Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Folliculotropic Mycosis Fungoides: Current Guidance and Experience from Clinical Practice
Authors Roccuzzo G, Mastorino L , Gallo G , Fava P, Ribero S, Quaglino P
Received 27 June 2022
Accepted for publication 30 August 2022
Published 13 September 2022 Volume 2022:15 Pages 1899—1907
DOI https://doi.org/10.2147/CCID.S273063
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Gabriele Roccuzzo,* Luca Mastorino,* Giuseppe Gallo, Paolo Fava, Simone Ribero, Pietro Quaglino
Department of Medical Sciences, Section of Dermatology, University of Turin, Turin, Italy
*These authors contributed equally to this work
Correspondence: Giuseppe Gallo, Department of Medical Sciences, Section of Dermatology, University of Turin, Via Cherasco 23, Torino, Turin, 10126, Italy, Tel +39 0116335843 ; +39 0116335034, Email [email protected]
Introduction: Folliculotropic mycosis fungoides (FMF) is the most frequent variant of mycosis fungoides (MF), with clinical features which differ from the classic form. As for therapeutic options, the latest guidelines on MF agree on a stage-driven strategy, in consideration of clinical presentation, symptom burden and patient’s comorbidities.
Materials and Methods: A search on MEDLINE, PubMed, Scopus and Cochrane Library was conducted to gather the latest evidence on FMF clinical management. Manuscripts published in the last five years (January 2017–April 2022) were included. Our single-center experience was also described.
Results: A total of 15 articles were analyzed, with a total of 432 patients (disease stage from IA to IVA2). The most widely-used treatment was psoralen ultra-violet A (PUVA) in monotherapy or in association with other drugs. Oral retinoid-based therapy was also described as a therapeutic option alone or in combination. Other therapy reported were based on Brentuximab Vedotin, Mogamulizumab, Carmustine, topical steroids, tazarotene and excimer laser, interferon, nitrogen mustard, imiquimod, systemic chemotherapy, extracorporeal photopheresis and stem cell transplantation.
Discussion: FMF is characterized by specific clinical-pathologic features. Advanced forms assume characteristics more similar to classic MF (infiltrated plaques and nodules), whilst early stages can present in a wide range of clinical forms (acneiform lesions, follicular-like keratoses, erythematous patches). As for therapeutic options, in absence of specific guidelines, a high number of treatments are described in clinical practice, with variable results. Phototherapy in all its forms, especially as PUVA, appears to have the greatest initial therapeutic success. Retinoids, although widely used, appear to be poorly effective in monotherapy, particularly acitretin. Combination treatment with phototherapy seems to be advisable. Ionizing treatments, such as radiotherapy and TSEBT, appear effective, at least in the short term. Overall, an integrated approach is mandatory for the inconstant course of the disease and its multidisciplinary nature.
Keywords: mycosis fungoides, folliculotropic mycosis fungoides, cutaneous lymphoma, primary cutaneous T-cell lymphoma, therapy
Introduction
Primary cutaneous lymphomas (PCL) are a group of non-Hodgkin’s lymphomas (NHL) characterized by monoclonal proliferation of malignant lymphocytes in the skin.1 Among them, around three-fourths are represented by cutaneous T-cell lymphoma (CTCL), with mycosis fungoides (MF) as the most common form. According to the 2016 revision of the World Health Organization (WHO) classification of lymphoid neoplasms,2 folliculotropic MF (FMF) is the most frequent variant of MF and is characterized by invasion of hair follicles by atypical T-cells, with clinical features which differ from classic MF3 (Figure 1). Recently, two distinct histopathologic patterns, referred to as early FMF (patch/thin plaque type) and advanced FMF (thick plaque/tumor type), have been described.4,5 The former is characterized by follicle-based patch/flat plaques, keratosis pilaris-like lesions, acneiform lesions and has good prognosis, like early-stage classic MF; the latter is distinguished by follicle-based infiltrated/thick plaques and/or tumors with worse prognosis. As for special populations, the FMF subtype has been recently found to be over-represented in solid organ transplant recipients and in paediatric patients, compared with other CTCL variants.6,7 Recent studies reported 10-year survival rates of 72% in skin-limited early stages, 28% in skin-limited advanced stages, and 2% in FMF with extracutaneous localizations at first presentation.5 As for therapeutic options, the latest guidelines on MF agree on a stage-driven strategy, in consideration of clinical presentation, symptom burden and patient’s comorbidities.8,9 In early stages (ie, IA, IB, and IIA), skin-directed therapies (SDTs), such as topical corticosteroids, UVB, PUVA, localized radiation therapy and mechlorethamine, represent the first therapeutic option.10,11 Those refractory to the first line may be considered for systemic therapies, such as retinoids, interferon alpha, total skin electron beam therapy (TSEB) or low-dose methotrexate, which conversely represent first-line treatments for stage IIB.10,11 Stage III patients may benefit from extracorporeal phototherapy (ECP), alone or in combination with skin-directed and other systemic therapies, whilst refractory and stage IV patients have been traditionally treated with chemotherapy regimens (gemcitabine, pegylated liposomal doxorubicin, CHOP and CHOP-like polychemotherapy).9,12 Lately, new monoclonal antibodies, such as anti-CD52 alemtuzumab, anti-CD30 brentuximab vedotin, anti-CCR4 mogamulizumab, and histone deacetylase inhibitors (though currently not approved in Europe), have represented promising options in the therapeutical armamentarium available to clinicians.13–16 According to the multi-center prospective international study PROCLIPI, real-life treatment decisions in MF patients are often influenced by the presence of the folliculotropic variant, as early-stage FMF patients are more likely to receive systemic first-line therapies.17 Moreover, recent findings have confirmed cutaneous stage to be the major predictive variable associated with disease-specific survival in FMF patients.18 Since FMF has been found to have worse prognosis and to be less responsive to SDTs, compared with classic MF, optimal treatment still needs to be defined.5 Herein we describe the experience of a tertiary referral center for CTCL and provide a synoptic overview of the articles concerning FMF treatment published in recent years.
Materials and Methods
A review of the literature was carried out according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. A search on MEDLINE, PubMed, Scopus and Cochrane Library was conducted using the combination of the following keywords and medical subject heading (MeSH) terms “folliculotropic mycosis fungoides”, “therapy”, “treatment”, via the Boolean term “AND”. Manuscripts published in the last five years, from January 2017 to April 2022, were included. Articles in a language other than English and/or not dealing with humans were excluded. No restriction related to article type was applied: case reports, letters to the editor, case series, and original articles were all included. Studies were selected if they provided information on therapy response in FMF. Two investigators extracted the data independently (GR, LM) and a third author (GG) was consulted in case of disagreements. Articles were first screened by reading the title and the abstract. Articles considered relevant were then read in their totality and those meeting the inclusion and exclusion criteria were selected. For each study, the following details were considered: authors, nation, type of article, number of patients, staging of disease, therapy response as CR (complete response), PR (partial response), PD (progressive disease) and NR (non-response), if available. Two authors (GR, LM) independently assessed the risk of bias of each included study, in accordance with methods recommended by National Institutes of Health Quality Assessment Tool for Case Series Studies.19 A third author (GG) was consulted in case of disagreements. Prisma flowchart is depicted in Figure 2.
 |
Figure 2 PRISMA flowchart. Notes: PRISMA figure adapted from Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. Creative Commons.43 |
Results
A total of 38 records was initially identified through a literature search, 19 of which were duplicates. After screening for eligibility and inclusion criteria, 15 articles were ultimately included20–32 (Table 1). Most publications were case reports (n=7), followed by case series (n=4), original articles (n=3), and clinical image (n=1). All the studies included were rated as level 4 or 5 evidence for clinical research as detailed in the Oxford Centre for Evidence-Based Medicine 2011 guidelines.33 A total of 432 patients with FMF were gathered. Treatment and type of response were evaluated within the limits of individual data exposure. Disease stages exhibited in the literature ranged from stage IA to IVA2; in 8 studies only earlier stages (maximum IIA) were evaluated, in 7 studies more advanced stages (from IIB) were also included. The most widely used treatment was Psoralen Ultra-Violet A (PUVA), reported in 8 studies, in 3 as monotherapy (1 case report with CR, 1 original article with CR 70% and PR 26%, 1 original article with ORR of 76%)5,24,25 and in 5 studies in association with other drugs: in 2 case reports PUVA was combined with bexarotene, with CR in both cases and subsequent stem cell transplantation following progression in one of them,27,28 whilst in another report it was associated with Interferon, with CR.31 This treatment, among others, was mentioned in the Japanese case series by Kamijo et al, yet it was less commonly used compared to UVB therapy (ie, 10% vs 50% of the patients in the cohort).29 In the Dutch experience, PUVA was administered as monotherapy to 31%, combined with retinoid or IFN-alpha to 9%, and with RT to 13% of the patients, respectively, with ORR of 76%.5 In the American experience, both phototherapy (47 patients) and bexarotene (53 oral, 33 topical) represented a common line of therapy in the 114-patient presented cohort.3 Oral retinoid-based therapy was also described as a therapeutic option in two other studies. Laggis et al reported, for acitretin in combination with RT or TSEB, response rates of 24% (CR), 48% (PR) and 28% (NR);32 single-agent isotretinoin (32 patients) was a common therapy in Wieser et al’s cohort,3 though response rates were specifically described only for radiation therapy protocols (ie, CR rates of 50%, 33.3%, 42.8% for local RT, TSEB and both, respectively). Complete and partial response rates of 62% and 38% in the local RT group and 53% and 43% in the TSEB group, respectively, were reported in the Dutch experience.5 Brentuximab vedotin was used in a case report with PR in an advanced stage IVA2 and in 8 patients in the American experience.3,20 Mogamulizumab was used in stages ranging from IB to IIA and in one case combined with RT, obtaining a CR.21,29 As for methotrexate, Jiang et al described one case with CR, while 18 patients received this treatment in the American cohort.3,22 Carmustine was used in a case series of 13 patients with stage IA to IIIB with PR 62% and CR 38%.23 Etoposide in combination with prednisone was described in a stage IA patient, with CR.30 Clobetasol in combination with tezarotene and excimer laser was used in a stage IA pediatric patient, with CR.26 Topical steroids were reported to be used in the American (97 patients), Dutch (22 patients) and Japanese cohorts.3,5,29 In these three reports, other less commonly used reported therapies were UVB, Denileukin diftitox, interferon, nitrogen mustard, imiquimod, systemic chemotherapy, extracorporeal photopheresis and stem cell transplantation3,5,29 (Figure 3).
 |
Table 1 Overview of Recently Published Articles on FMF Treatment |
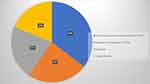 |
Figure 3 Most prescribed treatments in FMF patients according to our review. |
A Single-Center Experience
In 2021, 22 patients with a biopsy-proven diagnosis of FMF, according to the international criteria, were visited at the dermatologic clinic of the University of Turin, Italy. The diagnosis had been made on average 9 years earlier (range 0–21). A male prevalence (63.6%) and a mean age of 69 (range 28–89) were recorded in our cohort. The most represented stage was IB with 14 patients (63.6%), followed by IIB with 4 patients, IA with 3 patients and IIA with 1 patient each. Ten patients (45%) presented with severe pruritus as most relevant symptom. Six stage progressions were documented in the available records (five patients from IA to IB and one patient from IIA to IIB). As for the most used therapies, 17 patients (77.3%) received topical steroids, 13 patients (59.1%) UVB phototherapy, and 9 patients (40.1%) systemic steroids. As for oral retinoids, bexarotene and acitretin were prescribed to 10 (45.5%) and 12 (54.5%) patients, respectively. As for radiation therapy, 7 patients (31.8%) received local radiotherapy and 3 received TSEB (13.6%). Other recorded treatments were PUVA (3 patients), interferon, brentuximab vedotin, mogamulizumab (2 patients each), and methotrexate (1 patient). At the time of data collection, patients had already received on average 3 different treatments (range 2–7), either topical or systemic. The most experienced side effects were blood lipid disorders and cutaneous reactions with oral retinoids (51% of the patients), transient radiation-induced dermatitis after RT (80% of the patients), one case of mogamulizumab-induced rash, and one case of psoriasis onset during brentuximab vedotin treatment.34–37 Best overall response was assessed in conformity with standardized skin response definitions.38 A total of 7 CRs, 8 PRs, 3 SDs and 4 PDs were observed at the time of analysis. As for the complete responses, they were all achieved in early-stage patients (ie, 5 stage IB, 1 stage IA, 1 stage IIA), whilst PRs were achieved in six stage 1B and two stage 2B patients, respectively. Stable disease responses were seen in two stage 2B patients and one stage 1A patient, while progression affected three stage 1B patients and one stage 1A patient.
Discussion
Folliculotropic mycosis fungoides (FMF) represents the most common subtype of MF and is characterized by specific clinical-pathologic features.3,39,40 First, early and advanced stages differ in predilection of site, as the former more commonly affects the trunk and limbs, whilst the latter the head/neck region.4 Moreover, advanced forms assume characteristics more similar to classic MF, such as infiltrated plaques and nodules, whilst early stages can present in a wide range of clinical forms, such as acneiform lesions, follicular-like keratoses, or erythematous patches.4,5 As for histology, advanced forms seem to show more conspicuous cellular infiltrates (ie, eosinophils, plasma cells, lymphocytes with syringotropism), characterized by pronounced depth and density.4 Hodak et al highlighted how FMF can be distinguished in two distinct forms, a low-stage indolent form and a high-stage aggressive form, addressing the lack of long-term follow-up and small sample studies as major limitations to thoroughly grasp the nature of FMF forms.4
Looking at the recent data reported in the literature, more than half of the analyzed papers did not include advanced disease stages (≥IIB), thus showing generally higher ORR rates for the therapies reported.20–32 On the contrary, looking at those studies which included more advanced stages, such as the papers by van Santeen et al and Laggis et al, a significantly higher fluctuation of ORR could be observed (ie, PR and CR ranging from 25–24% to 48–51%, respectively).5,32 Our case history shows quite similar responses, with CR achieved mainly in early stages. Moreover, despite being traditionally considered an aggressive variant of MF, metastatic cases of FMF described in the literature in the last 5 years are in line with our case series and generally represent a small percentage of the patients. Regarding the treatments used in FMF, in the absence of specific guidelines and clinical prospective studies, a high number of treatments are described in clinical practice, with variable results.20–32 Phototherapy, retinoids (ie acitretin, bexarotene, isotretinoin) and topical steroids represent the most widely described non-ionizing therapies (Figure 3), with different results.3,5,24–32 Phototherapy in all its forms, especially as PUVA, appears to have the greatest initial therapeutic success, although it is more commonly proposed in the early stages of the disease. Both in the literature and in our case history, the first line of treatment is topical steroid therapy, often already initiated by the patient before the actual diagnosis of FMF. Retinoids, although widely used, appear to be poorly effective in monotherapy, particularly acitretin. Combination treatment with phototherapy seems to be advisable.27,28 Ionizing treatments such as radiotherapy and TSEBT, especially if combined with bexarotene, appear effective, at least in the short term, yet the need for coordination between different specialists and, in the case of TSEBT, between different centers can represent a limitation for their use in clinical practice3,5,32,41(Figure 3). Moreover, a TSEBT-based therapeutical approach has proved to significantly improve emotional domains of quality of life in MF patients.42 Overall, the large number of treatment options and high treatment resistance of FMF are reflected in the numerous therapeutic lines adopted in clinical practice. In contrast to classic MF, extra-corporeal photopheresis (ECP) does not seem to play a relevant part in the treatment of the folliculotropic variant, as it was adopted in only one study in our analysis.3 While in the literature it is often difficult to identify the various therapies adopted, our case series shows that patients undergo numerous treatments ranging from 2 to 7 with an average of 3.8. Overall, the design and poor-quality evidence of retrospective single-center experiences do not allow for the defining of clear therapeutic directions of FMF. Given the peculiar nature of FMF, specific guidelines based on prospective clinical studies are desirable to ensure significant and clear evidence on the treatments to be preferred in this variant. The inconstant course of the disease and the multidisciplinary nature of the feasible treatments mandatorily call for an integrated approach between the various specialists involved, such as dermatologists, oncologists, hematologists and radiotherapists.
Funding
There is no funding to report.
Disclosure
Gabriele Roccuzzo and Luca Mastorino share first authorship. The authors report no conflicts of interest in this work.
References
1. Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105(10):3768–3785. doi:10.1182/blood-2004-09-3502
2. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. doi:10.1182/blood-2016-01-643569
3. Wieser I, Wang C, Alberti-Violetti S, et al. Clinical characteristics, risk factors and long-term outcome of 114 patients with folliculotropic mycosis fungoides. Arch Dermatol Res. 2017;309(6):453–459. doi:10.1007/s00403-017-1744-1
4. Hodak E, Amitay-Laish I, Atzmony L, et al. New insights into folliculotropic mycosis fungoides (FMF): a single-center experience. J Am Acad Dermatol. 2016;75(2):347–355. doi:10.1016/j.jaad.2016.03.009
5. van Santen S, van Doorn R, Neelis KJ, et al. Recommendations for treatment in folliculotropic mycosis fungoides: report of the Dutch Cutaneous Lymphoma Group. Br J Dermatol. 2017;177(1):223–228. doi:10.1111/bjd.15355
6. Reiter O, Amitay-Laish I, Oren-Shabtai M, Feinmesser M, Ben-Amitai D, Hodak E. Paediatric mycosis fungoides - characteristics, management and outcomes with particular focus on the folliculotropic variant [published correction appears in J Eur Acad Dermatol Venereol. 2022 Jul;36(7):1143]. J Eur Acad Dermatol Venereol. 2022;36(5):671–679. doi:10.1111/jdv.17971
7. Shimshak S, Dai C, Comfere N, Sokumbi O. Characterization of primary cutaneous T-cell lymphoma following solid organ transplantation. Int J Dermatol. 2022. doi:10.1111/ijd.16300
8. Roccuzzo G, Giordano S, Fava P, et al. Immune check point inhibitors in primary cutaneous T-cell lymphomas: biologic rationale, clinical results and future perspectives. Front Oncol. 2021;11:733770. doi:10.3389/fonc.2021.733770
9. Trautinger F, Eder J, Assaf C, et al. European organisation for research and treatment of cancer consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome - Update 2017. Eur J Cancer. 2017;77:57–74. doi:10.1016/j.ejca.2017.02.027
10. Willemze R, Hodak E, Zinzani PL, Specht L, Ladetto M; ESMO Guidelines Committee. Primary cutaneous lymphomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv30–40. doi:10.1093/annonc/mdy133
11. Mehta-Shah N, Horwitz SM, Ansell S, et al. NCCN guidelines insights: primary cutaneous lymphomas, Version 2.2020. J Natl Compr Canc Netw. 2020;18(5):522–536. doi:10.6004/jnccn.2020.0022
12. Cariti C, Quaglino P, Lupia T, et al. Infections in Sézary syndrome: a retrospective cohort study of 113 patients. J Am Acad Dermatol. 2022;86(4):943–946. doi:10.1016/j.jaad.2021.03.079
13. de Masson A, Guitera P, Brice P, et al. Long- term efficacy and safety of alemtuzumab in advanced primary cutaneous T- cell lym- phomas. Br J Dermatol. 2014;170(3):720–724. doi:10.1111/bjd.12690
14. Prince HM, Kim YH, Horwitz SM, et al. Brentuximab vedotin or physician’s choice in CD30-positive cutaneous T-cell lymphoma (ALCANZA): an international, open- label, randomised, phase 3, multicentre trial. Lancet. 2017;390(10094):555–566. doi:10.1016/S0140-6736(17)31266-7
15. Kim YH, Bagot M, Pinter-Brown L, et al. Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): an international, open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2018;19(9):1192–1204. doi:10.1016/S1470-2045(18)30379-6
16. Duvic M, Talpur R, Ni X, et al. Phase 2 trial of oral vorinostat (Suberoylanilide Hydroxamic Acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL). Blood. 2007;109(1):31–39. doi:10.1182/blood-2006-06-025999
17. Quaglino P, Prince HM, Cowan R, et al. Treatment of early-stage mycosis fungoides: results from the PROspective Cutaneous Lymphoma International Prognostic Index (PROCLIPI) study [published correction appears in Br J Dermatol. 2021 Sep;185(3):685]. Br J Dermatol. 2021;184(4):722–730. doi:10.1111/bjd.19252
18. Charli-Joseph Y, Kashani-Sabet M, McCalmont TH, et al. Association of a proposed new staging system for folliculotropic mycosis fungoides with prognostic variables in a US cohort. JAMA Dermatol. 2021;157(2):157–165. doi:10.1001/jamadermatol.2020.4372
19. The National Heart Lung and Blood Institute. Quality assessment tool for case series studies; 2020. Available from: http://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/case_series.
20. Kinsella FA, Amel Kashipaz MR, Scarisbrick J, Malladi R. Donor-derived mycosis fungoides following reduced intensity haematopoietic stem cell transplantation from a matched unrelated donor. BMJ Case Rep. 2017;2017:bcr2016216331. doi:10.1136/bcr-2016-216331
21. Fujimura T, Furudate S, Tanita K, et al. Successful treatment of relapsed folliculotropic mycosis fungoides with mogamulizumab followed by intensity-modulated radiotherapy. J Dermatol. 2018;45(4):e84–e85. doi:10.1111/1346-8138.14111
22. Jiang Y, Xue R, Wang W, Chen Y. Low-dose methotrexate combined with superficial X-ray in the treatment of folliculotropic mycosis fungoides: a case report. Indian J Dermatol Venereol Leprol. 2017;83(6):710–713. doi:10.4103/ijdvl.IJDVL_1077_16
23. MacArthur KM, Jariwala N, Kim EJ, Rook AH. Topical carmustine as monotherapy or as multimodality therapy for folliculotropic mycosis fungoides. Acta Derm Venereol. 2017;97(3):373–374. doi:10.2340/00015555-2551
24. Amitay-Laish I, Prag-Naveh H, Dalal A, Pavlovsky L, Feinmesser M, Hodak E. Treatment of early folliculotropic mycosis fungoides with special focus on psoralen plus ultraviolet A. Acta Derm Venereol. 2018;98(10):951–955. doi:10.2340/00015555-3013
25. Boix-Vilanova J, Corral-Magaña O, Martin-Santiago A, Escalas J. Folliculotropic mycosis fungoides in a pediatric patient with response to psoralen-ultraviolet A phototherapy. Photodermatol Photoimmunol Photomed. 2019;35(1):54–56. doi:10.1111/phpp.12414
26. Wang RF, Sokumbi O, Chiu YE. Folliculotropic mycosis fungoides in a pediatric patient mimicking black dot tinea capitis. Pediatr Dermatol. 2019;36(3):386–387. doi:10.1111/pde.13768
27. Caccavale S, Vitiello P, Franco R, et al. Dermoscopic characterization of folliculotropic mycosis fungoides selectively localized on trunk and limbs. Int J Dermatol. 2019;58(10):e187–e189.
28. Doerschner M, Pekar-Lukacs A, Messerli-Odermatt O, et al. Interferon alfa-2a maintenance after salvage autologous stem cell transplantation in atypical mycosis fungoides with central nervous system involvement. Br J Dermatol. 2019;181(6):1296–1302. doi:10.1111/bjd.17535
29. Kamijo H, Sugaya M. Two distinct variants of mycosis fungoides (MF): folliculotropic MF and erythrodermic MF. J Dermatol. 2019;46(12):1136–1140. doi:10.1111/1346-8138.15114
30. Kurihara K, Shimauchi T, Kasuya A, Yatagai T, Ito T, Tokura Y. Multiple facial plaques of diffuse plane xanthoma arising from regressed tumours of folliculotropic mycosis fungoides. Clin Exp Dermatol. 2021;46(2):358–360. doi:10.1111/ced.14387
31. Valencia Ocampo OJ, Julio L, Zapata V, et al. Mycosis fungoides in children and adolescents: a series of 23 cases. Actas Dermosifiliogr. 2020;111(2):149–156. doi:10.1016/j.ad.2019.04.004
32. Laggis CW, Lamb A, Secrest AM, et al. Favourable outcomes in folliculotropic mycosis fungoides after multimodality treatment in a single institution. J Eur Acad Dermatol Venereol. 2021;35(1):e42–e45. doi:10.1111/jdv.16790
33. Oxford centre for evidence-based medicine 2011 levels of evidence; 2011. Available from: http://www.cebm.net/wp-content/uploads/2014/06/CEBM-Levels-of-Evidence-2.1.pdf.
34. Roccuzzo G, Fava P, Avallone G, et al. Time to next treatment and safety assessment in cutaneous-T-cell lymphomas: a retrospective analysis on patients treated with bexarotene and Acitretin [published online ahead of print, 2022 Jul 13]. Br J Dermatol. 2022. doi:10.1111/bjd.21772
35. Navi D, Riaz N, Levin YS, Sullivan NC, Kim YH, Hoppe RT. The Stanford University experience with conventional-dose, total skin electron-beam therapy in the treatment of generalized patch or plaque (T2) and tumor (T3) mycosis fungoides. Arch Dermatol. 2011;147(5):561–567. doi:10.1001/archdermatol.2011.98
36. Hirotsu KE, Neal TM, Khodadoust MS, et al. Clinical characterization of mogamulizumab-associated rash during treatment of mycosis fungoides or sézary syndrome. JAMA Dermatol. 2021;157(6):700–707. doi:10.1001/jamadermatol.2021.0877
37. Roccuzzo G, Cavallo F, Avallone G, et al. Guttate psoriasis in a patient with mycosis fungoides in treatment with Brentuximab vedotin: an unreported association. Dermatol Ther. 2022;35(4):e15309. doi:10.1111/dth.15309
38. Olsen EA, Whittaker S, Kim YH, et al. Clinical end points and response criteria in mycosis fungoides and Sezary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29(18):2598–2607. doi:10.1200/JCO.2010.32.0630
39. Gallo G, Pileri A, Starace M, et al. Clinical and trichoscopic features in 18 cases of Folliculotropic Mycosis Fungoides with scalp involvement. Sci Rep. 2021;11(1):10555. doi:10.1038/s41598-021-90168-9
40. Tomasini C, Kempf W, Novelli M, et al. Spiky follicular mycosis fungoides: a clinicopathologic study of 8 cases. J Cutan Pathol. 2015;42(3):164–172. PMID: 25355400. doi:10.1111/cup.12399
41. Elsayad K, Rolf D, Sunderkötter C, et al. Low-dose total skin electron beam therapy plus oral bexarotene maintenance therapy for cutaneous T-cell lymphoma. J Dtsch Dermatol Ges. 2022;20(3):279–285.
42. Elsayad K, Kroeger K, Greve B, et al. Low-dose total skin electron beam therapy: quality of life improvement and clinical impact of maintenance and adjuvant treatment in patients with mycosis fungoides or Sezary syndrome. Niedrigdosis-Ganzhautelektronenbestrahlung: verbesserung der Lebensqualität und klinische Auswirkungen der Erhaltungstherapie bei Patienten mit Mycosis fungoides oder Sézary-Syndrom. Strahlenther Onkol. 2020;196(1):77–84. doi:10.1007/s00066-019-01517-7
43. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

