Back to Journals » Research and Reports in Urology » Volume 15
Focal Irreversible Electroporation for Localized Prostate Cancer – Oncological and Safety Outcomes Using mpMRI and Transperineal Biopsy Follow-Up
Authors Gielchinsky I , Lev-Cohain N
Received 3 November 2022
Accepted for publication 18 January 2023
Published 22 January 2023 Volume 2023:15 Pages 27—35
DOI https://doi.org/10.2147/RRU.S393243
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Guglielmo Mantica
Ilan Gielchinsky,1,2 Naama Lev-Cohain3
1Research Branch, Meuhedet Health Services, Tel-Aviv, Israel; 2Department of Urology, Assaf Harofeh Medical Center, Tzrifin, Israel; 3Department of Radiology, Hadassah Medical Center, Jerusalem, Israel
Correspondence: Ilan Gielchinsky, Research Branch, Meuhedet Health Services, Tel-Aviv, Israel, Medica Medical Center, Habarzel 28 street, Suit 203, Tel-Aviv, Israel, Tel +972-50-4048489, Email [email protected]
Introduction: Irreversible electroporation (IRE) technology for prostate cancer (PC) generates consecutive electrical pulses between pairs of electrodes which move through tumorous cells, irreversibly perforate their membranes and eventually lead to cell death, while avoiding tissue thermal effect. The technique is used for primary focal lesions as well as for focal salvage cases. This series reports short term oncological control, quality of life and safety results.
Methods: Retrospective data were collected from 45 consecutive cases of primary (N=38) and salvage (N=7) PC patients treated with IRE. All patients had transperineal MRI/US fusion biopsy and PET-PSMA scan prior to treatment, to verify single lesion. Transperineal Nano-Knife IRE system was used in day-care theatre. Patients had 6 months mpMRI, blood PSA and 1 year confirmatory biopsy following procedure. Quality of life was recorded during the first year.
Results: Median primary subgroup analysis (N=38): age 69 years, initial PSA 5.6 ng/dL, lesion size 0.8 mL and ISUP Group 2 (1– 3). Median salvage subgroup analysis (N=7): age 76 years, initial PSA 11.9 ng/dL, lesion size 2.0 mL and ISUP Group 4 (1– 5). Median catheter time 5 (3– 7) days. No Clavien–Dindo> 1 complications were reported nor re-admissions, incontinence, strictures or fistulas. 5% of patients were given PDE-5i drugs. Primary group PSA dropped by 39%, mpMRI clearance in 84%, out-field new lesion in 12%, in-field lesion in 4%. Biopsy at 1 year: 4 patients had out-field clinically significant PC, thus 3 had re-IRE and 1 had radiation therapy. Salvage subgroup MRI clearance was 60%, and 52% remained on active surveillance by 1 year.
Conclusion: IRE treatment for focal PC is safe for primary and salvage cases, if done by a meticulously skilled and trained team, and under strict protocols. The short term oncological results are promising especially for primary lesions. Long term oncological results will be published over time.
Keywords: prostate cancer, irreversible electroporation, focal therapy, Nano-Knife
Introduction
Focal therapy for prostate cancer is a feasible option evolved with multiparametric MRI (mpMRI), PET-PSMA and biopsy methods (MRI/US fusion). Focal therapy aims at achieving equivalent oncological control as whole-gland treatments (radical prostatectomy and radiation therapy) while maintaining quality of life, and switching a patient who needs radical treatment to an active surveillance candidate.1 Irreversible electroporation (IRE) is a focal ablative technology that uses direct current between two or more needle electrode pairs. The multiple consecutive electrical pulses perforate the cell membrane, causing instability and cell death.2 IRE was shown to preserve vessels and nerves and the extracellular matrix.3 It can be used around the urethra, and it is not affected by vascular “heat sink”. The treatment is repeatable and does not prevent radical surgery if needed in the future.
IRE is used for primary solitary prostate lesions as well as salvage treatment for radiation therapy failure (external beam or brachytherapy). Phase I–II studies have shown that IRE is a safe and feasible focal ablative modality with low morbidity profile.1 Since IRE is novel and evolving technology, global high-quality data and protocols are essential.
Our series used strict pre- and post-treatment patient evaluation protocols, latest technologies and were executed by meticulously global post-fellowship programme trained personnel to provide high-quality data to be shared with others.
Methods
Ethical Approval
Institutional review board approval was obtained from Human Research Ethics Committee (“Helsinki Committee”) of Meuhedet Health Services, Tel-Aviv, Israel, for retrospective data analysis (Meuhedet 04-28-10-20). The study complies with the Declaration of Helsinki. Written informed consent was obtained from all patients to perform focal IRE treatment and biopsies. All images in this text are original, copyrighted to authors, anonymous and used under the consent of patients.
Study Design and Participants
Retrospective data were collected from 45 consecutive patient files treated with IRE in 2018–2022 for focal PC as primary treatment (N=38) or as salvage treatment following external beam radiation or brachytherapy failure (N=7). All patients were treated by a single surgeon, fully trained in IRE focal therapy by fellowship programme in a high volume IRE centre. Treatments and follow-up protocols were adjusted to St. Vincent’s Hospital and Garvan institute for medical research and Kinghorn Cancer Centre, NSW, Australia.4
Patients Evaluation, mpMRI and Biopsy
All patients were tested for PSA blood levels and had mpMRI done prior to biopsy. The pre-treatment scans were done in various centres using 3 Tesla devices and all were read by a single radiologist. The post-treatment scans were done in a single centre by Ingenia3.0T Philips and read by the same single radiologist. All scans were multiparametric with gadolinium injected, and T1, T2 and diffusion weighted images were used. Lesions which were interpreted as PIRADS 3–5 (Prostate Imaging and Reporting, and Data System, Version 2.1) were biopsied. The biopsy technique was transperineal MRI/US fused+Transperineal Template Mapping Biopsy (TTMB). The region of interest (ROI) was sampled by 4–6 cores, and then the prostate was divided into 10–14 areas, individually sampled transperineally (Apex Posterior R+L, Apex Anterior R+L, Base Posterior R+L, Base Posterior-Lateral R+L, Base lateral R+L, TZ R+L, Anterior Prostate R+L). All biopsies were done with the Bio-Jet system (DK) using the axial method by a single surgeon, fully trained in a fellowship programme and with experience of over 1000 transperineal MRI fusion biopsies.
Pathology analysis was done by senior uropathologists. All patients had pre-treatment PET-CT with Ga-68 PSMA to rule out secondary lesions and to correlate prostatic lesion with MRI and biopsy. Primary treatment was done for localized, single lesion, ISUP1-3 grade only, and for any ISUP grade in salvage cases. All patients were first offered standard radical treatments (radical prostatectomy and radiation therapy), and IRE was suggested for patients who refused to have these treatments. ISUP1 patients were few (10%), and were classified as intermediate risk PC due to high levels of blood PSA and unfavourable genomic test (Oncotype).
(Patients' pre-treatment data are summarized in Table 1.)
 |
Table 1 Patients Pre-Treatment Data |
IRE Treatment
All patients had general anaesthesia, and were positioned in Lithotomy. An indwelling catheter was inserted prior to treatment to avoid urine conduction. Biplanar transrectal ultrasound (Flex focus BK Medical, Denmark) was used combined with a MRI fusion system (Bio-Jet, DK), and transperineal grid. Irreversible electroporation was executed using the Nano-Knife system (Angio-Dynamics, NY, USA). The ultrasound and MRI holograms were fused by anatomic structures for the Z axis. Electrodes were inserted through the transperineal grid, with exposure of 1.5–2.0 cm, using 4–6 electrodes. The tumour was surrounded using 0.9 cm safety margins, and was verified in both axial and longitudinal views. The minimum electrode distance was 1.0 cm and maximum was 2.0 cm. Ten pulses were delivered to test the actual current and then 80 pulses were given after adjustments so that the current will be between 20 A and 40 A. The patients were discharged at the same day. Catheter was removed 5 days following the procedure (Figure 1). To note that in salvage cases the tumour volume was generally larger and borders less sharp on MRI hologram due to radiation treatment, thus larger areas were ablated and a 6 needle configuration was more frequently used.
Follow-Up Protocol
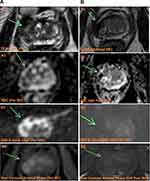
Patients had a PSA blood test and a mpMRI at 6 months (Figure 2). The scan was read by a single senior radiologist with formal fellowship in prostate MRI who had over 1000 reads by then. At 12 months patients had confirmatory transperineal MRI fused+Transperineal Template Mapping Biopsy.
PSA was tested every 6 months after, and mpMRI was done yearly. If mpMRI showed viable lesion (≥PIRADS3), the patient was biopsied. In case of PSA rise, the patient had an MRI scan+PET-PSMA to rule out metastatic disease. In any case of clinically significant disease the patient was offered treatment (re-IRE, radiation or robotic assisted radical prostatectomy, hormonal therapy). The patients were seen at the clinic by a single surgeon, every 6 months, and were asked periodically about quality of urination and sexual function.
Statistical Analysis and Image Graphics
Pre-treatment data, age, PSA level, prostate size, lesion size, PSA density and PIRADS score in each group were all analysed for median values and margins. Calculations of percentile changes over time were analysed as well. All were done by using Microsoft Excel statistical software. Images were edited using Preview software on MAC OS (Apple).
Results
Oncological Results
Primary Group
Median PSA 6 months after treatment was 3.4 ng/mL (0.8–16.3), showing a 39% drop. On follow-up mpMRI scan, 6 months post treatment (25/38), 84% of patients had no viable disease (PIRADS2) while 12% had an out-field new suspected lesion (PIRADS>3) and 4% had an in-field lesion. Transperineal, MRI fusion biopsy+full template was done (including the treated area) 1 year post treatment (or earlier if mpMRI showed viable lesion) for 13 patients. 69% shown no evidence of disease, 31% (N=4) had an out-field clinically significant PC (ISUP>2), None had in-field clinically significant PC. Overall by 1 year post treatment, 2 patients had re-IRE and 1 patient had WGT, thus 97% of patients were on AS. By 2 years out of 13 patients, 2 had WGT, 4 had re-IRE and overall 84% (11/13) remained on AS.
Salvage Group
Median PSA at 6 months was 22 ng/dL (4–34). 5/7 (71%) patients had mpMRI at 6 months. 60% showed no viable disease on mpMRI (PIRADS2) and (40%) had an out-field lesion (PIRADS>3). No viable disease was seen on mpMRI within the treated area (in-field). Natural history on salvage subgroup by 1 year showed 3 patients had HRT (1 due to lesion in seminal vesicle and 2 due to COVID-19 outbreak and inability to continue follow-up) and 4 patients (57%) were on active surveillance and without HRT.
Quality of Life (Both Groups N=45)
Catheter was left for a median 5 (5–7) days, none of the patients was admitted for overnight and none were re-admitted. No complications of Clavien–Dindo>1 were recorded. There were no cases of urinary incontinence (all patients had full continence immediately after catheter removal, with full control as they had prior to surgery) and no fistulas were recorded. Slight decrease in sexual function (erection) was reported by 2/38 patients in the primary subgroup, beyond their baseline (5.2%), treated effectively with PDE-5, ejaculation remained unchanged. Primary group oncological and QoL results are summarized in Table 2.
 |
Table 2 Short Term Oncological Results of the Primary Treatment Cohort |
Discussion
Prostate cancer is a major cause of disease and mortality among men, and each year 1.6 million men are being diagnosed and 366,000 die from prostate cancer.5 Whole gland treatments (radical prostatectomy, radiation treatment) carry the potential of quality of life deterioration (incontinence, impotence, urethral strictures, radiation cystitis, etc) and the risk of “over treatment” is considerable when comparing the incidence of prostate cancer and the mortality rate.
Focal therapy has become a realistic option once mpMRI and MRI/US fusion biopsies have become available. The aim of focal therapy is to achieve equivalent oncological control as whole gland therapy treatment, while preserving quality of life, and to switch a patient who needs radical treatment to an active surveillance candidate. IRE is a technology based on the concept of creating nanopores in the cell membrane, causing excessive permeability and disruption of the osmotic balance beyond capacity of cellular repair mechanism resulting in apoptosis.1 In contrast to HIFU (high intensity focal ultrasound), cryotherapy and other thermal focal technologies, IRE does not use thermal energy, thus, it is not affecting collagenous structures and not affected by vascular “heat sink effect” (heat buffering by proximal vassals to area of treatment). Furthermore, IRE is repeatable and does not prevent robotic assisted radical prostatectomy surgery if needed. IRE oncological and quality of life results were assessed in different series and protocols. In 2018, an Australian group published a series of 63 patients treated for localized prostate cancer (Gleason 6–7).4 No high grade adverse events were recorded. Quality of life (QoL) questionnaire analysis demonstrated no significant change from baseline physical, mental, bowel or urinary QoL. Mild decrease in sexual QoL (median score of 66 at baseline vs 54 at 6 months). As for oncological control, the group reported 70% decline in PSA level at 6–12 months. In-field control was increased from 87% to 97% once they increased their safety margins. In our series we used their protocol of updated safety margins in order to provide better in-field control. The need to increase safety margins was discussed in other series and is still not certain.6
In 2019 a group from Alabama, USA, analysed 471 cases of IRE treatment which included low risk (N=25), intermediate risk (N=88) and high risk (N=312) cases. Patients were treated focally (123), sub-whole gland (154), whole gland (134) or for recurrent disease (63). Urinary continence was preserved in all cases. IRE-related erectile dysfunction was reported in 3% of patients after 12 months. Kaplan–Meier estimation on recurrence rate at 5 years was reported as 5.6% for Gleason 6, 14.6% for Gleason 7 and 39.5% for Gleason 8–10, concluded as comparable to radical prostatectomy with better safety.3 In 2020, a prospective database of 123 patients treated with IRE for focal PCa was reported in order to assess QoL and short term oncological results. The patients were treated between the years 2013 and 2018. Median follow-up was 36 months. 91% had an intermediate risk and 9% low risk. 9.8% had ISUP1 lesion, 88% had ISUP2 lesion and 23% had ISUP3. The patients had follow-up of serial PSA tests, mpMRI and Transperineal Template Mapping Biopsy (TTMB) at 12 months. Failure free survival (FFS) was defined as progression to whole-gland or systemic treatment or metastasis/death. Functional outcomes were assessed. On post-treatment TTMB, in-field recurrence was present in 2.7–9.8% of patients. FFS at 3 years was 96.75%, metastasis free survival in 99% and overall survival 100%. A total of 8 patients required salvage treatment (12 repeat IRE, 6 whole gland therapy). NPV of mpMRI was 94% and sensitivity was 40% for detecting in-field residual disease at 6 months post treatment. 98.8% remained pad free and 76% had no change in erectile function.7 A 2020 systemic review of IRE reported an in-field recurrence rate of 0–39% and out-field of 6.4–24% and PSA level drop by 76% at 2 years.8
Addressing our data versus previously published results focuses on subgroup endpoints.
Our primary subgroup had an overall 39% median drop of PSA by 6 months. 25/38 patients had post-treatment mpMRI done and 4% showed in-field suspected lesion but a biopsy did not find clinically significant PC there. Out-field suspicious lesion was seen in 12% of patients, Biopsy confirmed 4 of them as having clinically significant PC. Three were re-treated with IRE and one was referred to radiation therapy.
Overall, 1 year post IRE, 97% avoided whole gland therapy and continued AS. By 2 years, another patient had been re-treated with IRE and another patient had radiation therapy.
As for quality of life, there were no re-admissions, strictures, fistulas or urinary complications. 2/38 patients reported slight decline in erection (5%) which was improved with PDE-5i.
The ability of mpMRI to be used as a follow-up tool after IRE was assessed in a publication from 2017. The negative predictive value of MRI in detection of large in-field lesions post IRE (≥ISUP3 or ISUP1≥6 mm) is 90% and for smaller lesions (≥ISUP2 or ISUP1≥4 mm) is 88%. For out-field lesions the negative predictive value is known to be 90% and 80% respectively.2
Our data show that mpMRI post IRE is an essential tool but cannot replace the 1 year confirmatory biopsy. We used 9 mm safety margin as suggested by Stricker et al, but this safety margin is still to be decided as suggested by other groups.6
The salvage subgroup consisted of patients who were treated with radiation therapy in the past (external or brachytherapy)+ADT, but had a localized prostate recurrence only. They had the full workup as the primary patients, but their results compared to the primary group were inferior.
At 1 year post IRE, 52% were on active surveillance (one was re-treated by IRE at 6 months). The rest of the patients went for HRT for several reasons, 1 due to seminal vesicle recurrence, 1 due to out-field lesion recurrence, that could theoretically be re-treated but due to COVID-19 situation avoided it, and 1 patient who asked to be treated with HRT due to PSA rise and did not wish to have mpMRI. There were no in-field lesions viable on mpMRI in these patients, thus raising the question of whether we should cover larger areas in salvage cases due to higher risk of out-field recurrence. Some groups report various success rate in salvage cases9 but a prospective international large trial of IRE after radiation (FIRE trial) is in progress now and might give us more information on planning of ablation in these cases.
As for QoL of salvage group, there were no complication of Clavien–Dindo>1, all patients remained continent and no fistulas or strictures were recorded. All salvage patients had reported severe ED before IRE treatment, due to prior radiation and age, so sexual function follow-up was less relevant in that group.
The overall oncological results and, more importantly, the safety results had much to do with the assembly of the unit. The surgeon who performed the IRE treatment was trained in a dedicated fellowship programme for over a year in a high volume IRE unit in Australia and was experienced with learning curve and safety protocols such as avoiding creating thermal effect (electrode spacing, number of electrode, pulse rate, voltage/cm³) and avoiding damage to the rectum or bladder. The MRI interpreter was trained in a fellowship programme in the USA and had over 1000 reads. The anaesthesiologist, technician and nursing staff were not changed in all procedures. The biopsy prior to procedure and the 1 year confirmatory biopsy was done in a transperineal manner (less chance for infection, better sampling of anterior prostate and apex) with a surgeon who had dedicated fellowship and had done over 1000 fusion biopsies by then. Our team was in constant contact with sister units in Australia and the Netherlands in order to adjust the treatment (such as increase of the safety margins). All of these measures are critical for the safety and oncological results in that technology.
Limitations
Quality of life was assessed by interview at the clinic every 6 months, with focus on quality of urination and sexual function. The use of dedicated questionnaires such as IPSS or SHIM score could result in better data analysis. Not all patients had confirmatory biopsy done due to their request.
Conclusions
IRE used for localized prostate cancer ablation is safe and effective if done by highly skilled personnel, using strict algorithm of selection, treatment and follow-up. Primary lesions treated by IRE showed high rates of success in oncological control. Salvage results were inferior to primary results but provided oncological control in a high percentage of cases, sparing the hormonal treatment. Safety profile was very high, with full urinary control and very minor compromise of sexual function. Overall short term oncological results are promising, long term data and larger series are important.
Funding
No funding reported for this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Blazevski A, Scheltema MJ, Amin A, Thompson JE, Lawrentschuk N, Stricker PD. Irreversible electroporation (IRE): a narrative review of the development of IRE from the laboratory to a prostate cancer treatment. BJU Int. 2020;125(3):369–378. doi:10.1111/bju.14951
2. Scheltema MJ, Chang JI, van den Bos W, et al. Preliminary diagnostic accuracy of multiparametric magnetic resonance imaging to detect residual prostate cancer following focal therapy with irreversible electroporation. Eur Urol Focus. 2017;5(4):585–591.
3. Guenther E, Klein N, Zapf S, et al. Prostate cancer treatment with Irreversible Electroporation (IRE): safety, efficacy and clinical experience in 471 treatments. PLoS One. 2019;14(4):e0215093. doi:10.1371/journal.pone.0215093
4. van den Bos W, Scheltema MJ, Siriwardana AR, et al. Focal irreversible electroporation as primary treatment for localized prostate cancer. BJU Int. 2018;121(5):716–724. doi:10.1111/bju.13983
5. Pernar CH, Ebot EM, Wilson KM, Mucci LA. The Epidemiology of Prostate Cancer. Cold Spring Harb Perspect Med. 2018;8(12):a030361. doi:10.1101/cshperspect.a030361
6. Enikeev D, Taratkin M, Morozov A, et al. Focal irreversible electroporation for localized prostate cancer management: prospective assessment of efficacy and safety. Minerva Urol E Nefrol Ital J Urol Nephrol. 2020;72(5):644–645.
7. Blazevski A, Scheltema MJ, Yuen B, et al. Oncological and quality-of-life outcomes following focal irreversible electroporation as primary treatment for localised prostate cancer: a biopsy-monitored prospective cohort. Eur Urol Oncol. 2020;3(3):283–290. doi:10.1016/j.euo.2019.04.008
8. Morozov A, Taratkin M, Barret E, et al. A systematic review of irreversible electroporation in localised prostate cancer treatment. Andrologia. 2020;52(10):e13789. doi:10.1111/and.13789
9. Scheltema MJ, van den Bos W, Siriwardana AR, et al. Feasibility and safety of focal irreversible electroporation as salvage treatment for localized radio-recurrent prostate cancer. BJU Int. 2017;120:51–58. doi:10.1111/bju.13991
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.