Back to Journals » Journal of Asthma and Allergy » Volume 13
FENOMA Study: Achieving Full Control in Patients with Severe Allergic Asthma
Authors Cabrejos S, Moreira A, Ramirez A, Quirce S , Soto Campos G , Dávila I , Campo P
Received 28 January 2020
Accepted for publication 10 April 2020
Published 6 May 2020 Volume 2020:13 Pages 159—166
DOI https://doi.org/10.2147/JAA.S246902
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Amrita Dosanjh
Sheila Cabrejos,1 Ana Moreira,2 Andreina Ramirez,2 Santiago Quirce,3 Gregorio Soto Campos,4 Ignacio Dávila,5 Paloma Campo6
1Allergy Service, Hospital Rafael Mendez de Lorca, Murcia, Spain; 2Novartis Farmacéutica, Barcelona, Spain; 3Department of Allergy, Hospital La Paz Institute for Health Research (IdiPAZ), Madrid, Spain, CIBER de Enfermedades Respiratorias, CIBERES, Madrid, Spain; 4Pneumology and Allergy Unit, Hospital Universitario de Jerez, Cádiz, Spain; 5Allergy Service, University Hospital of Salamanca and Institute for Biomedical Research of Salamanca (IBSAL), Salamanca, Spain, Biomedical and Diagnosis Science Department, Salamanca University School of Medicine, Salamanca, Spain; 6Allergy Unit, IBIMA-Regional University Hospital of Málaga, ARADyAL, Málaga, Spain
Correspondence: Ignacio Dávila
Allergy Service, University Hospital of Salamanca and Institute for Biomedical Research of Salamanca (IBSAL), Paseo de San Vicente, 58-182, Salamanca 37007, Spain
Tel +34923291100 (55373)
Email [email protected]
Introduction: A Spanish real-world study in patients with severe persistent asthma who achieved asthma control after a one-year treatment with omalizumab highlighted the phenotypic heterogeneity of these patients (FENOMA study). In this subanalysis, we describe the clinical improvement in patients with severe allergic asthma in this study (positive skin test and IgE level 30– 1500 IU/mL); n=240.
Patients and Methods: FENOMA was an observational, multicentre, retrospective study in 345 patients achieving asthma control according to Spanish guidelines (GEMA). Baseline demographic and asthma-related characteristics were collected. Outcomes analyzed were those included in asthma control definition plus changes in background treatments and in blood eosinophil count (%) and exhaled nitric oxide fraction [FeNO].
Results: At baseline, patients were aged 45.4± 15.0 years; 67% were women. Median (Q1;Q3) IgE levels were 302.5 (154.0; 553.5) IU/mL. After one-year treatment with omalizumab: 43.3% of patients had daytime symptoms vs 97.7% before treatment and 49.6% stopped taking oral corticosteroids. FEV1 increased a median of 12.0 (4.0; 23.0)%; P < 0.0001. The number of non-severe asthma exacerbations decreased a median of − 4.0 (− 7.0; 2.0); P < 0.0001. Median unplanned visits to primary care or specialists and days of school/workplace absenteeism decreased from 4.9 (2.0; 6.0), 1.0 (0.0; 3.0) and 0.0 (0.0; 14.0) to 0.0 (0.0; 1.0), 0.0 (0.0; 0.0) and 0.0 (0.0; 0.0), respectively. Median eosinophil blood count and FeNO decreased from 5.0 (3:0; 8.0)% to 3.0 (2.0; 5.5)% and from 36.0 (23:0; 53.0) ppb to 20.0 (13.0; 34.0) ppb, respectively.
Conclusion: This study highlights the asthma control achieved by patients with severe allergic asthma treated with omalizumab, with relevant benefits on the burden of the disease both on patients and the healthcare system.
Keywords: asthma, omalizumab, allergy, exacerbations, IgE
Introduction
Asthma is a heterogeneous disease for which recognizable clusters of demographic, clinical and/or pathophysiological characteristics – often called “phenotypes” or “endotypes”- have been defined.1,2 The treatment goal is achieving asthma control (i.e. good control of symptoms and minimization of the risk of exacerbations).1,3 However, despite treatment guidelines, asthma remains uncontrolled in many patients.4 Asthma severity is assessed according to the level of treatment required to achieve and maintain asthma control. However, the definition of severe or therapy-refractory asthma is not straightforward and has not been consistent across studies.5 Nowadays, the most accepted definition of severe asthma is that requiring high-dose inhaled corticosteroids (ICS) plus a second controller and/or systemic corticosteroids to prevent it from becoming “uncontrolled” or which remains “uncontrolled” despite this therapy (refractory).1,3 Patients with severe asthma are at risk of severe exacerbations and even death.1 Severe asthma has a profound personal, clinical and economic impact.1,3,6
Allergic asthma accounts for most cases of asthma, with immunoglobulin E (IgE) being integral to its physiopathology.7,8 Allergic asthma is associated with increased levels of circulating total and specific IgE, with a clear involvement both at the onset of the disease and during its chronic phase.7 Omalizumab, the first treatment addressing the allergic component of moderate-to-severe allergic asthma,9 is an anti-IgE monoclonal antibody that selectively binds to human IgE and prevents its binding to high-affinity IgE receptors.10 In the randomized, Phase III trial INNOVATE,11 omalizumab significantly reduced the rate of clinically significant and severe asthma exacerbations and emergency visits and significantly improved asthma-related quality of life, morning peak expiratory flow and asthma symptom scores vs. placebo in patients with severe persistent asthma, with a good safety profile.11 Omalizumab is indicated in Europe as an add-on therapy to improve asthma control only in patients with severe persistent allergic asthma.10 A meta-analysis of 25 non-controlled studies published up to 2015 and a recent systematic review (2018) that updates and extends this review up 42 of such studies confirm the effectiveness of omalizumab in the real-life setting.12,13
An observational, multicentre, retrospective study was conducted between 2015 and 2016 in Spain in 345 adult patients with severe persistent asthma who had achieved total disease control as soon as after one-year treatment with omalizumab (FENOMA study). This study underlined the heterogeneity of patients achieving this treatment goal with omalizumab in the real-life clinical setting.14 In the current study, we describe the clinical improvement of patients with allergic asthma (Immunoglobulin [Ig] E-mediated asthma) –i.e. in whom omalizumab was strictly administered following therapeutic indication10- who participated in the FENOMA study. Treatment-response biomarkers such as total IgE, peripheral blood eosinophil count and exhaled nitric oxide fraction (FeNO)8,15 were also assessed.
Methods
Study Design and Subjects
FENOMA ([FENOtipos de asMA], Asthma Phenotypes) was an observational, retrospective, real-life study, carried out in 69 Spanish centres. Design of this study has been previously published.14 Briefly, patients included where those ≥18 years, with severe persistent asthma (steps 5 or 6 according to the Spanish Guidelines on the Management of Asthma 2009),16 having started treatment with omalizumab (as per usual practice) between 1st January 2010 and 31st December 2013, and who had achieved control of the disease after finishing the first year of treatment with omalizumab. Asthma control was defined according to Spanish reference guidelines,16 which include fulfilling all of the following criteria: no daytime asthma symptoms or asthma symptoms ≤ twice a week, no limitation in activities of daily living, no night-time asthma symptoms/awakenings, no need for rescue medication or ≤ twice a week, normal pulmonary function (forced expiratory volume in 1 s [FEV1] ≥80% of the predicted value/peak expiratory flow [PEF] >80% of their personal best) and no severe or clinically significant exacerbations. The only exclusion criterion was having participated in a clinical trial during the period of analysis. Of the 345 patients included in the FENOMA study, 240 (69.6%) had allergic asthma (i.e. positive skin prick test or specific IgE and symptomatology associated with exposure), and should have serum IgE level between 30 and 1500 IU/mL. This population was included in the present study. A positive skin prick test was defined as an immediate hypersensitivity to commonly tested environmental aeroallergens. These are likely to have slightly varied among centers.
The study was conducted according to Good Clinical Practice (International Conference of Harmonization) guidelines and local regulation and was approved by the Independent Ethic Committee of the Hospital Universitari Germans Trias i Pujol, Barcelona, Spain. All participants provided written informed consent.
Information Collected
Medical records were reviewed between February 2015 and June 2016. Data collected before treatment (baseline) included: demographics and anthropometrics, smoking habit (never, former (≥1 year), current), clinical history including asthma-related co-morbidities (allergic rhinitis, sinus polyposis, atopic dermatitis), family history (atopy and mental/psychosocial disorders), total serum IgE level. Data collected before and after one-year treatment with omalizumab included: anthropometrics, severity and control of the disease (including data from the Spanish validated version of the Asthma Control Test [ACT]17,18 when available), pulmonary function (FEV1), the percentage of blood eosinophil count, FeNO, the number of non-severe exacerbations and the use of healthcare resources due to these episodes. The need for rescue medication and the treatment with ICS and oral corticosteroids (OCS) were also captured. OCS intake before receiving treatment with omalizumab was defined either as having a daily maintenance dose or a short course of treatment.
Study Outcomes
Primary outcome of this analysis was to describe the clinical and pulmonary improvement of patients that had achieved control disease after one-year treatment with omalizumab by means of i) incidence of daytime symptoms, ii) changes in the use of ICS or OCS, iii) need of rescue therapy, iv) pulmonary function (FEV1), iv) number of non-severe exacerbations and vi) use of healthcare resources: non-planed visits to primary care or specialists and the number of days of school or workplace absenteeism due to asthma worsening. Non-severe asthma exacerbations were defined as those that did not require OCS, emergency assistance or hospitalization. Secondary outcomes include the assessment of the level of asthma control by means of the ACT questionnaire and the percentage of blood eosinophil blood count before and after treatment and FeNO.
Statistical Analysis
Continuous variables are presented as mean, standard deviation (SD) and 95% confidence interval (CI), or median and quartile 1 (Q1) and 3 (Q3), as applicable. Categorical variables are reported as frequencies and percentages. Descriptive statistics were calculated for demographic and clinical characteristics. For statistical comparisons, the Wilcoxon signed-rank was used as applicable. All tests were 2-sided. A P value of <0.05 was considered to be statistically significant. All analyses were performed with the SAS statistical package (version 9.4; SAS Institute, Cary, NC).
Results
Patients Included and Baseline Characteristics
Demographic, anthropometric and clinical characteristics of patients who had achieved total control after one-year treatment with omalizumab are shown in Table 1. Mean (SD) age of patients was 45.4 (15.0) years. Sixty-seven percent (n=161) were female. Most patients (81.7%) had never smoked. Median time (Q1;Q3) between asthma and severe persistent asthma diagnoses was 9.8 (3.3;19.1) years. Median (Q1;Q3) time since diagnosis of severe persistent asthma was 5.6 (4.2;7.4) years. Forty percent of patients had a familial history of atopy. The most frequent comorbidity was allergic rhinitis (77.1%). Nearly half of patients (48.5%) showed hyper-reactivity to mites and 26.7% to pollens.
 |
Table 1 Demographic, Anthropometric and Clinical Characteristics at Baseline |
Asthma characteristics in the year before starting treatment with omalizumab are shown in Table 2. Almost all patients (98%; n=235) had daytime symptoms, with nearly half (48.7%; n=117) having daily symptoms and 17.1% (n=41) continuous symptoms. Also, almost all patients (92.3%; n=220) had night-time symptoms, with 35.8% (n=86) being more than once a week and 25.4% (n=61) frequents.
 |
Table 2 Asthma History in the Year Before Starting Treatment with Omalizumab |
Nearly 60% of patients (n=142) and 12.9% (n=31) had quite and much limitations in the activity of daily living, respectively. Ninety-seven percent (n=233) needed rescue medication, with 60.0% (n=144) needing medication everyday/several times a day. Median FEV1 was 71.5%. Median (Q1; Q3) number of non-severe asthma episodes and of severe or clinically significant exacerbations in the previous year was 5.0 (3.0; 8.5) and 3.0 (2.0; 4.0), respectively. The number of visits to emergency rooms, admission days to hospital or intensive care units due to these exacerbations is shown in Table 2. Median (Q1; Q3) total serum IgE levels were 302.5 (154.0; 553.5) IU/mL. Background treatments for asthma during the previous year are shown in Table 2. Mean (SD) initial dose of omalizumab was 325.9 (119.8) mg.
Outcomes After One-Year Treatment
More than half (56.7%, n=136) of patients had no daytime symptoms, while 35.0% (n=84) and 8.3% (n=20) had symptoms 1 and 2 days per week, respectively. Up to 49.6% (n=119) of the total population evaluated stopped taking OCS, only 8.3% (n = 20) of the patients continued to receive them after one year of treatment with omalizumab (P <0.0001). Of the patients with available information about dose modification, the reduction was not significant (P=0.3077). The proportion of patients taking ICS decreased significantly (from 99.6% to 90.2%; P <0.0001). The dose of ICS was reduced in 58.7% (n=141) patients (P=0.9062) and maintained in 37.5% (n=90) patients. More than half (55.4%, n=133) of patients needed no rescue medication, while 35.0% (n=84) and 9.6% (n=23) needed medication 1 and 2 days per week, respectively. FEV1 increased a median (Q1; Q3) of 12.0% (4.0; 23.0) (P <0.0001) (Table 3).
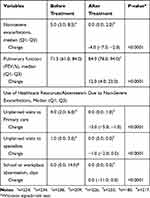 |
Table 3 Non-Severe Exacerbations, Pulmonary Function and Use of Healthcare Resources/Absenteeism Before and After One-Year Treatment with Omalizumab (n=240) |
The number of non-severe asthma exacerbations decreased a median (Q1; Q3) of −4.0 (−7.0; −2.0); P <0.0001. The number of unplanned visits to primary care or specialists, or days of school or workplace absenteeism before treatment is shown in Table 3. After one-year treatment with omalizumab, none of these took place (P <0.0001 vs. before treatment for school or workplace absenteeism).
Data regarding the ACT questionnaires were available in 101 (42.1%) and 117 (48.8%) patients before and after one-year treatment with omalizumab, respectively. Median score (Q1; Q3) was 14.0 (11.0; 16.0) before treatment and 22.0 (20.0; 24.0) after one-year treatment (change: 7.0 [5.0;10.0]).
The percentage of blood eosinophil count was available in 205 (85.4%) patients before treatment and in 132 (55.0%) after one-year treatment. Median (Q1; Q3) was 5.0% (3:0; 8.0) and 3.0 (2.0; 5.5)%, respectively (change: −1.0 [−3.0;0.0]). FeNO values were available in 98 (40.8%) patients before treatment and in 109 (45.4%) after one-year treatment. Median (Q1; Q3) was 36.0 (23.0; 53.0) ppb and 20.0 (13.0; 34.0) ppb, respectively (change: −14.5 [−26.0; 0.0]).
Discussion
The results of our study illustrate the clinical improvement of patients with severe persistent allergic asthma who achieved full asthma control as soon as after one-year treatment with omalizumab in a real-life setting. This achievement is relevant given the difficulty in optimally controlling asthma, especially severe asthma.19,20 It also adds evidence to the benefits of omalizumab as an add-on therapy to improve asthma control in patients with severe persistent allergic asthma under conditions of heterogeneity in patients, clinicians, sites, and treatment patterns, with results being consistent with those of other studies in the real-life setting.12,13
Several clinical indicators related to disease control according to reference guidelines – both objective and subjective – improved after one-year treatment with omalizumab. The incidence of daytime symptoms was greatly reduced, with nearly 57% of patients having no daytime symptoms compared to 2% having no symptoms or ≤ twice a week before starting treatment. Nearly 50% of patients needed no OCS, which is in agreement with other studies analyzing the effectiveness of omalizumab in patients with severe persistent allergic asthma in the real-life setting.12 The meta-analysis gathering 25 of these studies commented above estimated a relative risk (RR) reduction of 0.46 in the requirement of OCS after 12 months of omalizumab therapy, and reported significant and small-to-moderate reductions in ICS intake.12 Changes in the intake of OCS or ICS were not analyzed in the INNOVATE study.11 The use of rescue medication was also greatly reduced, with 55% of patients needing no rescue medication compared to 3% of patients needing no rescue medication or ≤ twice a week before starting treatment. The reduction in the proportion of patients needing rescue medication was not analyzed in the INNOVATE study nor in the meta-analysis of real-life evidence. However, a reduction of 0.5 puffs/day compared to placebo was reported in the INNOVATE study, although this was not statistically significant.11 FEV1 significantly improved in a magnitude similar to that reported in 11 of the 42 real-life studies (n=1814) that analyzed this clinical parameter after 1-year treatment (average of 12.4%).13
After one-year treatment with omalizumab, the number of non-severe exacerbations significantly decreased by five-fold (from a median [Q1; Q3] of 5.0 [3.0; 8.5] to 0.0 [0.0; 2.0]). Accordingly, the number of unplanned visits to primary or specialized care and of school or workplace absenteeism also decreased. Our results are in agreement with the reductions in healthcare resource and asthma-related absences from work/school reported in a long-term (up to 10-year) follow-up study in the real-life setting.21 This latter study also corroborated the overall safety profile of omalizumab,21 which was not analyzed in our study.
The ACT questionnaire revealed good asthma control, as assessed by both the physician and patient, with a change from 14 to 22 points in a scale from 5 to 25. This is in agreement with the favorable evaluation of treatment effectiveness by both, patients and investigators, in the INNOVATE study.11 Similarly, on average 73% of patients achieved a good or excellent rating in the “Global evaluation of treatment effectiveness” at 12 months treatment with omalizumab in the meta-analysis of studies in the real-life setting.12
It is worth noting that patients having achieved full asthma control after one-year treatment with omalizumab presented increased peripheral blood eosinophil percentage (5%) before starting treatment, which is quite frequent in patients with asthma.22 This observation is in agreement with the hypothesis that IgE would be the cause of allergic asthma, as it is involved early in the inflammatory cascade activating mast cells, basophils, eosinophils and dendritic cells, while eosinophilia would be the consequence of the whole process.22 A post hoc analysis of the INNOVATE study showed that omalizumab produced a greater reduction in exacerbation rate (primary outcome) in patients with higher (≥300 cells/µL) vs. lower baseline eosinophil count, and even more when IgE levels were also high (>75 IU/mL).23 In a pooled analysis of seven randomized controlled omalizumab trials, patients with pre-treatment total IgE ≥76 IU/mL were also more likely to experience clinically meaningful benefit to omalizumab and more patients had clinically meaningful responses.24 However, large studies in the real-life setting have shown that omalizumab response in patients with severe allergic asthma do not vary in patients with high or low (≤300 cells/µL) blood eosinophil count, with this finding being independent of the eosinophil cut-offs and of response definitions used.25,26 It should be noted that in our study eosinophil blood count was recorded as percentage, which limits its interpretation. A cut-off point of 2% has recently shown to be a strong predictor of eosinophilia (sensitivity of 87%; a specificity of 86%, P < 0.001), in smokers with asthma having well-controlled disease.27 Median IgE was 302.5 IU/mL in our series of well-controlled patients compared to 150 IU/mL in the INNOVATE study.11
The effect of omalizumab on inflammatory cells mediated by the reduction of the allergen-specific proliferative response of T-helper 2 cells (including the production of IL)22 may explain the reduction from 5% to 3% in eosinophil count observed after one-year treatment with omalizumab in our series. A similar reduction was also reported in the long-term follow-up study in the real-life setting commented above.21 These observations support the adequacy of targeting the cause of allergic asthma (IgE) instead of the consequence (eosinophilia) when treating severe allergic asthma.22 Novel therapies targeting IL-5 or its receptor are emerging for the treatment of severe eosinophilic asthma. However, their use is limited to patients with high blood eosinophil count (≥300 cells/µL) over 12 months.28 There is no blood eosinophil count threshold for the use of omalizumab.10
Similar to sputum and blood eosinophils, elevated FeNO is predictive of asthma exacerbations and asthma severity.8 In fact, FeNO levels before treatment were above the threshold of 25 ppb (FeNO levels <25 ppb are considered normal in adults)15 (median of 36.0 [23.0; 53.0] ppb). This observation is in agreement with the findings of a prospective, randomized, placebo-controlled study showing that patients with severe asthma and higher baseline FeNO had a greater reduction in exacerbation rate with omalizumab vs. patients with low baseline FeNO.21 After one-year treatment with omalizumab, FeNO levels decreased to levels below 25 ppb (20.0 [13.0; 34.0]) ppm. A similar reduction was also reported in the above-mentioned long-term study in the real-life setting.26 Nevertheless, the effectiveness of omalizumab has shown to be independent of baseline FeNO levels. It should be also considered that the reduction in exacerbation rate is not the only variable defining asthma control. Summing up, this study represents a unique real-life experience of omalizumab in patients who achieved total asthma control as soon as within 1 year, which provides information on the effectiveness of this drug in severe allergic persistent asthma. However, some limitations should be acknowledged. These include its single-arm retrospective nature, which implies relying on the accuracy and completeness of information included in the clinical records. This has especially affected biomarkers such as FeNO, which was not routinely assessed in the clinical practice at the time of the study, and the ACT questionnaire. The lack of a control group prevents corroborating findings about predictors of response.
The results of our study in patients with severe persistent allergic asthma who achieved disease control after one-year treatment with omalizumab highlight the clinical benefits achieved by these patients after this period, including the decrease of the use of medications with damaging side effects. They also support the effect of omalizumab on the reduction of the burden on healthcare systems.
Acknowledgments
The authors thank Beatriz Viejo, Ph.D. for providing medical writing support and editorial assistance that were funded by Novartis Pharmaceuticals, S.A. in accordance with Good Publication Practice guidelines. They also thank all the researchers participating in this study, all of them from Spanish hospitals: Ada Luz Andreu Rodríguez, Adalberto Pacheco Galván, Adolfo Baloira Villar, Aizea Mardones Charroalde, Alberto Levy Nahon, Alicia Padilla Galo, Ana Gómez-Bastero Fernández, Ana Montoro de Francisco, Ángel Blasco Sarramian, Ángel Ferrer Torres, Antonio León Jiménez, Antonio Moreno Fernández, Antonio Pablo Arenas Vacas, Astrid Crespo Lessmann, Beatriz Huertas Barbudo, Beatriz Rodríguez Jiménez, Carlos Almonacid Sánchez, Carlos Martínez Rivera, Carlos Sanjuas Benito, Celia Pinedo Sierra, Consuelo Fernández Rodríguez, Enrique Macias Fernández, Eva Martínez Moragón, Fernando Ruiz Mori, Francisco Javier Guerra, Gemma Jorro Martínez, Gerardo Pérez Chica, Irene de Lorenzo García, Isabel María Flores Martín, Isabel Molero Sancho, Jacinto Ramos González, Joan Serra Batlles, Joaquín Quiralte Enríquez, José Angel Carretero, José Antonio Gullón Blanco, José Carlos Orta Cuevas, Jose Fernando Florido López, Juan Guallar Ballester, Lucía Gimeno Casanova, Luis Carazo Fernández, Luis Mateos Caballero, María José Torres Jaén, Manuel Agustín Sojo González, Manuel García Marron, Mar Mosteiro Añon, María Angeles Peña, María Jesús Rodríguez Nieto, Maria Purificación Jiménez, Marta Reche Frutos, Mercedes Cimarra Alvarez, Miguel Angel Díaz Palacios, Miguel Angel Tejedor Alonso, Patricia Mata Calderón, Pedro Cabrera-Navarro, Pilar Cebollero Rivas, Pilar Serrano Delgado, Rafael Llatser Oliva, Ramón Rodríguez Pacheco, Rosa Irigay Canals, Ruperto González Pérez, Sandra Dorado Arenas, Teodoro Montemayor Rubio, Vanesa Vicens Zygmunt, Héctor Manuel González Expósito, Marina Blanco Aparicio.
Funding
This study was funded by the Novartis Farmacéutica, S.A.
Disclosure
S Cabrejos reports personal fees from Novartis, GSK, ALK Abelló, Stallergenes, HAL Allergy, Astra Zeneca, Inmunotek, and Diater collaborations. A Moreira is currently a Medical Advisor at Novartis Farmacéutica S.A. A Ramirez is currently a Medical Communication Advisor at Novartis Farmacéutica S.A. S Quirce reports personal fees and non-financial supports from ALK, AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Mundipharma, Novartis, Sanofi, and Teva (for the organization of educational events, advisory boards, and received speakers’ honoraria). G Soto Campos reports personal fees (advisory boards and speakers´ honoraria) from AstraZeneca, Boehringer Ingelheim, Novartis, GSK, Chiesi, Bial and Menarini. I Dávila reports payment for lectures including service on speakers´ bureaus from ALK-Abelló, Astra-Zeneca, Chiesi, Diater, GSK, Leti, Novartis, Sanofi, Stallergenes, Teva; consultancy from Allergy therapeutics, ALK-Abello, Astra-Zeneca, GSK, Merck, Novartis, Sanofi, and Stallergenes; grants from Merck and Thermo Fisher diagnostics. P Campo reports speaker fees from Bial and Novartis; advisory boards for Astra-Zeneca and GSK. The authors report no other conflicts of interest in this work.
References
1. Global Initiative for Asthma. Global strategy for asthma management and prevention 2019. Available from: https://ginasthma.org.
2. Lotvall J, Akdis CA, Bacharier LB, et al. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol. 2011;127:355–360. doi:10.1016/j.jaci.2010.11.037
3. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. doi:10.1183/09031936.00202013
4. Murphy KR, Meltzer EO, Blaiss MS, Nathan RA, Stoloff SW, Doherty DE. Asthma management and control in the United States: results of the 2009 asthma insight and management survey. Allergy Asthma Proc. 2012;33(1):54–64. doi:10.2500/aap.2011.32.3518
5. Lombardi C, Savi E, Ridolo E, Passalacqua G, Canonica GW. Is allergic sensitization relevant in severe asthma? Which allergens may be culprit? World Allergy Organ J. 2017;10(1):2. doi:10.1186/s40413-016-0138-8
6. Antonicelli L, Bucca C, Neri M, et al. Asthma severity and medical resource utilisation. Eur Resp J. 2004;23(5):723–729. doi:10.1183/09031936.04.00004904
7. Palomares O, Sanchez-Ramon S, Davila I, et al. dIvergEnt: how IgE axis contributes to the continuum of allergic asthma and anti-IgE therapies. Int J Mol Sci. 2017;18:6. doi:10.3390/ijms18061328
8. Tabatabaian F, Ledford DK. Omalizumab for severe asthma: toward personalized treatment based on biomarker profile and clinical history. J Asthma Allergy. 2018;11:53–61. doi:10.2147/JAA.S107982
9. Corren J, Kavati A, Ortiz B, et al. Patient-reported outcomes in moderate-to-severe allergic asthmatics treated with omalizumab: a systematic literature review of randomized controlled trials. Curr Med Res Opin. 2018;34(1):65–80. doi:10.1080/03007995.2017.1395734
10. Xolair (Omalizumab). Summary of product characteristics. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/xolair.
11. Humbert M, Beasley R, Ayres J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60(3):309–316. doi:10.1111/j.1398-9995.2004.00772.x
12. Alhossan A, Lee CS, MacDonald K, Abraham I. “Real-life” effectiveness studies of omalizumab in adult patients with severe allergic asthma: meta-analysis. J Allergy Clin Immunol Pract. 2017;5(5):1362–1370 e2. doi:10.1016/j.jaip.2017.02.002
13. MacDonald KM, Kavati A, Ortiz B, Alhossan A, Lee CS, Abraham I. Short- and long-term real-world effectiveness of omalizumab in severe allergic asthma: systematic review of 42 studies published 2008–2018. Expert Rev Clin Immunol. 2019;15(5):553–569. doi:10.1080/1744666X.2019.1574571
14. Campo Mozo P, Soto-Campos JG, Blanco Aparicio M, et al. Severe asthma phenotypes in patients controlled with omalizumab: a real-world study. Respir Med. 2019;159:105804. doi:10.1016/j.rmed.2019.105804
15. Hanania NA, Wenzel S, Rosen K, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013;187(8):804–811. doi:10.1164/rccm.201208-1414OC
16. Executive Committee GEMA 2009. GEMA 2009 (Spanish guidelines on the management of asthma). J Investig Allergol Clin Immunol. 2010;20(Suppl 1):1–59.
17. Juniper EF, O’Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14(4):902–907. doi:10.1034/j.1399-3003.1999.14d29.x
18. Vega JM, Badia X, Badiola C, et al. Validation of the Spanish version of the Asthma Control Test (ACT). J Asthma. 2007;44(10):867–872. doi:10.1080/02770900701752615
19. Cazzoletti L, Marcon A, Janson C, et al. Asthma control in Europe: a real-world evaluation based on an international population-based study. J Allergy Clin Immunol. 2007;120(6):1360–1367. doi:10.1016/j.jaci.2007.09.019
20. de Marco R, Cazzoletti L, Cerveri I, et al. Are the asthma guideline goals achieved in daily practice? A population-based study on treatment adequacy and the control of asthma. Int Arch Allergy Immunol. 2005;138(3):225–234. doi:10.1159/000088723
21. Mansur AH, Srivastava S, Mitchell V, Sullivan J, Kasujee I. Longterm clinical outcomes of omalizumab therapy in severe allergic asthma: study of efficacy and safety. Respir Med. 2017;124:36–43. doi:10.1016/j.rmed.2017.01.008
22. Matucci A, Vultaggio A, Maggi E, Kasujee I. Is IgE or eosinophils the key player in allergic asthma pathogenesis? Are we asking the right question? Respir Res. 2018;19(1):113. doi:10.1186/s12931-018-0813-0
23. Manga V, Humbert M, Djukanovic R, et al. Blood Eosinophils and serum IgE predict response to omalizumab in patients with severe allergic asthma: innovate trial post-hoc analysis. J Allergy Clin Immunol. 2016;137(2):AB16. doi:10.1016/j.jaci.2015.12.051
24. Bousquet J, Wenzel S, Holgate S, Lumry W, Freeman P, Fox H. Predicting response to omalizumab, an anti-IgE antibody, in patients with allergic asthma. Chest. 2004;125(4):1378–1386. doi:10.1378/chest.125.4.1378
25. Humbert M, Taillé C, Mala L, Le Gros V, Just J, Molimard M. Omalizumab effectiveness in patients with severe allergic asthma according to blood eosinophil count: the STELLAIR study. Eur Resp J. 2018;51(5):1702523. doi:10.1183/13993003.02523-2017
26. Casale TB, Luskin AT, Busse W, et al. Omalizumab effectiveness by biomarker status in patients with asthma: evidence from PROSPERO, a prospective real-world study. J Allergy Clin Immunol Pract. 2019;7(1):156–164 e1. doi:10.1016/j.jaip.2018.04.043
27. Papathanasiou E, Bakakos P, Hillas G, et al. Diagnostic accuracy of T2 biomarkers for the prediction of airway eosinophilia in treated smoking asthmatic patients with loss of asthma control. J Allergy Clin Immunol Pract. 2020;8(1):385–377.
28. Buhl R, Humbert M, Bjermer L, et al. Severe eosinophilic asthma: a roadmap to consensus. Eur Respir J. 2017;49(5):1700634. doi:10.1183/13993003.00634-2017
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
