Back to Journals » Therapeutics and Clinical Risk Management » Volume 19
Fibroblast Growth Factor-21 as a Potential Therapeutic Target of Nonalcoholic Fatty Liver Disease
Authors Raptis DD , Mantzoros CS, Polyzos SA
Received 19 October 2022
Accepted for publication 22 December 2022
Published 22 January 2023 Volume 2023:19 Pages 77—96
DOI https://doi.org/10.2147/TCRM.S352008
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Dimitrios D Raptis,1,2 Christos S Mantzoros,3,4 Stergios A Polyzos1
1First Laboratory of Pharmacology, School of Medicine, Aristotle University of Thessaloniki, Thessaloniki, Greece; 2Second Department of Internal Medicine, 424 General Military Hospital, Thessaloniki, Greece; 3Department of Internal Medicine, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA; 4Department of Internal Medicine, Boston VA Healthcare System, Harvard Medical School, Boston, MA, 02115, USA
Correspondence: Stergios A Polyzos, First Laboratory of Pharmacology, School of Medicine, Campus of Aristotle University of Thessaloniki, Thessaloniki, 54124, Greece, Tel +30 2310 999316, Email [email protected]
Abstract: Nonalcoholic fatty liver disease (NAFLD) is a highly prevalent disease without any approved treatment to-date despite intensive research efforts by researchers and pharmaceutical industry. Fibroblast growth factor (FGF)-21 has been gaining increasing attention as a possible contributing factor and thus therapeutic target for obesity-related metabolic disorders, including NAFLD, mainly due to its effects on lipid and carbohydrate metabolism. Most animal and human observational studies have shown higher FGF-21 concentrations in NAFLD than non-NAFLD, implying that FGF-21 may be increased to counteract hepatic steatosis and inflammation. However, although Mendelian Randomization studies have revealed that variations of FGF-21 levels within the physiological range may have effects in hyperlipidemia and possibly nonalcoholic steatohepatitis, they also indicate that FGF-21, in physiological concentrations, may fail to reverse NAFLD and may not be able to control obesity and other diseases, indicating a state of FGF-21 resistance or insensitivity that could not respond to administration of FGF-21 in supraphysiological concentrations. Interventional studies with FGF-21 analogs (eg, pegbelfermin, efruxifermin, BOS-580) in humans have provided some favorable results in Phase 1 and Phase 2 studies. However, the definite effect of FGF-21 on NAFLD may be clarified after the completion of the ongoing clinical trials with paired liver biopsies and histological endpoints. The aim of this review is to critically summarize experimental and clinical data of FGF-21 in NAFLD, in an attempt to highlight existing knowledge and areas of uncertainty, and subsequently, to focus on the potential therapeutic effects of FGF-21 and its analogs in NAFLD.
Keywords: hepatic fibrosis, hepatic inflammation, hepatic steatosis, hepatokine, nonalcoholic steatohepatitis, treatment
Introduction
Nonalcoholic fatty liver disease (NAFLD) has been closely associated with the metabolic syndrome (MetS); this has led to the recommendation of change in the disease nomenclature including a change to simply Fatty Liver Disease (FLD) or to metabolic (dysfunction)-associated fatty liver disease (MAFLD).1,2 In the setting of increasing prevalence of obesity and metabolic dysregulation in western societies, there has been a global increase in the prevalence of NAFLD, in parallel with type 2 diabetes mellitus (T2DM) and obesity epidemics. Notably, NAFLD may often remain underdiagnosed, provoking undesirable individual and public consequences.3 NAFLD demonstrates a wide histological spectrum, gradually ranging from simple nonalcoholic fatty liver (NAFL), to nonalcoholic steatohepatitis (NASH), hepatic fibrosis, liver cirrhosis and hepatocellular carcinoma (HCC) in a minority of patients, which appears in the absence of significant alcohol consumption, viral infection or any other specific hepatic disease.4 It is expected that NAFLD may possibly become the leading cause of liver transplantation in the near future.5 Despite its high prevalence and the extensive research in the field, the treatment of NAFLD remains an unmet medical need, since there is to-date no medication specifically licensed for NAFLD.6
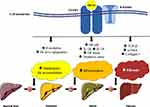
NAFL starts as a state of imbalance between factors favoring fat export vs factors favoring fat accumulation in the liver.4 Insulin resistance (IR) interplays with systemic and hepatic inflammation, through various adipokines, cytokines, hepatokines and other mediators, to increase the load of free fatty acids that divert from adipose tissue to the liver, but also hepatic de novo lipogenesis, thus favoring fat accumulation in the liver.7 Adipokines, cytokines, hepatokines and other mediators seem to contribute to the pathogenesis of the disease, thus being appealing therapeutic targets.8 Fibroblast growth factors (FGF) comprise a family of peptidic molecules, which are widely expressed; by activating FGF receptors (FGFR), FGFs contribute to the repair and regeneration of tissues, but also to metabolic homeostasis.9 FGF-21 has been gaining increasing interest, as a possible therapeutic target of obesity-related metabolic disorders, including NAFLD, owing to its impact on lipid and carbohydrate metabolism.10 FGF-21 is a hepatokine with pleiotropic properties.6 It mainly acts through FGFR1, which is a tyrosine kinase receptor and requires β-Klotho, a single-pass transmembrane glycoprotein, as co-receptor for its activation.11 FGF-21 improves insulin sensitivity by increasing glucose uptake in the skeletal muscle and brown adipose tissue. In the liver, FGF-21 enhances fatty acid β-oxidation and decreases hepatic de novo lipogenesis (Figure 1).6
In this review, we critically summarized experimental and clinical data of FGF-21 in NAFLD, trying to highlight existing knowledge and areas of uncertainty, and subsequently, we focus on the potential therapeutic effects of FGF-21 and its analogs in NAFLD.
Search Strategy
We performed a computerized literature search using the PubMed, not limited by publication time. A query was created by combining MeSH terms and non-MeSH terms: ((“non-alcoholic fatty liver disease”[Mesh]) OR (nonalcoholic fatty liver disease) OR (non-alcoholic fatty liver disease) OR (nonalcoholic steatohepatitis) OR (non-alcoholic steatohepatitis) OR NASH OR NAFL* OR MAFLD*) AND ((fibroblast growth factor-21) OR (fibroblast growth factor 21) OR FGF-21 OR FGF21 OR (FGF 21)). This query provided 329 results (last update: October 15, 2022). Subsequently, articles were selected based on the principles of evidence-based medicine, focusing mainly on original articles, preclinical and clinical. Search was extended to the reference list of some of the selected articles. Since this was a narrative review, some more articles were added on the discretion of the authors, when this was regarded as necessary for the flow of this review.
Experimental Studies
In the liver, the activation of FGF-21 pathway results in higher expression of genes involved in mitochondrial β-oxidation and in lower expression of genes involved in de novo lipogenesis (Figure 1). Therefore, by decreasing hepatic lipogenesis and rendering β-oxidation of fatty acids (FAs) more efficient, intrahepatic lipid accumulation is decreased.12 Furthermore, FGF-21 has been proposed to decrease hepatic inflammation by downregulating the nuclear factor kappaB pathway13 and decreasing pro-inflammatory cytokines (eg, interleukin-1β) and increasing anti-inflammatory cytokines (eg, interleukin-10).14 Moreover, FGF-21 seems to lessen intrahepatic oxidative stress and endoplasmic reticulum (ER) stress,15 which interplay with oxidative stress. Additionally, limited data reported that FGF-21 decreases the hepatic expression of tumor growth factor-β, α-smooth muscle actin and collagen I, implying a potentially beneficial effect on hepatic fibrosis (Figure 1). Some of these actions have been proposed to be partly mediated by the activation of AMP-activated protein kinase (AMPK)16 and peroxisome proliferator-activated receptor (PPAR)-α intracellular pathways.17
Further in vitro studies showed that treatment of mouse primary hepatocytes with palmitic acid upregulated FGF-21 mRNA, reportedly through increasing lipid-induced ER stress.18 Previous studies have also shown that ER stress may positively and directly induce the expression and secretion of FGF-21 in adipocytes,19 but also its expression in H4IIE hepatoma cells and in isolated rat hepatocytes.20 Consistently, treatment of human hepatocellular carcinoma (HepG2) cells with the ER stressors tunicamycin or thapsigargin increased FGF-21 mRNA and protein.15 More recently, sulforaphane, a sulfur-rich compound found in cruciferous vegetables, increased FGF-21 hepatic expression and attenuated the adverse effects of palmitic or oleic acid on HepG2 cells.21 Furthermore, lipopolysaccharide (LPS) administration in mice Hep1-6 cell line reduced the expression of FGFR1 in a dose-dependent manner.22 In this regard, administration of Bifidobacterium adolescentis, a species residing in human intestine, reduced the gut microbiota-derived LPS, increased hepatic FGFR1 and alleviated NAFL and NASH in mice on a choline-deficient high-fat (HF) diet (Table 1).22
 |
Table 1 Animal Studies Investigating the Effect of Relevant Intervention on FGF-21 or the Effect of FGE-21 Treatment on NAFLD |
The key findings of animal studies investigating the effects of relevant interventions on FGF-21 or the effect of FGF21 treatment on NAFLD are summarized in Table 1. Generally, higher circulating levels and hepatic expression of FGF-21 were observed in obese than lean rodents, those on HF diet (HFD) than chow diet, as well as those with than without NAFLD.23–30 Regarding specific histological lesions, higher circulating and/or hepatic FGF-21 were observed in more severe hepatic steatosis, inflammation and/or fibrosis.18,27,31 FGF-21 levels were also positively associated with hepatic fat content (HFC).26,30 FGF-21 treatment or FGF-21 analog treatment (eg, LY2405319, B1344, BMS-986171) or genetic FGF-21 upregulation (eg, adenovirus- or plasmid-mediated) improved hepatic histology, including steatosis, inflammation and/or fibrosis in most studies.22,27,31–37 More interestingly, other authors reported that monotherapy with FGF-21 or a glucagon-like peptide-1 receptor agonist (GLP-1RA) had an inferior effect on hepatic histology than a GLP-1-Fc-FGF-21 dual agonist,35 that the combination of an FGF-21 analog with a C–C motif chemokine receptor 2/5 (CCR2/5) inhibitor had also greater effect on hepatic steatosis and NASH than either monotherapy,38 and that a chimeric FGF-21/FGF-19 analog reduced liver weight more than FGF-21 monotherapy.39
Both exercise and chow diet (vs HFD or methionine-choline deficient [MCD] diet) were shown to reduce circulating and hepatic FGF-21.28–30,32,33,40,41 Limited relevant data also showed that: a) calorie restriction more efficiently increased hepatic FGF-21 than exercise;25 b) through upregulating FGF-21, which subsequently exerted its effects partly through the browning of white adipose tissue, female mice were protected more than male ones, when fed on a MCD diet;28 c) HFD accelerated the progression to NASH, independently from diabetes, especially in an FGF-21 deficient state;29 however, more studies are needed to consolidate these conclusions. Weight loss through bariatric surgery was also reported to decrease circulating and/or hepatic FGF-21 in line with the improvement in hepatic histology.42,43 Other authors reported that FGF-21 upregulation and improvement of hepatic histology were observed after treatment with exendin-4 (exenatide), which is a GLP-1RA,24 hydroxytyrosol, which is a phenolic antioxidant,44 a PPAR pan-agonist (MHY2013),45,46 curcumin, which is a member of the ginger family,47 bitter melon extract,48 sulforaphane, which is an organosulfur found in cruciferous vegetables,21 and estrogen (in ovariectomized mice).50 Taurine, which is an essential amino acid, was shown to reduce hepatic inflammation together with FGF-21.49 However, more mechanistic studies are required to consolidate these findings and show whether any of the reported effects of these compounds are mediated by FGF-21, thus elucidating the specific molecular pathways.
Considering the above, in vitro studies have shown that FGF-21 is upregulated in various hepatic cells and adipocytes, after treatment with long-chain FAs and this is partly mediated through ER stress. This has been validated in animal studies and may possibly represent a counteracting mechanism targeting to protect the liver from the overflow of FAs. For example, it has been supported that FGF-21 could serve as an insulin sensitizer, and this action may also be specific for the liver, where FGF-21 is mainly produced, but minimal for other tissues, eg, the adipose tissue.41 However, resistance or insensitivity to FGF-21 action in obesity and possibly related disorders, including NAFLD, has been speculated:23 this represents a state that the upregulated FGF-21 cannot exert its fully beneficial actions, eg, it is inadequate to counteract the diet-induced negative metabolic effects. In line, obese mice, after treatment with exogenous FGF-21, demonstrated an impairment of downstream FGF-21 molecular pathways, implying a resistant state.23 One potential mechanism for this supposedly FGF-21 resistance or insensitivity may be the above mentioned downregulation of FGFR1.22 Of course, further mechanistic studies are required to further investigate and validate FGF-21 resistance or insensitivity and to investigate potential ways to attenuate this.
Clinical Studies
Several observational studies evaluating the association between FGF-21 and NAFLD-related parameters (Table 2), and interventional studies, evaluating the effect of lifestyle or certain medications on FGF-21 in NAFLD patients (Table 3) or the effects of FGF-21 analogs on NAFLD-related parameters (Table 4) have been recently published. Ditto for ongoing interventional studies (Table 5).
 |
Table 2 Main Characteristics and Outcomes of Clinical Observational Studies |
 |
Table 3 Main Characteristics and Outcomes of Interventional Studies Evaluating the Effect of Lifestyle or Medications on Circulating FGF-21 in Patients with NAFLD |
 |
Table 4 Main Characteristics and Outcomes of the Interventional Studies Evaluating the Effects of FGF-21 Analogs on NAFLD |
 |
Table 5 Main Characteristics and Primary Outcomes of Ongoing Clinical Trials Targeting FGF-21 or Evaluating the Effects of FGF-21 Analogs on NAFLD |
Observational Studies
Clinical observational studies investigating the association between NAFLD and FGF-21 are summarized in Table 2. These are mainly cross-sectional and case–control studies, but there are also two cohort studies.
Most,51–57 but not all,58,59 cross-sectional and case–control studies reported higher circulating and/or hepatic FGF-21 concentrations in patients with than without NAFLD. However, a limitation of many of these studies was that NAFLD patients had higher body mass index (BMI) and/or waist circumference than controls, which may have distorted the association between FGF-21 and NAFLD, since FGF-21 is also upregulated in obesity.52,54,58,60 On the other hand, limited data from patients with NAFLD and T2DM or IR showed that FGF-21 may not be largely affected by T2DM53 or IR60 specifically in NAFLD patients, which may imply that NAFLD itself is a more important drive than T2DM or IR for FGF-21 upregulation. Other authors also speculated that FGF-21 is more closely associated with lipid metabolism than glucose metabolism and IR.52 However, more studies are needed to validate these observations and mechanistic studies to quantify the separate magnitude of NAFLD, obesity and T2DM on hepatic FGF-21 upregulation.
Most imaging-based studies also showed a positive association between FGF-21 concentrations and HFC.54,61 On the contrary, data derived from biopsy-proven studies on FGF-21 concentrations between NAFL and NASH are conflicting: some authors reported higher FGF-21 in NASH than NAFL,55,62 whereas others similar concentrations between NAFL and NASH.38,63,64 There is also one study showing lower FGF-21 concentration in children with NASH than non-NASH.59 Limited data on particular histological lesions are also conflicting. More specifically, some authors showed higher FGF-21 concentrations in more severe hepatic steatosis,63–65 inflammation,62 or fibrosis.62 However, other authors reported similar FGF-21 levels among different degrees of steatosis,62 inflammation64,65 and fibrosis.38,64,65
There is also a study investigating the association between single nucleotide polymorphisms (SNPs) of the FGF-21 gene with NAFLD: the rs499765 of FGF-21 gene was associated with higher circulating FGF-21 and the presence of NAFLD, whereas the rs2071699 and rs838136 were not associated with NAFLD.66 In a more recent genome-wide association study (GWAS) with large sample size, the rs2548957 of FGF-21 gene was shown to be associated with circulating FGF-21 concentrations, as well as with lower gamma-glutamyltransferase (GGT), but not HFC or NAFLD.67 Of course, more studies are needed to validate these findings and investigate whether they are of clinical implication.
The two cohort studies have also provided valuable information. In a 3-year prospective cohort study, FGF-21 concentrations at baseline were independently higher in individuals who developed NAFLD during the follow-up.68 Furthermore, an increase in FGF-21 concentrations during the follow-up (compared to baseline) was also associated with the development of NAFLD.68 Another prospective cohort study also showed that FGF-21 concentrations were positively and independently associated with the development of NAFLD.69 Both these cohort studies were performed in Chinese populations, thus the extrapolation of their results to other ethnic groups should be cautiously considered.
All the above considering, most observational studies showed higher circulating FGF-21 concentrations in patients with than without NAFLD and an association between FGF-21 with HFC. More importantly, baseline circulating FGF-21 and its changes in the long-term were also shown to be associated with the development of NAFLD. However, FGF-21 was not clearly associated with histological characteristics of more severe disease, since relevant studies provided conflicting data. This may be, at least partly, attributed to small sample sizes of biopsy-proven studies (Table 2), thus possibly being underpowered to show differences between NAFL and NASH or between different degrees or stages of severity of particular histological lesions (eg, hepatic inflammation, fibrosis). One appealing hypothesis, which however remains to be elucidated, would be that, in accordance with animal studies, FGF-21 is upregulated in human NAFLD as a potential counterbalancing mechanism; nonetheless, it remains unclear whether the hepatic FGF-21 upregulation is sufficient to counteract the progression of the disease to inflammation, fibrosis and cirrhosis, which requires long-term studies with large sample size, paired liver biopsies and histological endpoints.
Interventional Studies Investigating the Effect of Lifestyle or Medications on Circulating FGF-21
The main characteristics and outcomes of the interventional studies investigating the effect of lifestyle or medications on circulating FGF-21 in patients with NAFLD are summarized in Table 3. In one of the above mentioned studies showing higher circulating and hepatic FGF-21 in NAFLD patients than lean controls in a case–control fashion (Table 2),51 the authors also investigated the effect of fasting glucose load (oral glucose tolerance test) and ketogenic diet on circulating FGF-21.51 They practically showed no effect of these short-term interventions on FGF-21,51 which, however, need cautious interpretation, due to the small sample size and the uncontrolled nature of this study. Other authors reported that oral fat load reduced circulating FGF-21 in apparently healthy individuals, but this reduction was impaired in those with HFC>5% (ie, those with presumable NAFLD),70 possibly implying a type of FGF-21 resistance or intolerance in individuals with NAFLD. Taken together, these two studies suggest that a different effect of glucose51 or fat load70 on FGF-21 may be hypothesized, which, however, requires larger, directly comparative studies to be definitely shown. Notably, high protein diets, either animal or plant-based, reduced both FGF-21 and HFC in patients with T2DM and NAFLD.71 Nonetheless, this potential beneficial effect of high protein diets should be cautiously considered in the light of the potential adverse effect of high protein diet, especially those containing processed proteins and red meat, on other parameters of T2DM and NAFLD.72
Limited data from studies with lifestyle intervention provided conflicting results. A program combining exercise, diet and behavioral therapy in children,58 or another one with endurance exercise in elderly men73 were reported to decrease circulating FGF-21. This decrease of FGF-21 was positively associated with the reduction in HFC in the latter,58 but was not associated with NAFLD in the former study, despite its positive association with the decline in BMI.70 On the contrary, an open-label randomized controlled trial (RCT) in overweight/obese individuals with NAFLD reported no effect of a calorie-restricted diet or a common (characterized as “healthy”) diet on both circulating FGF-21 and NAFLD.74 These results warrant further research to definitely show whether the beneficial effect of lifestyle modifications on NAFLD75 may be partly mediated by FGF-21, as well as whether there are distinct effects in different ages.
There are also a few studies investigating the effect of relevant medications on FGF-21 and NAFLD. In an RCT comparing the combined effect of exenatide and pioglitazone vs pioglitazone monotherapy on circulating FGF-21 and HFC in patients with T2DM inadequately controlled with metformin, the combination treatment more effectively decreased both HFC and circulating FGF-21 compared with pioglitazone monotherapy.24 This may imply an additive effect of exenatide on pioglitazone treatment. It must be emphasized that pioglitazone has been recommended by most guidelines as an off-label treatment of NASH with significant fibrosis, although it has certain adverse effects, including weight gain.76 In this regard, it is important to note that exenatide attenuated the adverse effect of pioglitazone on weight gain in this study,24 highlighting the importance of the combination treatment in some patients with NAFLD.77 Another uncontrolled study, exploring the effect of liraglutide, an anti-diabetic medication with beneficial impact on hepatic steatosis and inflammation,78 on FGF-21, supported that circulating FGF-21 decreased only in patients with significant decrease in hepatic steatosis.79 Notably, there was positive association between change in FGF-21 and HFC in this study. However, whether FGF-21 partly mediates the beneficial effect of liraglutide on HFC needs studies of different design. It is also of note that tesamorelin, a synthetic growth hormone releasing hormone analog used in human immunodeficiency virus (HIV)-infected patients with lipodystrophy,80 marginally did not decrease FGF-21, but any change in FGF-21 was positively associated with change in HFC and fibrosis-4 (FIB-4), a non-invasive index of hepatic fibrosis.81 Of course, the design of this study cannot show a causative link between FGF-21 and improvement in hepatic steatosis or fibrosis. It should be noted that tesamorelin has not yet approved for the treatment of central obesity in non-HIV populations.
Considering the above, studies on lifestyle modification have not clearly shown whether FGF-21 may partly mediate their beneficial effect on NAFLD. The same also remains to be elucidated for pioglitazone and liraglutide, medications that have demonstrated beneficial effects on NAFLD. A distinct effect of glucose and fat load on FGF-21 in NAFLD may be implied by the existing data, but existing studies are considered too limited to draw definitive conclusions.
Interventional Studies Investigating the Effect of FGF-21 Analogs on NAFLD
The main characteristics and outcomes of the interventional studies investigating the effect of FGF-21 analogs on NAFLD are summarized in Table 4. There are two RCTs on the effect of pegbelfermin (BMS-986036), which is a recombinant PEGylated human FGF-21 analog, on NAFLD/NASH. Endogenous FGF-21 has short half-life, so the use of FGF-21 analogs seems to be a therapeutic alternative aiming at providing a more prolonged action.82 In a phase 2 RCT, pegbelfermin subcutaneous injection (1 mg/d, 5 mg/d, 20mg/d or 20 mg/week) in patients with T2DM and obesity, of whom 97% with presumable NAFLD, showed that the dose of 20 mg/d and 20 mg/week decreased N-terminal type III collagen propeptide (PRO-C3), regarded as a non-invasive index of hepatic fibrosis.82 Notably, pegbelfermin increased circulating adiponectin, but it failed to improve alanine aminotransferase (ALT) and aspartate aminotransferase (AST) more than placebo in this study, which, however, were not their primary endpoints. Pegbelfermin was shown to be safe and well tolerated, with the most common adverse effects being bruising (5%) and reactions (4%) at injection sites. Diarrhea, nausea and dyspepsia were also more frequent in the pegbelfermin groups than placebo and were mild to moderate in intensity.82
In a phase 2a RCT, pegbelfermin (10 mg/d or 20 mg/week) was subcutaneously administered in overweight/obese patients with biopsy-proven NASH and fibrosis stage F1-F3.83 After 16 weeks of treatment, hepatic fat fraction (evaluated by magnetic resonance imaging-proton density fat fraction) was similarly reduced in both treatment arms compared with placebo.83 Furthermore, treatment with both doses similarly reduced ALT, AST and PRO-C3 and increased adiponectin.83 This study did not show a beneficial effect of pegbelfermin on liver stiffness (evaluated by magnetic resonance elastography). However, this was measured in a subset of patients, thus being possibly underpowered toward this aim and the duration of the study may be short to show difference in liver stiffness, regarded as a non-invasive index of hepatic fibrosis. To this aim, there are two ongoing RCTs with NASH patients and F3 or cirrhosis (F4) and paired liver biopsies (Table 5), which are expected to clarify the effect of pegbelfermin on hepatic histology, including fibrosis.
Apart from pegbelfermin, efruxifermin (AKR-001), a long-acting Fc-FGF21 fusion protein, was investigated in NASH patients with NAS≥4 and F1-F3 in another phase 2a RCT (BALANCED).84 All doses of efruxifermin improved liver function tests and HFC compared with placebo. NAFLD activity score was also shown to improve after treatment, and there were also favorable effects on hepatic fibrosis (in patients with greater improvement in HFC).84 However, these secondary histological results should be cautiously interpreted, because liver biopsy was performed only in a subset of patients and was not performed in patients on placebo. As for pegbelfermin, most adverse events were considered to be mild-to-moderate, with gastrointestinal ones (eg, diarrhea, nausea, vomiting) being more frequent in the efruxifermin groups than placebo.84
LLF580, later renamed to BOS-580, is another long-acting Fc-FGF21 fusion protein, introduced to have longer duration of action compared with pegbelfermin and efruxifermin.85 In a phase 1 RCT with obese patients with hypertriglyceridemia, BOS-580 provided favorable results, ie, it improved HFC, liver function tests and markers of liver fibrosis, and increased adiponectin.85 As with other FGF-21 analogs, gastrointestinal adverse events were more frequent in BOS-580 groups than placebo.
It is also important to note that FGF-21 analogs showed a favorable effect on lipid profile in all the above mentioned trials. More specifically, FGF-21 analogs reduced total cholesterol, low-density lipoprotein cholesterol and triglyceride, whereas increased high-density lipoprotein cholesterol concentrations.82–85 This is regarded as important effect, because improvement in lipid profile may be associated with decrease in cardiovascular risk, which is high in patients with NAFLD.86
In summary, FGF-21 analogs have provided favorable results in phase 1 and phase 2 clinical trials. Of note, the hepatic effects of FGF-21 were observed without substantial decrease in body weight in most studies. Crucially, adiponectin was increased, despite unaffected body weight, at least after treatment with pegbelfermin and BOS-580. This is considered important, because adiponectin, which has been proposed to have beneficial role in the disease,87 may partly mediate the effects of FGF-21 in NASH. Of course, RCTs with paired liver biopsies are expected to clarify the histological effects of FGF-21 analogs in the liver.
Ongoing Interventional Studies
Based on favorable data from clinical trials with FGF-21 analogs in NASH, there are several RCTs that are ongoing, whose main characteristics and primary outcomes are summarized in Table 5. FALCON 1 and 2 are two phase 2b RCTs with paired liver biopsies, investigating the histological effects of pegbelfermin in NASH patients with F3 and F4 (compensated cirrhosis), respectively. Both studies seem to be completed and their results are expected to be published soon, elucidating the therapeutic effectiveness of pegbelfermin in patients with NASH. A phase 2a RCT is also ongoing, investigating mainly the safety of LLF580 (BOS-580) in obese participants with presumable NASH and fibrosis, after the favorable results of the phase 1 RCT.87 There are also two relevant RCTs, investigating the effects of resveratrol and pomegranate extract, respectively, on circulating FGF-21 and NAFLD (Table 5). In an era with no approved treatment for NAFLD,NaN the results of these studies are anticipated with considerable interest.
Discussion
NAFLD is a highly prevalent disease with global distribution without any approved medical treatment.4 This renders research for its pharmacological treatment appealing for both researchers and pharmaceutical industry. In this setting, research on FGF-21 has provided some promising results, which was the main focus of this review. Briefly, it seems that hepatic FGF-21 is upregulated in animal and human NAFLD (Tables 1 and 2), possibly as a counteracting mechanism against hepatic lipid accumulation. This mechanism may work under normal conditions, but may fail, when NAFLD is not reversed and its duration is prolonged, implying either a state of resistance/insensitivity or tolerance to FGF-21 action in obesity and possibly related disorders, including NAFLD, but this remains to be shown.23 Some existing early-phase trials provided favorable results, which may partly be mediated through adiponectin upregulation in the adipose tissue (Table 4); in this regard, clinical trials with histological endpoints, especially hepatic inflammation and fibrosis, would be needed to show with a level of certainty any definitive effect of FGF-21 analogs on the liver. Results of these trials are expected (Table 5). It should be underlined that adiponectin was early considered to be a beneficial adipokine for NAFLD;87 however, it is difficult to produce functionally active recombinant adiponectin, because its molecule needs complex post-translational modifications to be active.89 For this reason, medications that upregulate endogenous adiponectin are considered important for NAFLD. It should be also highlighted that there are also other FGF-21 analogs or FGF-21 receptor agonists or FGFR1/β-Klotho co-agonists in various preclinical stages of investigation, as they are elsewhere summarized in detail.11
It is of interest that hepatic FGF-21 and circulating FGF-21 are well correlated in rodent26 and human52 studies, implying that circulating FGF-21 concentrations reflect its hepatic concentrations. This is important and may have diagnostic implications, since liver biopsy, the current gold standard for the diagnosis and staging of the disease, cannot be performed in all NAFLD patients: the high prevalence of the disease and the low but existent rates of morbidity and mortality of liver biopsy are the main deterrent factors for the use of liver biopsy in a wide scale.90 In this regard, diagnostic accuracy studies showed a moderate accuracy of FGF-21 concentrations for the non-invasive diagnosis of NASH, which, however, increased, when a two-step approach91 or combination of FGF-21 with other parameters92 were used. Along these lines, in a recent meta-analysis of diagnostic accuracy studies, FGF-21 concentrations alone showed moderate sensitivity and specificity (0.62 and 0.78, respectively) for diagnosing NASH.93 However, both sensitivity and specificity increased (0.92 and 0.85, respectively), when FGF-21 concentrations were combined with other parameters, including cytokeratin-18.93 The above considering, more studies may possibly be needed to identify better combinations of parameters for the non-invasive diagnosis of NASH and, possibly more importantly, of hepatic fibrosis.
Although FGF-21 seems an appealing therapeutic target for NAFLD, there are also significant considerations, ie, bone and cardiovascular side effects. More specifically, increased bone density was shown in FGF21-null, whereas decreased trabecular bone volume was shown in FGF21-transgenic mice and diet-induced obese mice treated with FGF21.94 FGF21 was reported to switch the differentiation of bone marrow precursors to adipocytes rather than osteoblasts, thus resulting to impaired bone formation, as well as to induce the activation of osteoclasts, thus resulting to bone loss.94 However, data from clinical studies currently remain controversial, as elsewhere summarized in detail.95 Regarding cardiovascular safety, which is a major concern for patients with NAFLD, since cardiovascular diseases represent the first cause of death of NAFLD patients,86 there are limited data reporting an increase in heart rate and blood pressure after treatment with some FGF-21 analogs, which, however, were not shown by other authors.96,97 The clinical significance of these cardiovascular effects remains to be shown, as well as whether these effects outweigh the above mentioned beneficial effect of FGF-21 analogs on lipid profile, in terms of cardiovascular risk. These observations warrant further studies to evaluate the safety of FGF-21 analogs, focusing on cardiovascular and bone-related side effects. Until then, patients with metabolic bone diseases (eg, osteoporosis) or relevant cardiovascular diseases (eg, uncontrolled blood pressure or heart arrhythmias) may be reasonable to be excluded from the relevant clinical trials with FGF-21 analogs. An interesting concept may also be the co-administration of FGF-21 analogs together with GLP-1RA, since the latter have shown beneficial effects on NAFLD, but also on cardiovascular and bone metabolism.77 By this way, FGF-21 and GLP-1RA may have an additive or synergistic effect on the liver, and GLP-1RA may alleviate the possible adverse effects of FGF-21 on bone and cardiovascular diseases.
In conclusion, the hepatokine FGF-21 seems to play a role in the physiology of lipid metabolism, which renders it an appealing candidate for the treatment of some patients with NASH. There are favoring experimental and clinical results in phase 2 clinical trials. These have led to Phase 3 clinical trials, whose results may clarify whether FGF-21 analogs are beneficial in liver histology, especially in hepatic fibrosis. The cardiovascular and skeletal safety of FGF-21 analogs should be also further evaluated in clinical studies, in order to conclude whether any potential benefit outweighs possible risks.
Funding
Τhis review article did not receive any specific funding from any agency in the public, commercial, or not-for-profit sectors.
Disclosure
DDR, SAP and CSM report no conflicts of interest related to this work.
References
1. Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73:202–209. doi:10.1016/j.jhep.2020.03.039
2. Polyzos SA, Kang ES, Tsochatzis EA, et al. Commentary: nonalcoholic or metabolic dysfunction-associated fatty liver disease? The epidemic of the 21st century in search of the most appropriate name. Metabolism. 2020;113:154413. doi:10.1016/j.metabol.2020.154413
3. Lazarus JV, Mark HE, Villota-Rivas M, et al. The global NAFLD policy review and preparedness index: are countries ready to address this silent public health challenge? J Hepatol. 2022;76:771–780. doi:10.1016/j.jhep.2021.10.025
4. Makri E, Goulas A, Polyzos SA. Epidemiology, pathogenesis, diagnosis and emerging treatment of nonalcoholic fatty liver disease. Arch Med Res. 2021;52:25–37. doi:10.1016/j.arcmed.2020.11.010
5. Karlsen TH, Sheron N, Zelber-Sagi S, et al. The EASL-lancet liver commission: protecting the next generation of Europeans against liver disease complications and premature mortality. Lancet. 2022;399(10319):61–116. doi:10.1016/S0140-6736(21)01701-3
6. Polyzos SA, Kang ES, Boutari C, Rhee EJ, Mantzoros CS. Current and emerging pharmacological options for the treatment of nonalcoholic steatohepatitis. Metabolism. 2020;111S:154203. doi:10.1016/j.metabol.2020.154203
7. Polyzos SA, Kountouras J, Mantzoros CS. Adipose tissue, obesity and non-alcoholic fatty liver disease. Minerva Endocrinol. 2017;42:92–108. doi:10.23736/S0391-1977.16.02563-3
8. Polyzos SA, Kountouras J, Mantzoros CS. Adipokines in nonalcoholic fatty liver disease. Metabolism. 2016;65:1062–1079. doi:10.1016/j.metabol.2015.11.006
9. Ornitz DM, Itoh N. The fibroblast growth factor signaling pathway. WIREs Dev Biol. 2015;4:215–266. doi:10.1002/wdev.176
10. Fisher FM, Maratos-Flier E. Understanding the physiology of FGF21. Annu Rev Physiol. 2016;78(1):223–241. doi:10.1146/annurev-physiol-021115-105339
11. Jin L, Yang R, Geng L, Xu A. Fibroblast growth factor-based pharmacotherapies for the treatment of obesity-related metabolic complications. Annu Rev Pharmacol Toxicol. 2022. doi:10.1146/annurev-pharmtox-032322-093904
12. Kong Y, Zhao C, Tan P, et al. FGF21 reduces lipid accumulation in bovine hepatocytes by enhancing lipid oxidation and reducing lipogenesis via AMPK signaling. Animals. 2022;12(7):939. doi:10.3390/ani12070939
13. Lee KJ, Jang YO, Cha SK, et al. Expression of fibroblast growth factor 21 and β-klotho regulates hepatic fibrosis through the nuclear factor-κB and C-Jun n-terminal kinase pathways. Gut Liver. 2018;12:449–456. doi:10.5009/gnl17443
14. Li JY, Wang N, Khoso MH, et al. FGF-21 elevated IL-10 production to correct LPS-induced inflammation. Inflammation. 2018;41:751–759. doi:10.1007/s10753-018-0729-3
15. Jiang S, Yan C, Fang QC, et al. Fibroblast growth factor 21 is regulated by the IRE1α-XBP1 branch of the unfolded protein response and counteracts endoplasmic reticulum stress-induced hepatic steatosis. Int J Biol Chem. 2014;289(43):29751–29765. doi:10.1074/jbc.M114.565960
16. Chau MDL, Gao J, Yang Q, Wu Z, Gromada J. Fibroblast growth factor 21 regulates energy metabolism by activating the AMPK-SIRT1-PGC-1alpha pathway. Proc Natl Acad Sci USA. 2010;107(28):12553–12558. doi:10.1073/pnas.1006962107
17. Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARα and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5(6):426–437. doi:10.1016/j.cmet.2007.05.002
18. Tanaka N, Takahashi S, Zhang Y, et al. Role of fibroblast growth factor 21 in the early stage of NASH induced by methionine- and choline-deficient diet. Biochim Biophys Acta. 2015;1852:1242–1252. doi:10.1016/j.bbadis.2015.02.012
19. Wan XS, Lu XH, Xiao YC, et al. ATF4- and CHOP-dependent induction of FGF21 through endoplasmic reticulum stress. Biomed Res Int. 2014;2014:1–9.
20. Schaap FG, Kremer AE, Lamers WH, Jansen PLM, Gaemers IC. Fibroblast growth factor 21 is induced by endoplasmic reticulum stress. Biochimie. 2013;95:692–699. doi:10.1016/j.biochi.2012.10.019
21. Wu YK, Ren ZN, Zhu SL, et al. Sulforaphane ameliorates non-alcoholic fatty liver disease in mice by promoting FGF21/FGFR1 signaling pathway. Acta Pharmacol Sin. 2022;43:1473–1483. doi:10.1038/s41401-021-00786-2
22. Long X, Liu D, Gao Q, et al. Bifidobacterium adolescentis alleviates liver steatosis and steatohepatitis by increasing fibroblast growth factor 21 sensitivity. Front Endocrinol. 2021;12:773340. doi:10.3389/fendo.2021.773340
23. Fisher FM, Chui PC, Antonellis PJ, et al. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes. 2010;59(11):2781–2789. doi:10.2337/db10-0193
24. Samson SL, Sathyanarayana P, Jogi M, et al. Exenatide decreases hepatic fibroblast growth factor 21 resistance in non-alcoholic fatty liver disease in a mouse model of obesity and in a randomised controlled trial. Diabetologia. 2011;54(12):3093–3100. doi:10.1007/s00125-011-2317-z
25. Fletcher JA, Meers GM, Laughlin MH, Ibdah JA, Thyfault JP, Rector RS. Modulating fibroblast growth factor 21 in hyperphagic OLETF rats with daily exercise and caloric restriction. Appl Physiol Nutr Metab. 2012;37(6):1054–1062. doi:10.1139/h2012-091
26. Garcia-Caraballo SC, Comhair TM, Verheyen F, et al. Prevention and reversal of hepatic steatosis with a high-protein diet in mice. Biochim Biophys Acta Mol Basis Dis. 2013;1832:685–695. doi:10.1016/j.bbadis.2013.02.003
27. Fisher FM, Chui PC, Nasser IA, et al. Fibroblast growth factor 21 limits lipotoxicity by promoting hepatic fatty acid activation in mice on methionine and choline-deficient diets. Gastroenterology. 2014;147:1073–1083. doi:10.1053/j.gastro.2014.07.044
28. Lee YH, Kim SH, Kim SN, et al. Sex-specific metabolic interactions between liver and adipose tissue in MCD diet-induced non-alcoholic fatty liver disease. Oncotarget. 2016;7:46959–46971. doi:10.18632/oncotarget.10506
29. Liu X, Zhang P, Martin RC, et al. Lack of fibroblast growth factor 21 accelerates metabolic liver injury characterized by steatohepatities in mice. Am J Cancer Res. 2016;6:1011–1025.
30. Rusli F, Deelen J, Andriyani E, et al. Fibroblast growth factor 21 reflects liver fat accumulation and dysregulation of signalling pathways in the liver of C57BL/6J mice. Sci Rep. 2016;6:30484. doi:10.1038/srep30484
31. Lee JH, Kang YE, Chang JY, et al. An engineered FGF21 variant, LY2405319, can prevent non-alcoholic steatohepatitis by enhancing hepatic mitochondrial function. Am J Transl Res. 2016;8:4750–4763.
32. Gong Q, Hu Z, Zhang F, et al. Fibroblast growth factor 21 improves hepatic insulin sensitivity by inhibiting mammalian target of rapamycin complex 1 in mice. Hepatology. 2016;64:425–438. doi:10.1002/hep.28523
33. Park JG, Xu X, Cho S, et al. CREBH-FGF21 axis improves hepatic steatosis by suppressing adipose tissue lipolysis. Sci Rep. 2016;6:27938. doi:10.1038/srep27938
34. Cui A, Li J, Ji S, et al. The effects of B1344, a novel fibroblast growth factor 21 analog, on nonalcoholic steatohepatitis in nonhuman primates. Diabetes. 2020;69:1611–1623. doi:10.2337/db20-0209
35. Pan Q, Lin S, Li Y, et al. A novel GLP-1 and FGF21 dual agonist has therapeutic potential for diabetes and non-alcoholic steatohepatitis. EBioMedicine. 2021;63:103202. doi:10.1016/j.ebiom.2020.103202
36. Tang H, Li J, Zinker B, et al. Evaluation of a PEGylated fibroblast growth factor 21 variant using novel preclinical magnetic resonance imaging and magnetic resonance elastography in a mouse model of nonalcoholic steatohepatitis. J Magn Reson Imaging. 2022;56:712–724. doi:10.1002/jmri.28077
37. Yano K, Yamaguchi K, Seko Y, et al. Hepatocyte-specific fibroblast growth factor 21 overexpression ameliorates high-fat diet-induced obesity and liver steatosis in mice. Lab Invest. 2022;102:281–289. doi:10.1038/s41374-021-00680-9
38. Puengel T, Lefere S, Hundertmark J, et al. Combined therapy with a CCR2/CCR5 antagonist and FGF21 analogue synergizes in ameliorating steatohepatitis and fibrosis. Int J Mol Sci. 2022;23:6696. doi:10.3390/ijms23126696
39. Klaebel JH, Lykkesfeldt J, Tveden-Nyborg P. Efficacy of fibroblast growth factor 21 in non-alcoholic fatty liver disease in Guinea pigs. Basic Clin Pharmacol Toxicol. 2022;130:385–393. doi:10.1111/bcpt.13705
40. Xiao J, Bei Y, Liu J, et al. miR‐212 downregulation contributes to the protective effect of exercise against non‐alcoholic fatty liver via targeting
41. Markan KR, Naber MC, Ameka MK, et al. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes. 2014;63:4057–4063. doi:10.2337/db14-0595
42. Mosinski JD, Pagadala MR, Mulya A, et al. Gastric bypass surgery is protective from high-fat diet-induced non-alcoholic fatty liver disease and hepatic endoplasmic reticulum stress. Acta Physiol. 2016;217:141–151. doi:10.1111/apha.12640
43. Pei E, Liu Y, Jiang W, et al. Sleeve gastrectomy attenuates high fat diet-induced non-alcoholic fatty liver disease. Lipids Health Dis. 2018;17(1):243. doi:10.1186/s12944-018-0875-5
44. Pirozzi C, Lama A, Simeoli R, et al. Hydroxytyrosol prevents metabolic impairment reducing hepatic inflammation and restoring duodenal integrity in a rat model of NAFLD. J Nutr Biochem. 2016;30:108–115. doi:10.1016/j.jnutbio.2015.12.004
45. An HJ, Lee B, Kim DH, et al. Physiological characterization of a novel PPAR pan agonist, 2-(4-(5,6-methylenedioxybenzo[d]thiazol-2-yl)-2methylphenoxy)-2-methylpropanoic acid (MHY2013). Oncotarget. 2017;8:16912–16924. doi:10.18632/oncotarget.14818
46. An HJ, Lee B, Kim SM, et al. A PPAR pan agonist, MHY2013 alleviates age-related hepatic lipid accumulation by promoting fatty acid oxidation and suppressing inflammation. Biol Pharm Bull. 2018;41:29–35. doi:10.1248/bpb.b17-00371
47. Cunningham RP, Moore MP, Moore AN, et al. Curcumin supplementation mitigates NASH development and progression in female Wistar rats. Physiol Rep. 2018;6:e13789. doi:10.14814/phy2.13789
48. Yu Y, Zhang XH, Ebersole B, Ribnicky D, Wang ZQ. Bitter melon extract attenuating hepatic steatosis may be mediated by FGF21 and AMPK/Sirt1 signaling in mice. Sci Rep. 2013;3:3142. doi:10.1038/srep03142
49. Abd Elwahab AH, Ramadan BK, Schaalan MF, Tolba AM. A novel role of SIRT1/ FGF-21 in taurine protection against cafeteria diet-induced steatohepatitis in rats. Cell Physiol Biochem. 2017;43(2):644–659. doi:10.1159/000480649
50. Chukijrungroat N, Khamphaya T, Weerachayaphorn J, Songserm T, Saengsirisuwan V. Hepatic FGF21 mediates sex differences in high-fat high-fructose diet-induced fatty liver. Am J Physiol Endocrinol Metab. 2017;313(2):E203–E212. doi:10.1152/ajpendo.00076.2017
51. Dushay J, Chui PC, Gopalakrishnan GS, et al. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology. 2010;139(2):456–463. doi:10.1053/j.gastro.2010.04.054
52. Li H, Fang Q, Gao F, et al. Fibroblast growth factor 21 levels are increased in nonalcoholic fatty liver disease patients and are correlated with hepatic triglyceride. J Hepatol. 2010;53(5):934–940. doi:10.1016/j.jhep.2010.05.018
53. Li X, Fan X, Ren F, et al. Serum FGF21 levels are increased in newly diagnosed type 2 diabetes with nonalcoholic fatty liver disease and associated with hsCRP levels independently. Diabetes Res and Clin Pract. 2011;93:10–16. doi:10.1016/j.diabres.2011.02.034
54. Giannini C, Feldstein AE, Santoro N, et al. Circulating levels of FGF-21 in obese youth: associations with liver fat content and markers of liver damage. J Clin Endocrinol Metab. 2013;98:2993–3000. doi:10.1210/jc.2013-1250
55. Koliaki C, Szendroedi J, Kaul K, et al. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab. 2015;21:739–746. doi:10.1016/j.cmet.2015.04.004
56. Zhang X, Hu Y, Zeng H, et al. Serum fibroblast growth factor 21 levels is associated with lower extremity atherosclerotic disease in Chinese female diabetic patients. Cardiovasc Diabetol. 2015;14:32. doi:10.1186/s12933-015-0190-7
57. Flisiak-Jackiewicz M, Bobrus-Chociej A, Wasilewska N, Tarasow E, Wojtkowska M, Lebensztejn DM. Can hepatokines be regarded as novel non-invasive serum biomarkers of intrahepatic lipid content in obese children? Adv Med Sci. 2019;64:280–284. doi:10.1016/j.advms.2019.02.005
58. Reinehr T, Woelfle J, Wunsch R, Roth CL. Fibroblast growth factor 21 (FGF-21) and its relation to obesity, metabolic syndrome, and nonalcoholic fatty liver in children: a longitudinal analysis. J Clin Endocrinol Metab. 2012;97:2143–2150. doi:10.1210/jc.2012-1221
59. Alisi A, Ceccarelli S, Panera N, et al. Association between serum atypical fibroblast growth factors 21 and 19 and pediatric nonalcoholic fatty liver disease. Wang Y, ed. PLoS One. 2013;8:e67160. doi:10.1371/journal.pone.0067160
60. Sodhi K, Bracero L. Role of serum biomarkers in early detection of non-alcoholic steatohepatitis and fibrosis in West Virginian children. J Clin Cell Immunol. 2016;07:393. doi:10.4172/2155-9899.1000393
61. Yan H, Xia M, Chang X, et al. Circulating fibroblast growth factor 21 levels are closely associated with hepatic fat content: a cross-sectional study. Xu A, ed. PLoS One. 2011;6:e24895. doi:10.1371/journal.pone.0024895
62. Barb D, Bril F, Kalavalapalli S, Cusi K. Plasma fibroblast growth factor 21 is associated with severity of nonalcoholic steatohepatitis in patients with obesity and type 2 diabetes. J Clin Endocrinol Metab. 2019;104:3327–3336. doi:10.1210/jc.2018-02414
63. Yilmaz Y, Eren F, Yonal O, et al. Increased serum FGF21 levels in patients with nonalcoholic fatty liver disease. Eur J Clin Invest. 2010;40(10):887–892. doi:10.1111/j.1365-2362.2010.02338.x
64. Waluga M, Kukla M, Zorniak M, et al. Fibroblast growth factor-21 and omentin-1 hepatic mRNA expression and serum levels in morbidly obese women with non-alcoholic fatty liver disease. J Physiol Pharmacol. 2017;68(3):363–374.
65. Mutanen A, Heikkilä P, Lohi J, Raivio T, Jalanko H, Pakarinen MP. Serum FGF21 increases with hepatic fat accumulation in pediatric onset intestinal failure. J Hepatol. 2014;60:183–190. doi:10.1016/j.jhep.2013.09.003
66. Jiang S, Zhang R, Li H, et al. The single nucleotide polymorphism rs499765 is associated with fibroblast growth factor 21 and nonalcoholic fatty liver disease in a Chinese population with normal glucose tolerance. J Nutrigenet Nutrigenomics. 2014;7:121–129. doi:10.1159/000367943
67. Larsson SC, Michaëlsson K, Mola-Caminal M, Höijer J, Mantzoros CS. Genome-wide association and Mendelian randomization study of fibroblast growth factor 21 reveals causal associations with hyperlipidemia and possibly NASH. Metabolism. 2022;137:155329. doi:10.1016/j.metabol.2022.155329
68. Li H, Dong K, Fang Q, et al. High serum level of fibroblast growth factor 21 is an independent predictor of non-alcoholic fatty liver disease: a 3-year prospective study in China. J Hepatol. 2013;58:557–563. doi:10.1016/j.jhep.2012.10.029
69. Zhan L, Zhou H, Chen R. [A prospective study of serum fibroblast growth factor 21 changes in nonalcoholic fatty liver patients]. Zhonghua Gan Zang Bing Za Zhi. 2015;23:350–353. Chinese. doi:10.3760/cma.j.issn.1007-3418.2015.05.006
70. Matikainen N, Taskinen MR, Stennabb S, et al. Decrease in circulating fibroblast growth factor 21 after an oral fat load is related to postprandial triglyceride-rich lipoproteins and liver fat. Eur J Endocrinol. 2012;166:487–492. doi:10.1530/EJE-11-0783
71. Markova M, Pivovarova O, Hornemann S, et al. Isocaloric diets high in animal or plant protein reduce liver fat and inflammation in individuals with type 2 diabetes. Gastroenterology. 2017;152:571–585.e8. doi:10.1053/j.gastro.2016.10.007
72. Hassani Zadeh S, Mansoori A, Hosseinzadeh M. Relationship between dietary patterns and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2021;36:1470–1478. doi:10.1111/jgh.15363
73. Taniguchi H, Tanisawa K, Sun X, Kubo T, Higuchi M. Endurance exercise reduces hepatic fat content and serum fibroblast growth factor 21 levels in elderly men. J Clin Endocrinol Metab. 2016;101:191–198. doi:10.1210/jc.2015-3308
74. Asghari S, Rezaei M, Rafraf M, Taghizadeh M, Asghari-Jafarabadi M, Ebadi M. Effects of calorie restricted diet on oxidative/antioxidative status biomarkers and serum fibroblast growth factor 21 levels in nonalcoholic fatty liver disease patients: a randomized, controlled clinical trial. Nutrients. 2022;14:2509. doi:10.3390/nu14122509
75. Vachliotis I, Goulas A, Papaioannidou P, Polyzos SA. Nonalcoholic fatty liver disease: lifestyle and quality of life. Hormones. 2022;21(1):41–49. doi:10.1007/s42000-021-00339-6
76. Polyzos SA, Mantzoros CS. Adiponectin as a target for the treatment of nonalcoholic steatohepatitis with thiazolidinediones: a systematic review. Metabolism. 2016;65:1297–1306. doi:10.1016/j.metabol.2016.05.013
77. Makri ES, Makri E, Polyzos SA. Combination therapies for nonalcoholic fatty liver disease. J Pers Med. 2022;12:1166. doi:10.3390/jpm12071166
78. Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387(10019):679–690. doi:10.1016/S0140-6736(15)00803-X
79. Li X, Wu X, Jia Y, et al. Liraglutide decreases liver fat content and serum fibroblast growth factor 21 levels in newly diagnosed overweight patients with type 2 diabetes and nonalcoholic fatty liver disease. J Diabetes Res. 2021;2021:3715026. doi:10.1155/2021/3715026
80. Polyzos S, Perakakis N, Mantzoros C. Fatty liver in lipodystrophy: a review with a focus on therapeutic perspectives of adiponectin and/or leptin replacement. Metabolism. 2019;96:66–82. doi:10.1016/j.metabol.2019.05.001
81. Braun LR, Feldpausch MN, Czerwonka N, Torriani M, Grinspoon SK, Stanley TL. Fibroblast growth factor 21 decreases after liver fat reduction via growth hormone augmentation. Growth Horm IGF Res. 2017;37:1–6. doi:10.1016/j.ghir.2017.10.002
82. Charles ED, Neuschwander‐Tetri BA, Pablo Frias J, et al. Pegbelfermin (BMS‐986036), PEGylated FGF21, in patients with obesity and type 2 diabetes: results from a randomized phase 2 study. Obesity. 2019;27:41–49. doi:10.1002/oby.22344
83. Sanyal A, Charles ED, Neuschwander-Tetri BA, et al. Pegbelfermin (BMS-986036), a PEGylated fibroblast growth factor 21 analogue, in patients with non-alcoholic steatohepatitis: a randomised, double-blind, placebo-controlled, phase 2a trial. Lancet. 2019;392:2705–2717. doi:10.1016/S0140-6736(18)31785-9
84. Harrison SA, Ruane PJ, Freilich BL, et al. Efruxifermin in non-alcoholic steatohepatitis: a randomized, double-blind, placebo-controlled, phase 2a trial. Nat Med. 2021;27:1262–1271. doi:10.1038/s41591-021-01425-3
85. Rader DJ, Maratos-Flier E, Nguyen A, et al. LLF580, an FGF21 analog, reduces triglycerides and hepatic fat in obese adults with modest hypertriglyceridemia. J Clin Endocrinol Metab. 2022;107:e57–e70. doi:10.1210/clinem/dgab624
86. Polyzos SA, Kechagias S, Tsochatzis EA. Review article: non-alcoholic fatty liver disease and cardiovascular diseases: associations and treatment considerations. Aliment Pharmacol Ther. 2021;54:1013–1025. doi:10.1111/apt.16575
87. Polyzos SA, Kountouras J, Zavos C, Tsiaousi E. The role of adiponectin in the pathogenesis and treatment of non-alcoholic fatty liver disease. Diabetes Obes Metab. 2010;12:365–383. doi:10.1111/j.1463-1326.2009.01176.x
88. Mintziori G, Polyzos SA. Emerging and future therapies for nonalcoholic steatohepatitis in adults. Expert Opin Pharmacother. 2016;17:1937–1946. doi:10.1080/14656566.2016.1225727
89. Polyzos SA, Kountouras J, Zavos C. Adiponectin as a potential therapeutic agent for nonalcoholic steatohepatitis. Hepatol Res. 2010;40:446–447.
90. Polyzos SA, Mantzoros CS. Necessity for timely noninvasive diagnosis of nonalcoholic fatty liver disease. Metabolism. 2014;63:161–167. doi:10.1016/j.metabol.2013.10.010
91. Shen J, Chan HLY, Wong GLH, et al. Non-invasive diagnosis of non-alcoholic steatohepatitis by combined serum biomarkers. J Hepatol. 2012;56:1363–1370. doi:10.1016/j.jhep.2011.12.025
92. Yang M, Xu D, Liu Y, et al. Combined serum biomarkers in non-invasive diagnosis of non-alcoholic steatohepatitis. Lin HC, ed. PLoS One. 2015;10:e0131664. doi:10.1371/journal.pone.0131664
93. He L, Deng L, Zhang Q, et al. Diagnostic value of CK-18, FGF-21, and related biomarker panel in nonalcoholic fatty liver disease: a systematic review and meta-analysis. Biomed Res Int. 2017;2017:1–12.
94. Wei W, Dutchak PA, Wang X, et al. Fibroblast growth factor 21 promotes bone loss by potentiating the effects of peroxisome proliferator-activated receptor γ. Proc Natl Acad Sci U S A. 2012;109:3143–3148. doi:10.1073/pnas.1200797109
95. Vachliotis ID, Anastasilakis AD, Goulas A, Goulis DG, Polyzos SA. Nonalcoholic fatty liver disease and osteoporosis: a potential association with therapeutic implications. Diabetes Obes Metab. 2022;24:1702–1720. doi:10.1111/dom.14774
96. Talukdar S, Zhou Y, Li D, et al. A long-acting FGF21 molecule, PF-05231023, decreases body weight and improves lipid profile in non-human primates and type 2 diabetic subjects. Cell Metab. 2016;23:427–440.
97. Kim AM, Somayaji VR, Dong JQ, et al. (). Once‐weekly administration of a long‐acting fibroblast growth factor 21 analogue modulates lipids, bone turnover markers, blood pressure and body weight differently in obese people with hypertriglyceridaemia and in non‐human primates. Diabetes Obes Metab. 2017;19(12): 1762–1772. doi:10.1111/dom.13023
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.