dysfunction,89 and the amygdala is well known to be linked to social interaction.90 Felix-Ortiz and Tye reported that amygdala inputs to the ventral hippocampus bi-directionally modulate social behavior.90 These associations have implications for our study. To assess the importance of mTOR pathway in food allergy, and to determine whether it is a link between food allergies and autism-like behavioral, we evaluated components of the mTOR signaling pathway in several brain regions including hippocampus, hypothalamus, amygdala and PFC. We found mTOR signaling pathways were enhanced in the brain, especially in the amygdala. The levels of p70S6K, but not AKT and mTOR, were enhanced in PFC, amygdala, and hippocampus of allergic mice. The levels of p-AKT/AKT and p-mTOR/mTOR were increased in amygdala of allergic mice. The extent of p70S6K levels, p-AKT/AKT and p-mTOR/mTOR in amygdala were shown to negatively correlate with Lactobacillus abundance. Furthermore, the autism-like behavior was also shown to negatively correlate with p70S6K levels in PFC and hypothalamus. However, it is not entirely clear how is the mTOR pathway being activated in CMA mice and the downstream targets which require further investigation in the future.
Overall, these results demonstrate that mTOR signaling pathway and gut microbiota have a very important role in allergic response and autism-like behavior. The results of the study are encouraging, but more data are needed to better define the potential of regulating the gut microbiome-brain axis to fight allergen-induced autism-like behavioral. Furthermore, in clinical practice, Lactobacillus is one of the most commonly used probiotics and may help provide an effective strategy to prevent or treat FA and FA-related conditions based on modulation of the intestinal microbiota.91,92 A clinical study to examine the effectiveness of probiotics in allergen-induced autism-like behavioral and other neurodevelopmental disorders may be useful.
Conclusion
In conclusion, our results indicate that the mTOR signaling pathway and Lactobacillus might be the critical link in regulating gut-immune-brain axis in the intestinal, immunological, and psychiatric ASD-like symptoms seen in our model of CMA-induced autism. Our findings show that mTOR signaling pathway proteins may be potential targets, while the intestinal bacterium Lactobacillus may be used as a probiotic to prevent and treat allergen-induced autism-like behavioral and other neurodevelopmental disorders (Figure 9). It is a compelling hypothesis that changes of gut microbiota, especially Lactobacillus attenuation and mTOR signaling activation in the brain, may be an important mechanism linking allergic reactions and autistic behaviors. They warrant further investigation.
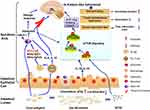 |
Figure 9 Gut microbiota and mTOR signaling pathway: plays an important role in cow’s mike allergy-associated ASD. |
Acknowledgments
We thank Henry Ehrlich for reading this manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Key scientific research projects of colleges and universities in Henan Province (20A360009, 22A360003). Henan youth talent support project (2022HYTP049). Study of integrative Medicine for Immunology and Wellness (to XML at New York Medical College). Henan Province scientific and technological project (21210230344).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Prescott SL, Allen KJ. Food allergy: riding the second wave of the allergy epidemic. Pediatr Allergy Immunol. 2011;22(2):155–160. doi:10.1111/j.1399-3038.2011.01145.x
2. Wood RA, Sicherer SH, Vickery BP, et al. The natural history of milk allergy in an observational cohort. J Allergy Clin Immunol. 2013;131:805–812. doi:10.1016/j.jaci.2012.10.060
3. Sicherer SH, Sampson HA. Food allergy: a review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J Allergy Clin Immunol. 2018;141(1):41–58. doi:10.1016/j.jaci.2017.11.003
4. Ford LS, Bloom KA, Nowak-Węgrzyn AH, Shreffler WG, Masilamani M, Sampson HA. Basophil reactivity, wheal size, and immunoglobulin levels distinguish degrees of cow’s milk tolerance. J Allergy Clin Immunol. 2013;131(1):
5. Sicherer SH. Epidemiology of food allergy. J Allergy Clin Immunol. 2011;127(3):594–602. doi:10.1016/j.jaci.2010.11.044
6. Bunyavanich S, Shen N, Grishin A, et al. Early-life gut microbiome composition and milk allergy resolution. J Allergy Clin Immunol. 2016;138(4):1122–1130. doi:10.1016/j.jaci.2016.03.041
7. Kim JS, Sicherer SH. Living with food allergy: allergen avoidance. Pediatr Clin North Am. 2011;58(2):459–470. doi:10.1016/j.pcl.2011.02.007
8. Flom JD, Sicherer SH. Epidemiology of cow’s milk allergy. Nutrients. 2019;11(5):1051. doi:10.3390/nu11051051
9. Smith NA, Germundson DL, Combs CK, Vendsel LP, Nagamoto-Combs K. Astrogliosis associated with behavioral abnormality in a non-anaphylactic mouse model of cow’s milk allergy. Front Cell Neurosci. 2019;13:320. doi:10.3389/fncel.2019.00320
10. Germundson DL, Smith NA, Vendsel LP, Kelsch AV, Combs CK, Nagamoto-Combs K. Oral sensitization to whey proteins induces age- and sex-dependent behavioral abnormality and neuroinflammatory responses in a mouse model of food allergy: a potential role of mast cells. J Neuroinflammation. 2018;15(1):120. doi:10.1186/s12974-018-1146-0
11. Addolorato G, Marsigli L, Capristo E, Caputo F, Dall’Aglio C, Baudanza P. Anxiety and depression: a common feature of health care seeking patients with irritable bowel syndrome and food allergy. Hepatogastroenterology. 1998;45(23):1559–1564.
12. Parker G, Watkins T. Treatment-resistant depression: when antidepressant drug intolerance may indicate food intolerance. Aust N Z J Psychiatry. 2002;36(2):263–265. doi:10.1046/j.1440-1614.2002.00978.x
13. Shanahan L, Zucker N, Copeland WE, Costello EJ, Angold A. Are children and adolescents with food allergies at increased risk for psychopathology? J Psychosom Res. 2014;77(6):468–473. doi:10.1016/j.jpsychores.2014.10.005
14. Ferro MA, Van Lieshout RJ, Ohayon J, Scott JG. Emotional and behavioral problems in adolescents and young adults with food allergy. Allergy. 2016;71(4):532–540. doi:10.1111/all.12829
15. Fattorusso A, Di Genova L, Dell’Isola GB, Mencaroni E, Esposito S. Autism spectrum disorders and the gut microbiota. Nutrients. 2019;11(3):521. doi:10.3390/nu11030521
16. Fowlie G, Cohen N, Ming X. The perturbance of microbiome and gut-brain axis in autism spectrum disorders. Int J Mol Sci. 2018;19(8):2251. doi:10.3390/ijms19082251
17. Srikantha P, Mohajeri MH. The possible role of the microbiota-gut-brain-axis in autism spectrum disorder. Int J Mol Sci. 2019;20(9):2115. doi:10.3390/ijms20092115
18. Mayer EA, Tillisch K, Gupta A. Gut/brain axis and the microbiota. J Clin Invest. 2015;125(3):926–938. doi:10.1172/JCI76304
19. Sharon G, Cruz NJ, Kang DW, et al. Human gut microbiota from autism spectrum disorder promote behavioral symptoms in mice. Cell. 2019;177(6):1600–1618.e17. doi:10.1016/j.cell.2019.05.004
20. De Angelis M, Francavilla R, Piccolo M, De Giacomo A, Gobbetti M. Autism spectrum disorders and intestinal microbiota. Gut Microbes. 2015;6(3):207–213. doi:10.1080/19490976.2015.1035855
21. de Theije CG, Wopereis H, Ramadan M, et al. Altered gut microbiota and activity in a murine model of autism spectrum disorders. Brain Behav Immun. 2014;37:197–206. doi:10.1016/j.bbi.2013.12.005
22. Kang DW, Adams JB, Gregory AC, et al. Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome. 2017;5(1):10. doi:10.1186/s40168-016-0225-7
23. Bunyavanich S, Berin MC. Food allergy and the microbiome: current understandings and future directions. J Allergy Clin Immunol. 2019;144(6):1468–1477. doi:10.1016/j.jaci.2019.10.019
24. Iweala OI, Nagler CR. The microbiome and food allergy. Annu Rev Immunol. 2019;37:377–403. doi:10.1146/annurev-immunol-042718-041621
25. Shu SA, Yuen AWT, Woo E, et al. Microbiota and food allergy. Clin Rev Allergy Immunol. 2019;57(1):83–97. doi:10.1007/s12016-018-8723-y
26. Bunyavanich S. Food allergy: could the gut microbiota hold the key? Nat Rev Gastroenterol Hepatol. 2019;16(4):201–202. doi:10.1038/s41575-019-0123-0
27. Berni Canani R, Paparo L, Nocerino R, et al. Gut microbiome as target for innovative strategies against food allergy. Front Immunol. 2019;10:191. doi:10.3389/fimmu.2019.00191
28. Abdel-Gadir A, Stephen-Victor E, Gerber GK, et al. Microbiota therapy acts via a regulatory T cell MyD88/RORγt pathway to suppress food allergy. Nat Med. 2019;25(7):1164–1174. doi:10.1038/s41591-019-0461-z
29. Winden KD, Ebrahimi-Fakhari D, Sahin M. Abnormal mTOR activation in autism. Annu Rev Neurosci. 2018;41:1–23. doi:10.1146/annurev-neuro-080317-061747
30. Ganesan H, Balasubramanian V, Iyer M, et al. mTOR signalling pathway - A root cause for idiopathic autism? BMB Rep. 2019;52(7):424–433. doi:10.5483/BMBRep.2019.52.7.137
31. Wu J, de Theije CGM, da Silva SL, et al. Dietary interventions that reduce mTOR activity rescue autistic-like behavioral deficits in mice. Brain Behav Immun. 2017;59:273–287. doi:10.1016/j.bbi.2016.09.016
32. Steinmetz AB, Stern SA, Kohtz AS, Descalzi G, Alberini CM. Insulin-like growth factor II targets the mTOR pathway to reverse autism-like phenotypes in mice. J Neurosci. 2018;38(4):1015–1029. doi:10.1523/JNEUROSCI.2010-17.2017
33. Cao X, Liu K, Liu J, et al. Dysbiotic gut microbiota and dysregulation of cytokine profile in children and teens with autism spectrum disorder. Front Neurosci. 2021;15:635925. doi:10.3389/fnins.2021.635925
34. Nadeem A, Ahmad SF, Al-Harbi NO, et al. Imbalance in pro-inflammatory and anti-inflammatory cytokines milieu in B cells of children with autism. Mol Immunol. 2022;141:297–304. doi:10.1016/j.molimm.2021.12.009
35. Shipman L. Allergy: neonatal IL-33 drives allergy. Nat Rev Immunol. 2017;17(2):80–81. doi:10.1038/nri.2016.149
36. Galand C, Leyva-Castillo JM, Yoon J, et al. IL-33 promotes food anaphylaxis in epicutaneously sensitized mice by targeting mast cells. J Allergy Clin Immunol. 2016;138(5):1356–1366. doi:10.1016/j.jaci.2016.03.056
37. de Theije CG, Wu J, Koelink PJ, et al. Autistic-like behavioural and neurochemical changes in a mouse model of food allergy. Behav Brain Res. 2014;261:265–274. doi:10.1016/j.bbr.2013.12.008
38. Villa C, Costa J, Oliveira MBPP, Mafra I. Bovine milk allergens: a comprehensive review. Compr Rev Food Sci Food Saf. 2018;17(1):137–164. doi:10.1111/1541-4337.12318
39. Srivastava KD, Siefert A, Fahmy TM, Caplan MJ, Li XM, Sampson HA. Investigation of peanut oral immunotherapy with CpG/peanut nanoparticles in a murine model of peanut allergy. J Allergy Clin Immunol. 2016;138(2):536–543.e4. doi:10.1016/j.jaci.2016.01.047
40. Srivastava KD, Qu C, Zhang T, Goldfarb J, Sampson HA, Li XM. Food allergy herbal formula-2 silences peanut-induced anaphylaxis for a prolonged posttreatment period via IFN-gamma-producing CD8+ T cells. J Allergy Clin Immunol. 2009;123(2):443–451. doi:10.1016/j.jaci.2008.12.1107
41. Wu J, de Theije CG, da Silva SL, et al. mTOR plays an important role in cow’s milk allergy-associated behavioral and immunological deficits. Neuropharmacology. 2015;97:220–232. doi:10.1016/j.neuropharm.2015.04.035
42. Liu J, Dietz K, DeLoyht JM, et al. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat Neurosci. 2012;15(12):1621–1623. doi:10.1038/nn.3263
43. Qu C, Srivastava K, Ko J, Zhang TF, Sampson HA, Li XM. Induction of tolerance after establishment of peanut allergy by the food allergy herbal formula-2 is associated with up-regulation of interferon-gamma. Clin Exp Allergy. 2007;37(6):846–855. doi:10.1111/j.1365-2222.2007.02718.x
44. Cao LH, Qiao JY, Huang HY, et al. PI3K-AKT signaling activation and icariin: the potential effects on the perimenopausal depression-like rat model. Molecules. 2019;24(20):3700. doi:10.3390/molecules24203700
45. Saad MA, El-Sahar AE, Sayed RH, Elbaz EM, Helmy HS, Senousy MA. Venlafaxine mitigates depressive-like behavior in ovariectomized rats by activating the EPO/EPOR/JAK2 signaling pathway and increasing the serum estradiol level. Neurotherapeutics. 2019;16(2):404–415. doi:10.1007/s13311-018-00680-6
46. Chen XQ, Li CF, Chen SJ, et al. The antidepressant-like effects of Chaihu Shugan San: dependent on the hippocampal BDNF-TrkB-ERK/Akt signaling activation in perimenopausal depression-like rats. Biomed Pharmacother. 2018;105:45–52. doi:10.1016/j.biopha.2018.04.035
47. Deacon RM, Rawlins JN. T-maze alternation in the rodent. Nat Protoc. 2006;1(1):7–12. doi:10.1038/nprot.2006.2
48. Sharma S, Haselton J, Rakoczy S, Branshaw S, Brown-Borg HM. Spatial memory is enhanced in long-living Ames dwarf mice and maintained following kainic acid induced neurodegeneration. Mech Ageing Dev. 2010;131(6):422–435. doi:10.1016/j.mad.2010.06.004
49. Papale LA, Zhang Q, Li S, Chen K, Keleş S, Alisch RS. Genome-wide disruption of 5-hydroxymethylcytosine in a mouse model of autism. Hum Mol Genet. 2015;24(24):7121–7131.
50. Tremblay MW, Jiang YH. DNA methylation and susceptibility to autism spectrum disorder. Annu Rev Med. 2019;70:151–166. doi:10.1146/annurev-med-120417-091431
51. Madrid A, Chopra P, Alisch RS. Species-specific 5 mC and 5 hmC genomic landscapes indicate epigenetic contribution to human brain evolution. Front Mol Neurosci. 2018;11:39. doi:10.3389/fnmol.2018.00039
52. Gould GG, Hensler JG, Burke TF, Benno RH, Onaivi ES, Daws LC. Density and function of central serotonin (5-HT) transporters, 5-HT1A and 5-HT2A receptors, and effects of their targeting on BTBR T+tf/J mouse social behavior. J Neurochem. 2011;116(2):291–303. doi:10.1111/j.1471-4159.2010.07104.x
53. Strati F, Cavalieri D, Albanese D, et al. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome. 2017;5(1):24. doi:10.1186/s40168-017-0242-1
54. Modi M, Sahin M. Tau: a novel entry point for mTOR-based treatments in autism spectrum disorder? Neuron. 2020;106(3):359–361. doi:10.1016/j.neuron.2020.04.019
55. Shin HS, See HJ, Jung SY, et al. Turmeric (Curcuma longa) attenuates food allergy symptoms by regulating type 1/type 2 helper T cells (Th1/Th2) balance in a mouse model of food allergy. J Ethnopharmacol. 2015;175:21–29. doi:10.1016/j.jep.2015.08.038
56. Benede S, Berin MC. Mast cell heterogeneity underlies different manifestations of food allergy in mice. PLoS One. 2018;13(1):e0190453. doi:10.1371/journal.pone.0190453
57. Kara M, Beser OF, Konukoglu D, et al. The utility of TNF-α, IL-6 and IL-10 in the diagnosis and/or follow-up food allergy. Allergol Immunopathol (Madr). 2020;48(1):48–55. doi:10.1016/j.aller.2019.04.011
58. Chen TK, Lee JH, Yu HH, et al. Association between human IL-10 gene polymorphisms and serum IL-10 level in patients with food allergy. J Formos Med Assoc. 2012;111(12):686–692. doi:10.1016/j.jfma.2011.11.027
59. Heuer L, Ashwood P, Schauer J, et al. Reduced levels of immunoglobulin in children with autism correlates with behavioral symptoms. Autism Res. 2008;1(5):275–283. doi:10.1002/aur.42
60. Onore C, Careaga M, Ashwood P. The role of immune dysfunction in the pathophysiology of autism. Brain Behav Immun. 2012;26(3):383–392. doi:10.1016/j.bbi.2011.08.007
61. Jyonouchi H, Geng L, Streck DL, Dermody JJ, Toruner GA. MicroRNA expression changes in association with changes in interleukin-1ß/interleukin10 ratios produced by monocytes in autism spectrum disorders: their association with neuropsychiatric symptoms and comorbid conditions (observational study). J Neuroinflammation. 2017;14(1):229. doi:10.1186/s12974-017-1003-6
62. Salmond RJ, Mirchandani AS, Besnard AG, Bain CC, Thomson NC, Liew FY. IL-33 induces innate lymphoid cell-mediated airway inflammation by activating mammalian target of rapamycin. J Allergy Clin Immunol. 2012;130(5):1159–1166.e6. doi:10.1016/j.jaci.2012.05.018
63. Jia XH, Cao B, An YQ, Zhang XL, Wang C. Rapamycin ameliorates lipopolysaccharide-induced acute lung injury by inhibiting IL-1β and IL-18 production. Int Immunopharmacol. 2019;67:211–219. doi:10.1016/j.intimp.2018.12.017
64. Pandolfo G, Genovese G, Casciaro M, et al. IL-33 in mental disorders. Medicina (Kaunas). 2021;57(4):315. doi:10.3390/medicina57040315
65. Businaro R, Corsi M, Azzara G, et al. Interleukin-18 modulation in autism spectrum disorders. J Neuroinflammation. 2016;13:2. doi:10.1186/s12974-015-0466-6
66. Xu GF, Snetselaar LG, Jing J, Liu B, Strathearn L, Bao W. Association of food allergy and other allergic conditions with autism spectrum disorder in children. JAMA Netw Open. 2018;1(2):e180279. doi:10.1001/jamanetworkopen.2018.0279
67. Wang CC, Lin HC, Chan YH, Gean PW, Yang YK, Chen PS. 5-HT1A-receptor agonist modified amygdala activity and amygdala-associated social behavior in a valproate-induced rat autism model. Int J Neuropsychopharmacol. 2013;16(9):2027–2039. doi:10.1017/S1461145713000473
68. Barnes NM, Ahern GP, Becamel C, et al. International union of basic and clinical pharmacology. CX. Classification of receptors for 5-hydroxytryptamine; pharmacology and function. Pharmacol Rev. 2021;73(1):310–520.
69. Zhubi A, Chen Y, Dong E, Cook EH, Guidotti A, Grayson DR. Increased binding of MeCP2 to the GAD1 and RELN promoters may be mediated by an enrichment of 5-hmC in autism spectrum disorder (ASD) cerebellum. Transl Psychiatry. 2014;4(1):e349. doi:10.1038/tp.2013.123
70. Fargeas MJ, Fioramonti J, Bueno L. Involvement of capsaicin-sensitive afferent nerves in the intestinal motor alterations induced by intestinal anaphylaxis in rats. Int Arch Allergy Immunol. 1993;101(2):190–195. doi:10.1159/000236518
71. Aguilera AC, Dagher IA, Kloepfer KM. Role of the microbiome in allergic disease development. Curr Allergy Asthma Rep. 2020;20(9):44. doi:10.1007/s11882-020-00944-2
72. Kong XJ, Liu J, Cetinbas M, et al. New and preliminary evidence on altered oral and gut microbiota in individuals with autism spectrum disorder (ASD): implications for ASD diagnosis and subtyping based on microbial biomarkers. Nutrients. 2019;11(9):2128. doi:10.3390/nu11092128
73. Feehley T, Plunkett CH, Bao R, et al. Healthy infants harbor intestinal bacteria that protect against food allergy. Nat Med. 2019;25(3):448–453. doi:10.1038/s41591-018-0324-z
74. Daillère R, Vétizou M, Waldschmitt N, et al. Enterococcus hirae and Barnesiella intestinihominis facilitate cyclophosphamide-induced therapeutic immunomodulatory effects. Immunity. 2016;45(4):931–943. doi:10.1016/j.immuni.2016.09.009
75. Li D, Wang P, Wang P, Hu X, Chen F. The gut microbiota: a treasure for human health. Biotechnol Adv. 2016;34(7):1210–1224. doi:10.1016/j.biotechadv.2016.08.003
76. Adak A, Khan MR. An insight into gut microbiota and its functionalities. Cell Mol Life Sci. 2019;76(3):473–493. doi:10.1007/s00018-018-2943-4
77. Chabé M, Lokmer A, Ségurel L. Gut protozoa: friends or foes of the human gut microbiota? Trends Parasitol. 2017;33(12):925–934. doi:10.1016/j.pt.2017.08.005
78. Stefka AT, Feehley T, Tripathi P, et al. Commensal bacteria protect against food allergen sensitization. Proc Natl Acad Sci U S A. 2014;111(36):13145–13150. doi:10.1073/pnas.1412008111
79. Liu S, Li E, Sun Z, et al. Altered gut microbiota and short chain fatty acids in Chinese children with autism spectrum disorder. Sci Rep. 2019;9(1):287. doi:10.1038/s41598-018-36430-z
80. Tomova A, Husarova V, Lakatosova S, et al. Gastrointestinal microbiota in children with autism in Slovakia. Physiol Behav. 2015;138:179–187. doi:10.1016/j.physbeh.2014.10.033
81. Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA. Low relative abundances of the mucolytic bacterium Akkermansia muciniphila and Bifidobacterium spp. in feces of children with autism. Appl Environ Microbiol. 2011;77(18):6718–6721. doi:10.1128/AEM.05212-11
82. Hughes HK, Rose D, Ashwood P. The gut microbiota and dysbiosis in autism spectrum disorders. Curr Neurol Neurosci Rep. 2018;18(11):81. doi:10.1007/s11910-018-0887-6
83. van Sadelhoff JHJ, Perez Pardo P, Wu J, et al. The gut-immune-brain axis in autism spectrum disorders; a focus on amino acids. Front Endocrinol (Lausanne). 2019;10:247. doi:10.3389/fendo.2019.00247
84. Yamaki K, Yoshino S. Preventive and therapeutic effects of rapamycin, a mammalian target of rapamycin inhibitor, on food allergy in mice. Allergy. 2012;67(10):1259–1270. doi:10.1111/all.12000
85. Gernez Y, Tirouvanziam R, Reshamwala N, et al. Modulation of mTOR effector phosphoproteins in blood basophils from allergic patients. J Clin Immunol. 2012;32(3):565–573. doi:10.1007/s10875-012-9651-x
86. Shin NR, Lee JC, Lee HY, et al. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut. 2014;63(5):727–735. doi:10.1136/gutjnl-2012-303839
87. Jung MJ, Lee J, Shin NR, et al. Chronic repression of mTOR complex 2 induces changes in the gut microbiota of diet-induced obese mice. Sci Rep. 2016;6:30887. doi:10.1038/srep30887
88. Noureldein MH, Eid AA. Gut microbiota and mTOR signaling: insight on a new pathophysiological interaction. Microb Pathog. 2018;118:98–104. doi:10.1016/j.micpath.2018.03.021
89. DeLong GR. Autism, amnesia, hippocampus, and learning. Neurosci Biobehav Rev. 1992;16(1):63–70. doi:10.1016/S0149-7634(05)80052-1
90. Felix-Ortiz AC, Tye KM. Amygdala inputs to the ventral hippocampus bidirectionally modulate social behavior. J Neurosci. 2014;34(2):586–595. doi:10.1523/JNEUROSCI.4257-13.2014
91. Canani RB, Di Costanzo M, Bedogni G, et al. Extensively hydrolyzed casein formula containing Lactobacillus rhamnosus GG reduces the occurrence of other allergic manifestations in children with cow’s milk allergy: 3-year randomized controlled trial. J Allergy Clin Immunol. 2017;139(6):1906–1913.e4. doi:10.1016/j.jaci.2016.10.050
92. Berni Canani R, Sangwan N, Stefka AT, et al. Lactobacillus rhamnosus GG-supplemented formula expands butyrate-producing bacterial strains in food allergic infants. ISME J. 2016;10(3):742–750. doi:10.1038/ismej.2015.151
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.