Back to Journals » Clinical Ophthalmology » Volume 13
Five-year outcomes of intravitreal drug therapy for neovascular age-related macular degeneration in eyes with baseline vision 20/60 or better
Authors Khanani AM , Gahn GM, Koci MM, Dang JM, Brown SM, Hill LF
Received 17 October 2018
Accepted for publication 28 December 2018
Published 13 February 2019 Volume 2019:13 Pages 347—351
DOI https://doi.org/10.2147/OPTH.S191170
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Arshad M Khanani,1 Greggory M Gahn,2 Micaela M Koci,2 Jonathan M Dang,2 Sandra M Brown,3 Lauren F Hill4
1Sierra Eye Associates, Reno, NV, USA; 2School of Medicine, University of Nevada, Reno, NV, USA; 3Cabarrus Eye Center, Concord, NC, USA; 4Hill Statistical Consulting, Morrison, CO, USA
Objective: To assess outcomes in treatment-naive eyes with neovascular age-related macular degeneration (nAMD) and good baseline visual acuity (VA) treated using a treat-and-extend (T&E) regimen with intravitreal aflibercept, ranibizumab, or bevacizumab.
Design: Single center, retrospective, observational case series.
Participants: Ninety-one patients (93 eyes) with nAMD and baseline VA ≥20/60 followed for ≥1 year after the first intravitreal injection. Minimum of 6 (first year) and 3 (subsequent years) and maximum of 12 injections per 12 calendar months.
Intervention: Intravitreal aflibercept 2.0 mg, ranibizumab 0.5 mg, or bevacizumab 1.25 mg. Three monthly injections. Treatment interval extended in 2-week increments after resolution of macular edema and reduced in 2-week increments if edema recurred; maximum interval of 12 weeks. Medication changed if edema recurred during and persisted after three monthly injections of original agent.
Main outcome measures: VA maintenance over time. Total number of injections received by year of treatment.
Results: Ninety-three eyes were analyzed. Pretreatment VA was 20/20–20/25 (N=16), 20/30–20/40 (N=47), and 20/50–20/60 (N=30). Mean follow-up was 3.2 years. Follow-up by year was 93, 73, 65, 44, and 26 eyes for years 1–5, respectively. Mean number of injections during years 1–5 was 7.9, 5.9, 5.6, 5.9, and 6.0, respectively; mode number of injections was 7, 5, 3, 6, and 4, respectively. For years 1–5, percent of all eyes at or above baseline was 70%, 66%, 65%, 59%, and 58%, respectively; percent ≥20/60 was 86%, 88%, 86%, 84%, and 77% for years 1–5. For eyes with baseline VA ≥20/40, percent of eyes at or above baseline was 83%, 82%, 81%, 68% and 76% for years 1–5, respectively.
Conclusion: Using a T&E intravitreal injection protocol, more than 75% of treatment-naive eyes with nAMD and baseline VA ≥20/60 can maintain VA ≥20/60 over 5 years.
Keywords: neovascular age-related macular degeneration, ranibizumab, aflibercept, bevacizumab, treat and extend protocol, visual acuity
Introduction
Neovascular age-related macular degeneration (nAMD) is a leading cause of visual acuity (VA) loss in older adults. The development of anti-vascular endothelial growth factor (anti-VEGF) agents has converted this formerly disabling disease into a condition which can be managed successfully for years, but at substantial treatment burden to patients and caretakers. The original clinical trials on anti-VEGF treatment used monthly intravitreal injections for all eyes. Treat-and-extend (T&E) protocols based on an individualized treatment interval can result in decreased treatment burden.1–3
The prevalence of optical coherence tomography (OCT) has led to earlier detection of macular changes by ophthalmologists and optometrists and the rapid referral for treatment of nAMD. Patients are often referred with relatively mild VA loss. In many states including Nevada, a VA of 20/40 in at least one eye is required for an unrestricted drivers license. In the United States, a driver’s license is almost always essential for continued independent living, and older patients very highly value this privilege. Patients who gain a strong functional benefit from anti-VEGF treatment for nAMD are more likely to be compliant with a lifelong treatment regimen. The efficacy of a T&E protocol in a patient population with driving privileges is therefore of particular interest.
This study reports the 5-year outcomes of a T&E protocol applied by one retina specialist in a typical private practice setting, in eyes with baseline VA of 20/60 or better.
Methods
This was a retrospective chart review of all patients in a private retina practice with a new diagnosis of nAMD who began intravitreal anti-VEGF therapy between August 2010 and August 2016. Institutional review board exemption was received from the Western Institutional Review Board. To protect patient confidentiality, data were compiled using the medical record number as the patient identifier. Patients were identified through a search of the billing database for diagnostic codes for nAMD. Exclusion criteria were previous anti-VEGF therapy, other macular disease such as diabetic retinopathy, baseline Snellen VA less than 20/60, follow-up for less than 12 months, fewer than 6 injections during first 12 months of treatment, fewer than 3 injections during each subsequent 12-month treatment interval, and more than 12 injections in 12 months at any time.
All examinations and intravitreal injections were performed by one physician (AMK). The diagnosis of nAMD was made based on clinical examination and evidence of sub- and intraretinal fluid on macula OCT.
VA was measured with Snellen letters using projectors and recorded in customary Snellen notation. Data from the first examination which occurred after the specified treatment interval (12, 24 months, etc) were used for analysis. All VAs reported are best-corrected VAs. Fluorescein angiography was performed with a Topcon (Topcon Inc., Tokyo, Japan) or an Optos fundus camera (Optos Inc., Marlborough, MA, USA). Optical coherence tomography was performed with a Heidelberg Spectralis device (Heidelberg Engineering GmbH, Heidelberg, Germany).
Choice of initial anti-VEGF medication was made by the surgeon. The first three injections were given at 1-month intervals to all patients including those with complete resolution of intra- and subretinal fluid after fewer than three injections. Eyes with persistent fluid after three injections continued to receive monthly injections until fluid resolved. Injection interval was then increased by 2 weeks for each interval to a maximum interval of 12 weeks. If fluid recurred, the injection interval was shortened by 2 weeks (eg, from 8 to 6 to 4 weeks) until fluid again resolved. A consolidating injection was given at the same interval and then treatment was extended again. The minimum interval between injections was 4 weeks. Medication was switched to a different anti-VEGF agent when fluid persisted after three monthly injections of an initially successful drug. The choice of the second agent was made by the surgeon.
Results
A total of 317 charts were identified through the practice billing database; of these 226 patients had one or more exclusion criteria. A total of 93 eyes of 91 patients were analyzed. Mean patient age was 81 years (range: 60–98 years); there were 36 men and 55 women.
Mean follow-up for all eyes was 3.2 years. Distribution of baseline VA is given in Table 1. Number of injections by baseline VA and treatment year is given in Table 2. For all eyes, mean number of injections during years 1–5 was 7.9, 5.9, 5.6, 5.9, and 6.0, respectively; mode number of injections was 7, 5, 3, 6, and 4, respectively.
  | Table 1 Baseline visual acuity |
  | Table 2 Number of injections by baseline visual acuity (VA) and treatment year |
A total of 2,031 injections were performed (Table 3). There were no serious adverse ocular events: no eye developed endophthalmitis, retinal detachment, retinal tear, macular hole, or vitreous hemorrhage.
  | Table 3 Choice of first and second anti-VEGF agent |
Number of eyes at or above baseline VA over time, stratified by baseline VA and treatment duration, is given in Table 4. An eye that declined below baseline at one time point but subsequently regained to baseline would be counted again. Percent of all eyes at or above baseline VA by year of treatment was as follows: 69.9% (65/93), 65.8% (48/73), 64.6% (42/65), 59.1% (26/44), and 57.7% (15/26) for years 1–5, respectively. Percent of all eyes at or above 20/60 by year of treatment was as follows: 86.0% (80/93), 87.7% (64/73), 86.2% (56/65), 84.1% (37/44), and 76.9% (20/26) for years 1–5, respectively. For eyes with baseline VA 20/40 or better, percent of all eyes 20/40 or better by year was as follows: 82.5% (52/63), 81.6% (40/49), 81.4% (35/43), 67.7% (21/31), and 76.5% (13/17) for years 1–5, respectively.
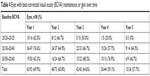  | Table 4 Eyes with best-corrected visual acuity (BCVA) maintenance or gain over time |
Discussion
The approval of ranibizumab in 2006 transformed the management of nAMD. Effective treatment of this formerly blinding disease allows thousands of older individuals to maintain functional VA and independence. As with other disruptive medical advances, ophthalmologists and health care delivery experts did not initially consider or prepare for the markedly increased work requirements of anti-VEGF treatment.
A patient with nAMD requires continuous treatment indefinitely. As the postwar generation ages and average life expectancy increases, ophthalmologists who treat nAMD will see an influx of new patients that exceeds attrition due to death or discontinuation of therapy. To decrease treatment burden and maintain good VA, a number of T&E injection protocols have been investigated. These studies typically report mean VA over time and the percent of eyes with gain or loss of more than 15 Early Treatment Diabetic Retinopathy (ETDRS) letters. However, when treating patients with better baseline VA, a ceiling effect occurs making this metric less informative.
Raja et al4 reported 14 eyes of 14 patients with baseline VA better than 6/12 (better than 20/40) treated with ranibizumab with three monthly loading doses followed by monthly clinic examinations with decision for subsequent treatment made on unspecified clinical grounds. Follow-up was 12 months. Mean number of injections was 7.5 (range: 3–12). Thirteen eyes maintained acuity above 6/12 (20/40) and one eye declined to 6/12 (20/40). Sixty-four percent of eyes were at or above baseline VA at 12 months, and no eye was worse than 20/40.
El-Molayess et al5 reported 30 eyes of 30 patients with baseline VA better than 70 ETDRS letters (20/40 or better) treated for 12 months with intravitreal bevacizumab using an OCT-guided injection protocol. Mean baseline VA was 78 letters (~20/30). Injections were performed at 6-week intervals until there was no evidence of subretinal fluid on macula OCT. Patients were then examined every 4–6 weeks but injections were performed only when macula OCT suggested recurrent neovascularization or leakage. An average of 4.4 injections was performed over 12 months. At 12 months, 28 eyes (93.3%) had VA between 20/20 and 20/40 and 20 eyes (66.7%) had a VA of 20/25 or better.
Rahimy et al6 reported 30 eyes of 30 patients, with pretreatment VA better than 20/40, injected with ranibizumab or bevacizumab using a T&E protocol; average follow-up was 1.44 years. Twenty eyes were followed for 24 months. The authors found that 81% of eyes maintained VA of 20/40 or better at 1 year and 75% did so at 2 years (this result allowed for loss of VA from better than 20/40 to 20/40). Mean number of injections was 7.8 during year 1 and 6.1 during year 2. Our results are comparable, with 82% of eyes with baseline VA of 20/40 remaining 20/40 or better at both 1 and 2 years of treatment.
Our study represents the largest and longest evaluation of a continuous T&E protocol applied by one retina specialist in community practice to eyes with good baseline VA. Using our T&E protocol, 58% of eyes followed for 5 years were at or above baseline VA at 5 years. Since any decline (eg, from 20/25 to 20/30) was classified as vision loss, it is important to consider decline below functional thresholds. At 5 years, 77% of all eyes were at or above 20/60. Mean number of injections for all eyes during year 1 was 7.9, which declined to ~6 injections for subsequent years. This is ~50% reduction in treatment burden for patients and caregivers compared to a fixed monthly injection protocol. Our results reflect real-world factors such as treatment interruption due to illness or travel causing an injection interval greater than 3 months (ie, fewer than four injections per 12 calendar months). Nuanced care, including a change in the anti-VEGF agent when indicated, is required.
Life function benefits of nAMD treatment are also important to consider. Patients who maintain sufficient vision in one eye to obtain a restricted or unrestricted driver’s license can provide for their own needs such as shopping and trips to physicians and the pharmacy. In the United States, loss of driving privileges profoundly affects a patient’s independence and the time burden of caregivers who must provide transportation. Visual acuity limits for driving vary by state and can be complex based on the presence or absence of progressive disease and – if both eyes have subnormal acuity – the VA in the worse eye. We chose to analyze eyes with baseline VA 20/60 or better because 20/50 to 20/60 is the VA range at which most states start to apply driving restrictions. After 5 years of treatment, more than 75% of our patients retained VA 20/60 or better in the treated eye, which would make them eligible for a restricted drivers license in Nevada. For eyes with baseline VA 20/40 or better, ~80% remained at or above baseline through three years of treatment, making these patients eligible for an unrestricted drivers license. The lifetime cost of anti-VEGF therapy must be weighed against this powerful benefit. Our T&E injection protocol maintains or improves VA in the majority of eyes with good baseline VA over 5 years of treatment, at a considerable reduction in treatment burden compared to monthly injections.
Disclosure
Dr Khanani has received speaker and consultation fees from Genentech and Novartis; Ms Hill is a full-time statistical contractor for Genentech. The authors report no other conflicts of interest in this work.
References
Shienbaum G, Gupta OP, Fecarotta C, Patel AH, Kaiser RS, Regillo CD. Bevacizumab for neovascular age-related macular degeneration using a treat-and-extend regimen: clinical and economic impact. Am J Ophthalmol. 2012;153(3):468–473. | ||
Silva R, Berta A, Larsen M, et al. Treat-and-extend versus monthly regimen in neovascular age-related macular degeneration: results with ranibizumab from the trend study. Ophthalmology. 2018;125(1):57–65. | ||
Rayess N, Houston SK, Gupta OP, Ho AC, Regillo CD. Treatment outcomes after 3 years in neovascular age-related macular degeneration using a treat-and-extend regimen. Am J Ophthalmol. 2015;159(1):3–8. | ||
Raja MS, Saldana M, Goldsmith C, Burton BJ. Ranibizumab treatment for neovascular age-related macular degeneration in patients with good baseline visual acuity (better than 6/12): 12-month outcomes. Br J Ophthalmol. 2010;94(11):1543–1545. | ||
El-Mollayess GM, Mahfoud Z, Schakal AR, Salti HI, Jaafar D, Bashshur ZF. Intravitreal bevacizumab in the management of neovascular age-related macular degeneration: effect of baseline visual acuity. Retina. 2013;33(9):1828–1835. | ||
Rahimy E, Rayess N, Ho AC, Regillo CD. Treatment outcomes for neovascular age-related macular degeneration patients with initial vision better than 20/40 using a TREAT-AND-EXTEND regimen. Retina. 2016;36(5):875–880. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
