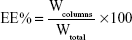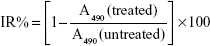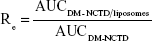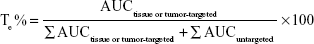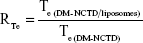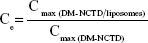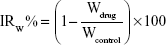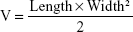Back to Journals » International Journal of Nanomedicine » Volume 11
Folate receptor-targeted liposomes loaded with a diacid metabolite of norcantharidin enhance antitumor potency for H22 hepatocellular carcinoma both in vitro and in vivo
Authors Liu M, Liu L, Wang X, Shuai W, Hu Y, Han M, Gao J
Received 22 September 2015
Accepted for publication 5 February 2016
Published 4 April 2016 Volume 2016:11 Pages 1395—1412
DOI https://doi.org/10.2147/IJN.S96862
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lei Yang
Min-Chen Liu,1 Lin Liu,1 Xia-Rong Wang,1 Wu-Ping Shuai,2 Ying Hu,3 Min Han,1 Jian-Qing Gao1
1Institute of Pharmaceutics, College of Pharmaceutical Sciences, 2First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, 3Zhejiang Pharmaceutical College, Ningbo, People’s Republic of China
Abstract: The diacid metabolite of norcantharidin (DM-NCTD) is clinically effective against hepatocellular carcinoma (HCC), but is limited by its short half-life and high incidence of adverse effects at high doses. We developed a DM-NCTD-loaded, folic acid (FA)-modified, polyethylene glycolated (DM-NCTD/FA-PEG) liposome system to enhance the targeting effect and antitumor potency for HCC at a moderate dose based on our previous study. The DM-NCTD/FA-PEG liposome system produced liposomes with regular spherical morphology, with mean particle size approximately 200 nm, and an encapsulation efficiency >80%. MTT cytotoxicity assays demonstrated that the DM-NCTD/FA-PEG liposomes showed significantly stronger cytotoxicity effects on the H22 hepatoma cell line than did PEG liposomes without the FA modification (P<0.01). We used liquid chromatography–mass spectrometry for determination of DM-NCTD in tissues and tumors, and found it to be sensitive, rapid, and reliable. In addition, the biodistribution study showed that DM-NCTD liposomes improved tumor-targeting efficiency, and DM-NCTD/FA-PEG liposomes exhibited the highest efficiency of the treatments (P<0.01). Meanwhile, the results indicated that although the active liposome group had an apparently increased tumor-targeting efficiency of DM-NCTD, the risk to the kidney was higher than in the normal liposome group. With regard to in vivo antitumor activity, DM-NCTD/FA-PEG liposomes inhibited tumors in H22 tumor-bearing mice better than either free DM-NCTD or DM-NCTD/PEG liposomes (P<0.01), and induced considerably more significant cellular apoptosis in the tumors, with no obvious toxicity to the tissues of model mice or the liver tissue of normal mice, as shown by histopathological examination. All these results demonstrate that DM-NCTD-loaded FA-modified liposomes might have potential application for HCC-targeting therapy.
Keywords: active targeting, PEGylated liposome, diacid metabolite of norcantharidin, hepatic cancer
Introduction
Hepatocellular carcinoma (HCC) is the sixth-most prevalent cancer and one of the leading causes of cancer-related death worldwide.1 Unfortunately, only approximately 20% of patients with HCC are eligible for curative treatments that may achieve long-term complete response and improved survival, as most patients present with advanced or unresectable disease and are suitable only for palliative approaches.2–4 Systemic chemotherapy is commonly used as a palliative treatment to improve survival of patients with HCC.3
Among the chemotherapeutic options for HCC are natural products, which have been used in cancer therapy by skilled Chinese practitioners for thousands of years.5 Norcantharidin (NCTD), a demethylation derivative of cantharidin, obtained from the dried body of the Chinese blister beetle (Mylabris spp.), is one such natural anticancer agent.6–8 In a previous study,9 we found that NCTD was unstable and subject to ring opening and hydrolysis. The diacid metabolite (DM) of NCTD is a stable form of NCTD.9,10 Clinical studies have shown that DM-NCTD is effective against HCC as an inhibitor of PP1 and PP2A.11 Recent studies have also indicated that DM-NCTD administered orally or intravenously has potential applications in cancer chemotherapy.12,13 Currently, the clinical application of DM-NCTD is limited by its short half-life (t½), as it is eliminated rapidly. Even high doses of DM-NCTD are unable to maintain a high level of circulating activity,9 and such doses are prone to causing serious adverse effects, including intense irritation of the urinary organs, leading to nephrotoxicity and inflammation.14–16 To improve the safety and efficacy of this drug treatment, many new alternative formulations, such as microspheres, microemulsions, and nanoparticles, have been studied.10,17,18
Liposomes from naturally occurring phospholipids are biocompatible carriers, and their application in drug-delivery systems is known to reduce drug toxicity and increase therapeutic efficacy.19 Liposomes are one of the only two families of therapeutic nanocarriers that have been approved for clinical practice.20 In particular, polyethylene glycolated (PEGylated) liposomes, also known as sterically stabilized liposomes, significantly prolong the circulation time of drugs in vivo by reducing phagocytosis by the reticuloendothelial system and thus improving the efficacy of the cancer therapy.21–23 Although sterically stabilized liposomes achieve more drug accumulation in the tumor region through an enhanced permeability and retention (EPR) effect,24–26 a passive targeting effect cannot guarantee increased cellular uptake of the drug(s).27–29 Therefore, insufficient uptake at tumor sites will decrease the therapeutic benefit of the administered drug dose, and nonspecific association with healthy tissues can lead to toxic adverse effects, limiting the maximum dosage that can be applied safely. This limitation prevents drug-loaded liposomal preparations from achieving their potential therapeutic effects. One strategy to achieve cancer-targeted drug delivery is the utilization of unique molecular markers that are specifically overexpressed within the cancerous tissues.
The folate receptor (FR) is a 38 kDa glycosylphosphatidylinositol membrane-anchored glycoprotein that is overexpressed in various cancers.30 Conversely, FR expression in normal tissues is considerably reduced compared with tumor tissues. The distinct expression pattern of FR in normal and malignant tissues makes it an ideal target for drug delivery. The natural ligand of FR, folic acid (FA), exhibits highly selective affinity for FR, and has been extensively explored as the targeting ligand for chemotherapeutic nanoparticle delivery because of its inherent high affinity, small size, and nontoxicity.31–34 Therefore, FA specifically promotes cancer-cell uptake through FR-mediated endocytosis.35–37
Therapeutic evaluation of DM-NCTD liposomes in tumors is essential to improving cancer therapy. In a previous study, we confirmed the prolonged circulation characteristics of related parameters and increased the relative bioavailability (Fr) of DM-NCTD liposomes (compared with DM-NCTD as the reference formulation) in Kunming mice at a moderate dose (2 mg/kg) converted from the clinical dosage used in the People’s Republic of China (PRC).9,38 Although the results indicated that a liposomal drug-delivery system could have the potential to overcome the shortcomings of DM-NCTD by improving Fr and increasing therapeutic efficacy, further in vitro and in vivo studies on tumor-targeted FR-mediated DM-NCTD liposome therapy are still needed to confirm these results. Therefore, the aim of this study was to develop tumor-targeted delivery for DM-NCTD and confirm its targeting characteristics at a moderate dose. We prepared DM-NCTD encapsulated in PEG liposomes (DM-NCTD/PEG liposomes) and in FA-PEG liposomes (DM-NCTD/FA-PEG liposomes) to be assessed as tumor-targeting carriers for DM-NCTD. In addition, the DM-NCTD liposomes were characterized, and the characteristics of in vitro DM-NCTD release from the DM-NCTD liposomes were investigated. Furthermore, the biodistribution of DM-NCTD in H22 tumor-bearing mice was assessed to reveal the tumor-targeting effect of DM-NCTD liposomes. Both the in vitro and in vivo antineoplastic activity of DM-NCTD liposomes were studied, including their cytotoxicity against H22 cells in vitro and their tumor inhibition in vivo in H22 tumor-bearing mice. Additionally, tumor-cell apoptosis and the preliminary toxicity of the various formulations in the tissues was assessed by terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick-end labeling (TUNEL) assay and hematoxylin and eosin (H&E) staining.
Materials and methods
Materials
The materials used were the same as those described in our previous study.38 NCTD (purity >98%) was purchased from J&K Scientific (Beijing, PRC). DM-NCTD was converted from NCTD (1.11:1, molar ratio). Ribavirin was used as the internal reference standard (IS) (purchased from the National Institutes for Food and Drug Control, Beijing, PRC), while 1,2-distearoyl-sn-glycero-3-phosphatidylcholine (DSPC), 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(PEG)-2000] (DSPE-PEG2000), and DSPE-PEG2000-FA were all purchased from Resenbio (Xi’an, PRC). All other chemicals were analytical grade.
The H22 murine hepatoma cell line was obtained from the Shanghai Cell Center of the Chinese Academy of Medical Science (Shanghai, PRC). Equal numbers of male and female Kunming mice, 20–25 g in weight, were supplied by the Experimental Animal Center, Zhejiang University (Hangzhou, PRC). All animal-handling procedures were performed according to the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and followed the guidelines of the Animal Welfare Act. All animal experiments were approved by the Ethical Committee of Animal Experimentation of Zhejiang University.
Preparation of DM-NCTD liposomes
DM-NCTD/FA-PEG liposomes were prepared using reverse-phase evaporation.38 The main procedure was as follows: DSPC, cholesterol, DSPE-PEG2000, and DSPE-PEG2000-FA (2:1:0.11:0.017 molar ratio) were all dissolved in chloroform. To obtain an emulsion, the lipid solution was mixed with phosphate-buffered saline (PBS) containing DM-NCTD and then homogenized in a sonicator (JY92-II; Ningbo Science Biotechnology Co Ltd, Ningbo, PRC). The obtained emulsion was evaporated by a rotary evaporator (RE-52C; Shanghai Yaguang Instrument Co Ltd, Shanghai, PRC) under negative pressure at 60°C to form the liposome suspensions. The resulting mixture was then homogenized again in a sonicator for 20 minutes to form well-proportioned liposomes. The resulting liposome suspension was successively extruded through a polycarbonate membrane with pore size of 0.22 μm. The DM-NCTD/PEG liposomes were prepared using the same procedure, but without DSPE-PEG2000-FA.
Characterization of DM-NCTD liposomes
Size, zeta potential, and morphology of liposomes
The size and zeta potential of the DM-NCTD liposomes were measured using a Zetasizer 3000HS (Malvern Instruments, Malvern, UK) in accordance with the manufacturer’s protocol. Dispersion Technology software, version 4.20 (Malvern Instruments) was used to analyze the effective diameter. The measurements were performed in triplicate. The vesicle shape of the different liposome formulations was evaluated by high-resolution transmission electron microscopy (Tecnai G2 F20 S-Twin; FEI, Hillsboro, OR, USA).
Determination of the encapsulation efficiency of the DM-NCTD liposomes
Encapsulation efficiency (EE) was determined using a previously reported method.39 Briefly, 1 mL of the liposome dispersion was eluted with PBS (pH 7.4) through a Sephadex G-50 column (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) to remove the unloaded DM-NCTD. The quantity of entrapped drug was determined by disrupting the liposome dispersion with methanol. The drug concentration in the liposomes was measured by high-performance liquid chromatography (HPLC; Agilent 1200; Agilent Technologies, Santa Clara, CA, USA). The EE of DM-NCTD was estimated as follows:
|
|
where Wcolumns is the measured amount of DM-NCTD in the liposome suspension after separation in the column and Wtotal is the measured amount of DM-NCTD in an equal volume of the liposome suspension before separation in the column.
The amount of DM-NCTD in the liposomes was measured using HPLC at room temperature. A Zorbax SB-C18 column (4.6×250 mm, 5 μm) was employed. The mobile phase was methanol and 0.025 M potassium dihydrogen phosphate (11:89 v/v), and the pH of the aqueous phase was adjusted with H3PO4 to 3. The flow rate was 1.0 mL/min. The wavelength of the ultraviolet detector was 210 nm. The injection volume was 20 μL.
In vitro drug-release assay
Drug release from the DM-NCTD liposomes was measured by dialysis. Briefly, 10 mL of DM-NCTD liposomes was placed into a dialysis bag (2,000 Da molecular weight cutoff), which was then placed into a beaker containing 50 mL of medium and stirred for 48 hours at 37°C±0.5°C. At various time points, aliquots were withdrawn from the beaker and replaced with equal volumes of the medium. Aliquots (10 mL) of the DM-NCTD liposomes were mixed with the same amount of medium as the total dosage, which was determined by disrupting the liposome dispersions with methanol. The DM-NCTD concentrations were then measured by HPLC, as described in the previous section. To allow comparison with DM-NCTD, the release medium was PBS (pH 7.4).
EE and particle-size stability in vitro
Liposome stability under in vitro storage conditions is an important criterion for both in vitro and in vivo biomedical applications. To investigate the EE and particle-size stability of DM-NCTD liposomes in PBS (pH 7.4) at 4°C in the dark, three batches of DM-NCTD liposomes (DM-NCTD/PEG liposomes and DM-NCTD/FA-PEG liposomes) were assessed at various time points (1, 7, 15, and 30 days) by the methods described in previous sections.
In vitro cytotoxicity of the DM-NCTD liposomes
The in vitro antineoplastic activity of the DM-NCTD liposomes was determined by their cytotoxicity to H22 cells, using the 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. Cells in logarithmic growth were seeded into 96-well plates at a density of 104 cells per well and incubated for 24 hours, then the medium was exchanged for fresh medium. The cells were then treated with 100 μL of sterile samples (DM-NCTD, DM-NCTD/PEG liposomes, and DM-NCTD/FA-PEG liposomes) at a range of concentrations (0.8, 4, 20, 100, and 500 μg/mL). Next, the cells were incubated for 24 and 48 hours. After incubation, 20 μL of a 5 mg/mL MTT solution in PBS was added to each well, and the plate was incubated for an additional 4 hours. The supernatant was then carefully removed, and 100 μL of dimethyl sulfoxide was added to each well. After the formazan crystals had dissolved completely, optical density at 490 nm (A490) was determined with a model 680 Microplate Reader (Bio-Rad Laboratories Inc, Hercules, CA, USA). The inhibition rate (IR) of the treated cells was defined as follows:
|
|
with A490 values for the treated and untreated cells.
Validation of analytical method in vivo
A Sciex 4000 Q-Trap tandem quadrupole mass spectrometer, equipped with an electrospray ionization source (Thermo Fisher Scientific, Waltham, MA, USA), a Paradigm MS4B series for HPLC (Bruker Corporation, Billerica, MA, USA), and an HTC PAL autosampler (CTC Analytics AG, Zwingen, Switzerland), was used for the liquid chromatography tandem mass spectrometry (LC-MS/MS) analysis. The apparatus and LC-MS/MS conditions were the same as those described in our previous study.9
Whole tissues and tumor samples were collected from the mice and stored at -80°C until use. The samples were homogenized in saline (tissue or tumor:water ratio of 1:4, w/v). A 100 μL aliquot of each sample was combined with 57 μL of 1 M hydrochloric acid and 43 μL of IS (100 μg/mL), followed by the addition of 300 μL of acetone and vortexing for 5 minutes. Finally, the mixture was centrifuged at 12,000 rpm for 25 minutes at 4°C. A 200 μL aliquot of the supernatant was transferred to a 2 mL polypropylene centrifuge tube and evaporated using a condensation dryer. The residue was reconstituted with the appropriate amount of water containing 0.1% formic acid and then centrifuged at 12,000 rpm for 15 minutes at 4°C. Finally, 5 μL of the supernatant was injected for analysis. A thorough and complete validation method for assaying the DM-NCTD mouse samples was performed in accordance with the US Food and Drug Administration (FDA) guidelines.40
Biodistribution study
Preparation of the H22 tumor-bearing mouse model
The in vivo antineoplastic study was performed as previously described, with a few modifications.41,42 Briefly, after serial subcultivation of the H22 cells for 7 days, mice with viable H22 ascites tumors were killed by cervical dislocation under sterile conditions. The ascites were withdrawn and mixed with physiological saline to dilute the cell density to 1×107/mL. The tumor-cell ascites were injected subcutaneously into the right hind limb of each mouse at a dose of approximately 0.1 mL/10 g.
Biodistribution study in H22 tumor-bearing mice
When the tumor volume reached approximately 300 mm3, the H22 tumor-bearing mice were randomly assigned to one of three groups: group 1 received DM-NCTD, group 2 received DM-NCTD/PEG liposomes, and group 3 received DM-NCTD/FA-PEG liposomes. All groups were injected with 2 mg/kg DM-NCTD through the tail vein. The dose was converted from the clinical dosage used in the PRC (10–30 mg/60 kg per single dose for adults, and the ratio of adult to mice 1:9.01), and has been reported previously in a therapeutic anticancer study.43
After drug administration, the H22 tumor-bearing mice were killed at 1, 2, 4, and 8 hours. The tumors or excised tissues (heart, liver, spleen, lung, and kidney) were collected, blotted with a paper towel, rinsed in saline, blotted again to remove excess fluid, weighed, and homogenized as described in the “Validation of analytical method in vivo” section. Meanwhile, the concentration of DM-NCTD in each sample was measured by LC-MS/MS as described in the same section.
The area under the curve (AUC) from 0 to 8 hours and peak concentration (Cmax) in the tissues and tumors were calculated using a noncompartment model with PK Solver version 2.0 (China Pharmaceutical University, Nanjing, People’s Republic of China). Based on the AUC and Cmax values, four targeting parameters – relative intake rate (Re), tissue/tumor-targeting efficacy (Te), relative targeting efficiency (RTe), and peak concentration ratio (Ce) – were calculated to evaluate the tissue or tumor-specific targeting of the DM-NCTD liposomes, as per the equations below:44
|
|
|
|
|
|
|
|
In vivo antineoplastic activity and preliminary toxicity of the DM-NCTD liposomes
After the preparation of the H22 tumor-bearing mouse model described, the model mice were randomly assigned to one of the four different experimental groups: the control group (PBS) or the groups that were administered DM-NCTD, DM-NCTD/PEG liposomes, or DM-NCTD/FA-PEG liposomes. Moreover, to investigate the preliminary toxicity of the DM-NCTD and DM-NCTD liposomes on normal liver tissue, Kunming mice were randomly assigned to the same four experimental groups mentioned.
Each mouse was injected with 2 mg/kg DM-NCTD through the tail vein. For the model mice, tumor growth was investigated in H22 tumor-bearing mice injected with DM-NCTD or DM-NCTD liposomes on days 1–9 after H22 cells were injected on day 0 (n=10). The normal mice were each injected on days 1–9 with DM-NCTD or DM-NCTD liposomes as planned (2 mg/kg, n=10). The mice were killed by cervical dislocation on day 10, and then subcutaneous tumors of the model mice were carefully collected and weighed. The IR on tumor weight (IRw) was assessed as follows:
|
|
where Wdrug and Wcontrol are the tumor weights of the drug-administered and control groups, respectively. Tumor size was measured every day on days 1–10 with a Vernier caliper, and the individual tumor volumes (V) were calculated by the following formula:
|
|
The mice were also weighed every day.
Finally, the mice were killed and the major organs (heart, liver, spleen, lung, and kidney) and tumors were harvested and fixed in a 4% formaldehyde solution for 24 hours at room temperature. Paraffin wax sections were prepared from the harvested tissues. Tumor-cell apoptosis was detected by TUNEL assay according to the manufacturer’s protocol.45 The sections were treated with proteinase K and 0.3% H2O2 for 10 minutes, then the sections were incubated in the TUNEL reaction mixture. Finally, the sections were stained with a diaminobenzidine solution for 10 minutes at room temperature. Photographs were captured under a microscope (Olympus, ×200; Tokyo, Japan). The tissues were stained with H&E according to the manufacturer’s instructions and observed under bright-field microscopy (Olympus, ×200).
Statistical analysis
Data are expressed as mean ± standard deviation. Statistical significance was set at P<0.05.
Results and discussion
Dose of DM-NCTD
In our previous study, we confirmed the pharmacokinetic profiles of different-level doses of DM-NCTD in normal saline solution after administration to beagles, which could be helpful in identifying a rational dose range for DM-NCTD in the preclinical study.9 The pharmacokinetic study revealed that the risk of DM-NCTD intoxication may increase at high doses, and these results were consistent with the reported adverse effects for adults administered the high clinical dosage in the PRC. It is precisely because of these reasons that our study focused on increasing the t½ and decreasing the potential adverse effects of DM-NCTD when the drug was used at a moderate dose. In the current study, we confirmed that the liposome groups exhibited an apparently longer circulation time following intravenous administration to mice (2 mg/kg), while the Fr of DM-NCTD increased.38 We also investigated the tumor-targeting efficiency, antineoplastic activity, and preliminary toxicity evaluation at the moderate dose on the basis of previous research, and our results provide a reference for further research into evaluating the risk of the high dose of DM-NCTD liposomes in further studies.
Characterization of the DM-NCTD liposomes
Size, zeta potential, and morphology
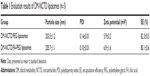
The average sizes of the DM-NCTD/PEG liposomes and DM-NCTD/FA-PEG liposomes were approximately 203 and 205 nm, respectively; these sizes are preferable for tumor accumulation via the EPR effect.24–26 Moreover, the zeta potential (5.9 and 6.9 mV, respectively) analyses demonstrated that both types of drug-loaded liposome exhibited approximately neutral surface charge, which would minimize undesirable nonspecific protein adsorption (Table 1).27 No significant changes were observed in any of the characteristics following the addition of FA to the liposomes. The particle-size distribution and zeta potential of the DM-NCTD/FA-PEG liposomes are presented in Figure 1A and B, while transmission electron microscopy (Figure 1C) demonstrates the regular spherical morphology of the DM-NCTD/FA-PEG liposomes. The EE values were both >80% (Table 1).
In vitro drug release
In vitro DM-NCTD-release data are presented in Figure 1D. DM-NCTD was quickly and completely released into PBS (pH 7.4). The release of DM-NCTD from the liposomes demonstrated that DM-NCTD liposomes released DM-NCTD in a sustained manner within 48 hours compared with DM-NCTD.
EE and particle-size stability in vitro
Previous studies have shown that DM-NCTD is a stable form of NCTD both in vitro and in vivo,7,9,10 and retains the main structure–activity relationships of NCTD.7,38 However, investigations into the stability of DM-NCTD liposomes are essential, because liposomal stability is a prerequisite for achieving long circulation time and tumor-targeted delivery, which is strongly influenced by the hydrophilic stealth layer, zeta potential, lipid composition, particle size, and distribution.46 In the current study, DSPE-PEG2000 was used as the definitive functional phospholipid in the liposomal formulation to maintain a long circulation time and specific stability. This compound is also used in the FDA-approved PEGylated liposomal doxorubicin.46,47
In the stability studies, the results demonstrated that the particle size of the liposomes ranged from 203.5 to 206 nm in the DM-NCTD/PEG liposome group and from 205.7 to 208 nm in the DM-NCTD/FA-PEG liposome group (Figure 1E). In addition, the EE values ranged from 82.3% to 80.5% in the DM-NCTD/PEG liposome group and from 80.1% to 79% in the DM-NCTD/FA-PEG liposome group (Figure 1F). No significant changes were observed in the two groups, indicating that the liposomes were stable for 1 month at 4°C in the dark.
In vitro cytotoxicity of the DM-NCTD liposomes
First, the cytotoxicity of the blank liposomes was investigated. The concentration of the blank liposomes was equal to the concentration of the drug-loaded liposomes used in the cytotoxicity assay. The blank liposomes were incubated with H22 cells for 24 and 48 hours. Over 95% of the cells survived during the incubation period, indicating that the blank liposomes did not exhibit cytotoxicity against H22 cells.
H22 cells were incubated with DM-NCTD, DM-NCTD/PEG liposomes, or DM-NCTD/FA-PEG liposomes for 24 and 48 hours to analyze the cytotoxicity of these treatments, as depicted in Figure 2. The DM-NCTD concentrations in the liposomes were equal to those of the free DM-NCTD (0.8, 4, 20, 100, and 500 μg/mL). Both dose-dependent and time-dependent cytotoxicity were observed. All of the groups exhibited cytotoxicity against H22 cells, as shown in Figure 2. In this study, half-maximal inhibitory concentration values revealed that free DM-NCTD was the most efficacious among the three treatments in vitro at 24 and 48 hours (P<0.01) (Table 2). The results demonstrated that the cytotoxicity of DM-NCTD/PEG liposomes and DM-NCTD/FA-PEG liposomes increased more rapidly compared with DM-NCTD from 24 to 48 hours. Figure 2 shows that the DM-NCTD/PEG liposome group exhibited the lowest cytotoxicity of the three groups, and that the unmodified liposomes displayed very little cytotoxicity at doses of 4 and 20 μg/mL at 24 hours and no cytotoxicity at a dose of 0.8 μg/mL at both 24 and 48 hours. However, the FA-modified liposomes performed better than the unmodified liposomes, particularly at low doses at 24 hours (4 or 20 μg/mL at 24 hours, P<0.01).
In the different dose groups at 24 and 48 hours, the free drug exhibited the greatest antitumor effect of the three groups, and the FA-modified group was better than the unmodified group, indicating that DM-NCTD accumulated more easily than the other groups in H22 cells, owing to its intracellular antitumor role, as previously described.48–51 We believe that the cellular internalization mechanism of the free drug and drug-loaded liposomes contributed to this phenomenon. For the free drug, the low molecular weight (186.16 Da) of DM-NCTD might assist in its internalization into cells by direct diffusion, thus explaining why the free drug accumulated more quickly than the liposomes in the cells. For the unmodified group, the low IR is related to its sustained drug release. Reports indicate that PEGylation significantly reduces the cellular uptake and endosomal/lysosomal escape of liposomes and interferes with the tumor retention and antitumor efficacy of liposome-based drug-delivery systems,27–29 indicating that encapsulated DM-NCTD may be internalized via diffusion after extracellular release from liposomes. Finally, with regard to the FA-modified liposomal group, FA is an active targeting moiety and natural element that specifically promotes uptake of these liposomes by cancer cells through FR-mediated endocytosis.35–37 Although the results for cytotoxicity of the FA-modified liposomal drug-delivery system are consistent with the previous reports mentioned, the hypothesized cellular internalization mechanism of DM-NCTD liposomes still needs to be confirmed.
Validation of the analytical method in vivo
LC-MS/MS methods may overcome the disadvantages that have been reported with the current DM-NCTD analytical methods, including low resolution of HPLC ultraviolet,52 instability of gas chromatography–MS,53 high lower limit of quantification, and long chromatography run times.54 In the current study, an LC-MS/MS method with multiple reaction monitoring was developed and validated for the determination of DM-NCTD in mouse tissues and tumor, using ribavirin as the IS.
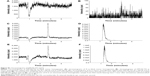
Specificity was assessed by analyzing three different samples of the blank matrix with and without DM-NCTD and IS, as well as the in vivo samples. No endogenous interference was noted from the tumor homogenates or from the tissue homogenates of heart, liver, spleen, lung, and kidney, thus ensuring the high specificity of the LC-MS/MS method. In this study, we used the tumor homogenates as an example (Figure S1). The retention times of DM-NCTD and the IS were approximately 2.8 and 1.4 minutes, respectively. Inter- and intraday precision, matrix effect, and extraction recovery for three quality-control samples from five replicates are presented in Table S1. The stability of DM-NCTD was within 15%, as indicated by the relative standard deviation for the quality-control samples (n=5). As shown in Tables S2–S4, no significant difference was observed under the different stability conditions, indicating that DM-NCTD was stable under the storage, disposition, and analysis conditions used. The method was sufficiently sensitive for biodistribution analysis of DM-NCTD in H22 tumor-bearing mice.
In vivo biodistribution in H22 tumor-bearing mice
FA-modified liposomes were used as biocompatible carriers to reduce the potential toxicity of DM-NCTD, prolong its retention time,38 and increase its tumor-targeting ability.31–34 In the current study, the tissue- and tumor-targeting characteristics of DM-NCTD and DM-NCTD liposomes were evaluated through biodistribution. Figure 3 presents the results of tissue- and tumor-distribution in samples obtained at 1, 2, 4, and 8 hours after injection of DM-NCTD, DM-NCTD/PEG liposomes, or DM-NCTD/FA-PEG liposomes into the mouse tail vein (2 mg/kg, n=6). Free DM-NCTD was present in the tissues and tumors, with particularly high levels noted in the liver, spleen, and kidney. The highest concentrations were achieved at 2 hours. However, the concentration in the tumor was very low and was rapidly eliminated. Compared with DM-NCTD, DM-NCTD liposomes improved the tumor-targeting efficiency, and DM-NCTD/FA-PEG liposomes exhibited the highest efficiency of the three treatments (Tables 3 and 4).
The noncompartment parameters of AUC0–8, t½, and Cmax for DM-NCTD liposomes and free DM-NCTD in the tissues and tumors of H22 tumor-bearing mice after intravenous administration are presented in Table 3. Re, Te, RTe, and Ce were calculated using AUC0–8 and Cmax, and the results are presented in Table 4. The DM-NCTD/PEG liposome and DM-NCTD/FA-PEG liposome groups exhibited excellent tumor-targeting efficiency (Re 4.86 and 9.25, Te 12.81% and 24.44%, RTe 2.36 and 4.50, and Ce 4.78 and 9.24, respectively) compared with the DM-NCTD group. Given that liposomes have passive targeting ability owing to their small size (approximately 200 nm), an EPR effect is achieved, and the particles accumulate relatively easily into well-vascularized tumors through vascular fenestrations.24–26 The results in Table 3 show that the AUC0–8, t½, and Cmax of tumor tissue were significantly prolonged in both the unmodified and FA-modified liposomal groups (P<0.01), which indicates that the EPR effects might be helpful for this kind of liposomal group loaded with DM-NCTD-targeting tumors. In addition, the FA-modified group exhibited increased tumor-targeting efficiency compared with the other groups, owing to its active targeting mechanism (Table 4).35–37
Overall, in comparison with DM-NCTD, we found that both DM-NCTD/PEG liposomes and DM-NCTD/FA-PEG liposomes were able to decrease the efficiency of kidney-specific targeting (Re 0.46 and 0.94, Te 6.93% and 14.32%, RTe 0.22 and 0.46, and Ce 0.37 and 0.87, respectively), as shown in Table 4, which helped to decrease the potential nephrotoxicity of DM-NCTD. Meanwhile, with regard to DM-NCTD/PEG liposomes and DM-NCTD/FA-PEG liposomes, it should be noted that not only did tumor-targeting efficiency increase but the efficiency of kidney-specific targeting was also improved (AUC0–8 ranged from 586.98±29.48 to 1,209.74±36.17 ng·h/mL, and Cmax from 157.23±13.25 to 371.35±16.10 ng/mL), as shown in Table 3 (P<0.01). The results revealed that although the active liposome group had an apparent increase in the tumor-targeting efficiency of DM-NCTD, the risk of DM-NCTD intoxication to kidneys was higher than in the normal liposome group, which is consistent with our previous report.38
In the future, we will need to perform a comprehensive and thorough safety evaluation of FA-modified liposome-loaded DM-NCTD. Based on the results of the current study, we will perform a further study on the antineoplastic activity and preliminary toxicity of DM-NCTD and its liposomes.
In vivo antineoplastic activity and preliminary toxicity of the DM-NCTD liposomes
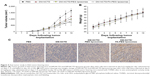
To provide in vivo evidence for the antitumor potential of the DM-NCTD liposomes, their antitumor efficacy was investigated using H22 tumor-bearing mice. Tumor growth was measured using a Vernier caliper, and the growth curve is presented in Figure 4A. All of the experimental formulations were effective in preventing tumor growth compared with PBS treatment. Mice treated with the DM-NCTD/FA-PEG liposomes displayed stronger tumor inhibition compared with those treated with either DM-NCTD or DM-NCTD/PEG liposomes (P<0.01).
The IRw in response to the treatments was also determined (Table 5). DM-NCTD liposomes exhibited excellent antitumor activity, with an IRw of 40.41% and 67.81% for unmodified and FA-modified liposomes, respectively, which was considerably enhanced compared with free DM-NCTD. Figure 4B demonstrates a similar weight-change range of the mice during the experiment. Therefore, none of the treatments influenced the weight of the model mice as a result of the tumor-specific toxicity of DM-NCTD liposomes. We conclude that delivery of DM-NCTD in the FA-PEG liposomes improves tumor inhibition in vivo.
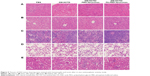
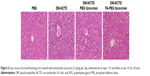
Tumor-cell apoptosis and the preliminary tissue toxicity of the various formulations was assessed by TUNEL assays and H&E staining. DM-NCTD/FA-PEG liposomes induced more significant increases in the levels of tumor-cell apoptosis compared with DM-NCTD/PEG liposomes and free drug (Figure 4C), and none of the groups exhibited obvious toxicity to the tissues of model mice (Figure 5) or to the liver tissue of normal mice (Figure 6). The investigations also revealed that the moderate dose used did not result in any obvious adverse effects on kidneys or other tissues of the model mice, including the groups treated with DM-NCTN or DM-NCTD liposomes. However, although there were no obvious adverse effects on kidneys during the in vivo antineoplastic activity in this study in mice administered the moderate dose, it remains unclear whether the liposomes could decrease the adverse effects of DM-NCTD when used at high doses, which requires further study. Meanwhile, histopathological examination alone is not enough to evaluate toxicity to kidney function; there still needs to be further evaluation of the toxicity to kidneys and other tissue function after the in vivo study.
Conclusion
The current study describes a novel method using DM-NCTD-loaded FA-modified liposomes as a tumor-targeted drug-delivery system. The liposomes exhibited excellent stability and showed sustained drug release. The LC-MS/MS method used to determine the concentrations of DM-NCTD in tissues and tumors is sensitive, rapid, and reliable. In addition, the biodistribution study showed that DM-NCTD liposomes improved tumor-targeting efficiency, and DM-NCTD/FA-PEG liposomes exhibited the highest efficiency of the treatments (P<0.01). Meanwhile, the results revealed that although the active liposome group had an apparent increase in the tumor-targeting efficiency of DM-NCTD, the risk of DM-NCTD intoxication to kidneys was higher than in the normal liposome group (P<0.01). Compared with DM-NCTD/PEG liposomes, DM-NCTD/FA-PEG liposomes exhibited enhanced cytotoxicity toward H22 cells (P<0.01) and a significant antitumor effect in H22 tumor-bearing mice (P<0.01), with more significant tumor-cell apoptosis and no obvious toxicity to the tissues of model mice or to the liver tissue of normal mice, as demonstrated by the results of histopathological examination. The results of this research demonstrate that DM-NCTD-loaded FA-modified liposomes might be a potential tumor-targeting drug-delivery system for HCC treatment.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81403113, 81373346), Fundamental Research Funds for the Central Universities (2015QNA7034), and Zhejiang Provincial Program for the Cultivation of High-Level Innovative Health Talents.
Disclosure
The authors report no conflicts of interest in this work.
References
Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379(9822):1245–1255. | ||
Rahbari NN, Mehrabi A, Mollberg NM, et al. Hepatocellular carcinoma: current management and perspectives for the future. Ann Surg. 2011;253(3):453–469. | ||
Thomas MB, O’Beirne JP, Furuse J, Chan AT, Abou-Alfa G, Johnson P. Systemic therapy for hepatocellular carcinoma: cytotoxic chemotherapy, targeted therapy and immunotherapy. Ann Surg Oncol. 2008;15(4):1008–1014. | ||
Abou-Alfa GK, Venook AP. The impact of new data in the treatment of advanced hepatocellular carcinoma. Curr Oncol Rep. 2008;10(3):199–205. | ||
Wang S, Wu X, Tan M, et al. Fighting fire with fire: poisonous Chinese herbal medicine for cancer therapy. J Ethnopharmacol. 2012; 140(1):33–45. | ||
Wang GS. Medical uses of Mylabris in ancient China and recent studies. J Ethnopharmacol. 1989;26(2):147–162. | ||
Bertini I, Calderone V, Fragai M, Luchinat C, Talluri E. Structural basis of serine/threonine phosphatase inhibition by the archetypal small molecules cantharidin and norcantharidin. J Med Chem. 2009; 52(15):4838–4843. | ||
Carrel JE, Eisner T. Cantharidin: potent feeding deterrent to insects. Science. 1974;183(4126):755–757. | ||
Liu M, Ma X, Jin Z, Li W, Guo M, Li F. Determination and pharmacokinetic study of the diacid metabolite of norcantharidin in beagle plasma by use of liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2013;405(28):9273–9283. | ||
Ding XY, Hong CJ, Liu Y, et al. Pharmacokinetics, tissue distribution, and metabolites of a polyvinylpyrrolidone-coated norcantharidin chitosan nanoparticle formulation in rats and mice, using LC-MS/MS. Int J Nanomedicine. 2012;7:1723–1735. | ||
Bei YY, Chen XY, Liu Y, et al. Novel norcantharidin-loaded liver targeting chitosan nanoparticles to enhance intestinal absorption. Int J Nanomedicine. 2012;7:1819–1827. | ||
Zhao Q, Qian Y, Li R, et al. Norcantharidin facilitates LPS-mediated immune responses by up-regulation of AKT/NF-κB signaling in macrophages. PloS One. 2012;7(9):e44956. | ||
Lin X, Zhang B, Zhang K, et al. Preclinical evaluations of norcantharidin-loaded intravenous lipid microspheres with low toxicity. Expert Opin Drug Deliv. 2012;9(12):1449–1462. | ||
Hill TA, Stewart SG, Ackland SP, et al. Norcantharimides, synthesis and anticancer activity: synthesis of new norcantharidin analogues and their anticancer evaluation. Bioorg Med Chem. 2007;15(18):6126–6134. | ||
Lixin W, Haibing H, Xing T, Ruiying S, Dawei C. A less irritant norcantharidin lipid microspheres: formulation and drug distribution. Int J Pharm. 2006;323(1–2):161–167. | ||
Li DC, Zhong XK, Zeng ZP, et al. Application of targeted drug delivery system in Chinese medicine. J Control Release. 2009;138(2):103–112. | ||
Liu X, Heng WS, Li Q, Chan LW. Novel polymeric microspheres containing norcantharidin for chemoembolization. J Control Release. 2006;116(1):35–41. | ||
Zhang L, Sun X, Zhang ZR. An investigation on liver-targeting microemulsions of norcantharidin. Drug Deliv. 2005;12(5):289–295. | ||
Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4(2):145–160. | ||
Sanhai WR, Sakamoto JH, Canady R, Ferrari M. Seven challenges for nanomedicine. Nat Nanotechnol. 2008;3(5):242–244. | ||
Papahadjopoulos D, Allen TM, Gabizon A, et al. Sterically stabilized liposomes: improvements in pharmacokinetics and antitumor therapeutic efficacy. Proc Natl Acad Sci U S A. 1991;88(24):11460–11464. | ||
Bae YH, Park K. Targeted drug delivery to tumors: myths, reality and possibility. J Control Release. 2011;153(3):198–205. | ||
Li SD, Huang L. Stealth nanoparticles: high density but sheddable PEG is a key for tumor targeting. J Control Release. 2010;145(3):178–181. | ||
Maruyama K. Intracellular targeting delivery of liposomal drugs to solid tumors based on EPR effects. Adv Drug Deliv Rev. 2011;63(3):161–169. | ||
Yuan F, Dellian M, Fukumura D, et al. Vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size. Cancer Res. 1995;55(17):3752–3756. | ||
Maeda H, Sawa T, Konno T. Mechanism of tumor-targeted delivery of macromolecular drugs, including the EPR effect in solid tumor and clinical overview of the prototype polymeric drug SMANCS. J Control Release. 2001;74(1–3):47–61. | ||
Li Y, Liu R, Yang J, et al. Enhanced retention and anti-tumor efficacy of liposomes by changing their cellular uptake and pharmacokinetics behavior. Biomaterials. 2015;41:1–14. | ||
Yuan YY, Mao CQ, Du XJ, Du JZ, Wang F, Wang J. Surface charge switchable nanoparticles based on zwitterionic polymer for enhanced drug delivery to tumor. Adv Mater. 2012;24(40):5476–5480. | ||
Hong BJ, Chipre AJ, Nguyen ST. Acid-degradable polymer-caged lipoplex (PCL) platform for siRNA delivery: facile cellular triggered release of siRNA. J Am Chem Soc. 2013;135(47):17655–17658. | ||
Chen C, Ke J, Zhou XE, et al. Structural basis for molecular recognition of folic acid by folate receptors. Nature. 2013;500(7463):486–489. | ||
Gabizon A, Horowitz AT, Goren D, Tzemach D, Shmeeda H, Zalipsky S. In vivo fate of folate-targeted polyethylene-glycol liposomes in tumor-bearing mice. Clin Cancer Res. 2003;9(17):6551–6559. | ||
Huang Y, Yang T, Zhang W, et al. A novel hydrolysis-resistant lipophilic folate derivative enables stable delivery of targeted liposomes in vivo. Int J Nanomedicine. 2014;9:4581–4595. | ||
Gao JQ, Lv Q, Li LM, et al. Glioma targeting and blood-brain barrier penetration by dual-targeting doxorubincin liposomes. Biomaterials. 2013;34(22):5628–5639. | ||
Zhao XB, Lee RJ. Tumor-selective targeted delivery of genes and antisense oligodeoxyribonucleotides via the folate receptor. Adv Drug Deliv Rev. 2004;56(8):1193–1204. | ||
Antony AC. The biological chemistry of folate receptors. Blood. 1992;79(11):2807–2820. | ||
Park EK, Lee SB, Lee YM. Preparation and characterization of methoxy poly(ethylene glycol)/poly(epsilon-caprolactone) amphiphilic block copolymeric nanospheres for tumor-specific folate-mediated targeting of anticancer drugs. Biomaterials. 2005;26(9):1053–1061. | ||
Wang J, Liu W, Tu Q, et al. Folate-decorated hybrid polymeric nanoparticles for chemically and physically combined paclitaxel loading and targeted delivery. Biomacromolecules. 2011;12(1):228–234. | ||
Liu MC, Ma XQ, Xu Y, Peng LH, Han M, Gao JQ. Liquid chromatography-tandem mass spectrometry evaluation of the pharmacokinetics of a diacid metabolite of norcantharidin loaded in folic acid-targeted liposomes in mice. J Pharm Biomed Anal. 2016;119:76–83. | ||
Dipali SR, Kulkarni SB, Betageri GV. Comparative study of separation of non-encapsulated drug from unilamellar liposomes by various methods. J Pharm Pharmacol. 1996;48(11):1112–1115. | ||
US Department of Health and Human Services. Analytical procedures and methods validation for drugs and biologics: guidance for industry. 2015. Available from: www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm386366.pdf. Accessed January 27, 2016. | ||
Shimura N, Sogawa Y, Kawakita Y, Ikekita M, Yamazaki N, Kojima S. Radioiodination of glycoprotein-conjugated liposomes by using the Bolton-Hunter reagent and biodistribution in tumor-bearing mice. Nucl Med Biol. 2002;29(4):491–496. | ||
Xiong XB, Huang Y, Lu WL, et al. Enhanced intracellular delivery and improved antitumor efficacy of doxorubicin by sterically stabilized liposomes modified with a synthetic RGD mimetic. J Control Release. 2005;107(2):262–275. | ||
Wang Q, Zhang L, Hu W, et al. Norcantharidin-associated galactosylated chitosan nanoparticles for hepatocyte-targeted delivery. Nanomedicine. 2010;6(2):371–381. | ||
Luo LH, Zheng PJ, Nie H, et al. Pharmacokinetics and tissue distribution of docetaxel liposome mediated by a novel galactosylated cholesterol derivatives synthesized by lipase-catalyzed esterification in non-aqueous phase. Drug Deliv. Epub 2015 Jul 14. | ||
Liu JP, Feng L, Zhang MH, et al. Neuroprotective effect of liuwei dihuang decoction on cognition deficits of diabetic encephalopathy in streptozotocin-induced diabetic rat. J Ethnopharmacol. 2013;150(1):371–381. | ||
Gabizon A, Shmeeda H, Barenholz Y. Pharmacokinetics of PEGylated liposomal doxorubicin: review of animal and human studies. Clin Pharmacokinet. 2003;42(5):419–436. | ||
Wang T, Yin X, Lu Y, Shan W, Xiong S. Formulation, antileukemia mechanism, pharmacokinetics, and biodistribution of a novel liposomal emodin. Int J Nanomedicine. 2012;7:2325–2337. | ||
Chang C, Zhu Y, Tang X, Tao W. The anti-proliferative effects of norcantharidin on human HepG2 cells in cell culture. Mol Biol Rep. 2011;38(1):163–169. | ||
Chen YJ, Chang WM, Liu YW, et al. A small-molecule metastasis inhibitor, norcantharidin, downregulates matrix metalloproteinase-9 expression by inhibiting Sp1 transcriptional activity in colorectal cancer cells. Chem Biol Interact. 2009;181(3):440–446. | ||
Kok SH, Cheng SJ, Hong CY, et al. Norcantharidin-induced apoptosis in oral cancer cells is associated with an increase of proapoptotic to antiapoptotic protein ratio. Cancer Lett. 2005;217(1):43–52. | ||
Zhang S, Li G, Ma X, et al. Norcantharidin enhances ABT-737-induced apoptosis in hepatocellular carcinoma cells by transcriptional repression of Mcl-1. Cell Signal. 2012;24(9):1803–1809. | ||
Zhang JL, Cui M, He Y, Yu HL, Guo DA. Chemical fingerprint and metabolic fingerprint analysis of Danshen injection by HPLC-UV and HPLC-MS methods. J Pharm Biomed Anal. 2005;36(5):1029–1035. | ||
Wei C, Teng Y, Wang B, et al. Separation and identification of norcantharidin metabolites in vivo by GC-MS method. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879(20):1741–1747. | ||
Wei CM, Zhang R, Wang BJ, Yuan GY, Guo RC. Determination and pharmacokinetic study of norcantharidin in human serum by HPLC-MS/MS method. Biomed Chromatogr. 2008;22(1):44–49. |
Supplementary materials
  | Table S2 Stability test of DM-NCTD in different conditions (n=5) |
  | Table S3 Repeated freeze–thaw stability test of DM-NCTD (n=5) |
  | Table S4 The auto-sampler placed stability test of DM-NCTD (n=5) |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.