Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
Feasibility of an Activity Control System in Patients with Diabetes: A Study Protocol of a Randomised Controlled Trial
Authors Montagut-Martínez P , García-Arenas JJ, Romero-López M, Rodríguez-Rodríguez N, Pérez-Cruzado D , González-Lama J
Received 5 April 2022
Accepted for publication 9 July 2022
Published 2 September 2022 Volume 2022:15 Pages 2683—2691
DOI https://doi.org/10.2147/DMSO.S369464
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ming-Hui Zou
Pedro Montagut-Martínez,1 Jose Joaquin García-Arenas,2 Matilde Romero-López,3 Nicomedes Rodríguez-Rodríguez,3 David Pérez-Cruzado,2,4 Jesús González-Lama3,5
1Health Sciences PhD Program, Universidad Católica de Murcia UCAM, Murcia, 30107, Spain; 2Department of Occupational Therapy, Universidad Catolica de Murcia UCAM, Murcia, 30107, Spain; 3Cabra Clinical Management Unit, Sur de Córdoba Health Management Area, Cordoba, Spain; 4Institute of Biomedicine of Malaga (IBIMA), Málaga, Spain; 5Maimonides Biomedical Research Institute of Cordoba (IMIBIC), Reina Sofia University Hospital, University of Cordoba, Cordoba, Spain
Correspondence: David Pérez-Cruzado, Department of Occupational Therapy, Universidad Catolica de Murcia UCAM, Campus de los Jerónimos n° 135, Guadalupe, Murcia, 30107, Spain, Tel +34 653141109, Email [email protected]
Purpose: Patients with diabetes mellitus have an increased risk of developing various serious health problems that could be life-threatening. These problems are associated with the difficulty of these patients in managing their lifestyle, which may even lead to the abandonment of treatment. The present study was designed to evaluate the feasibility of a multipurpose activity control solution for home activity (home activity control system), which will provide information on the activities of daily living carried out outside in real time, to improve adherence to each of the therapeutic objectives agreed on with the diabetic patient.
Patients and Methods: A pilot randomised controlled feasibility study will be carried out to evaluate a home activity control system (Beprevent) in managing patients with type 2 diabetes mellitus. Twenty patients with type 2 diabetes mellitus will be included (10 in the intervention group and 10 in the control group). Data on satisfaction with the tool will be collected from professionals and patients, as well as other clinical/epidemiological data from their digital health records and several questionnaires, at baseline and six months. In addition, data will also be recorded regarding the degree of adherence to the behaviors agreed on with the patients before starting the study to assess changes throughout the study and their relationship with clinical results (glycosylated haemoglobin (HbA1c), cholesterol, etc), and to compare these outcomes between two study groups.
Discussion: This project involves the incorporation of telemedicine in the management of patients with diabetes. Thus, according to the currently published bibliography, the use of smart devices in this population could help improve the quality of life of these people, reduce medical visits and improve adherence to home care patterns for diabetes mellitus. There are currently no published clinical trials or protocols that monitor activities of daily living in patients with diabetes individually using artificial intelligence (AI) devices.
Keywords: artificial intelligence, diabetes mellitus, self-care, telemedicine
Introduction
Patients with diabetes are at increased risk of developing a variety of severe health problems, leading to high healthcare costs, decreased quality of life, and increased mortality.1 Optimizing the management of the patient with diabetes is a public health priority.2 According to the diabetes atlas of the International Diabetes Federation (IDF), it is estimated that in 2017 there were 451 million people (between 18 and 99 years old) with diabetes worldwide, and it is expected that by 2045 that number will reach 693 million.3 One of the essential aspects in the fight against this disease is to optimize lifestyles, including a healthy diet, regular physical activity, not smoking, and maintaining an adequate body weight.4 Another essential aspect in the fight against this pathology is the involvement of the patient in the management of his or her disease, focusing the recommendations on the patient and agreeing with him or her on both the therapeutic objectives and the best way to achieve them. Thus, it seems that interventions in patients with diabetes that are individualized according to the needs of each of them may be associated with long-term beneficial clinical results.5
In the last decade, a diabetes management paradigm has been transformed with the progressive incorporation of new technologies. New technology is gaining great interest because the amount of data that can be obtained from patients with this pathology has grown exponentially. There is ample evidence of the use of various methods of new technologies in this field, such as the analysis of general questionnaires6,7 or in particular domains, such as early diagnosis,8 as detailed in a recent review on the application of new technologies in the management and support in decision-making of patients with diabetes.9 Decision support systems (DSSs) consist of tools focused on helping patients and/or healthcare professionals manage different therapies for diabetes. These systems usually have monitoring tools that facilitate the systematic recording of information on diet, physical activity, medications, glucose measurements, and other factors, and combine them with others to support both patients and healthcare providers, with the general objective of improving therapeutic results. Thus, the consulted bibliography proposes that the treatment of diabetes for the modification of the lifestyles of this population must be carried out individually, adapting to the individual characteristics of each patient.10,11 Similar studies have been carried out, in which individualized programs of patient empowerment and adherence to therapy have been designed through the automatic generation of feedback messages, without obtaining a record of whether the patient carried out these tasks on the same day.9,12,13
Materials and Methods
Both investigators and external reviewers followed the SPIRIT reporting guidelines as the basis for trial registration and study evaluation.14
The main objective of this study is to evaluate the feasibility of a home activity control system focused on therapeutic adherence in treating people with type 2 diabetes mellitus.
Beprevent is a multipurpose activity control solution at home (home activity control system) that reports on people’s routines through a mobile application; Its target population is made up mostly of elderly, dependent people, people with chronic or mental illnesses or some type of physical and/or mental disability who live alone. It consists of “providing intelligence” to objects that are used daily in the interior of a house, offering a map of the routines and habits of the user. It consists of a central device with an electrical power cable and adhesive labels, and it is easy to place on everyday objects, which, through an app, informs us of the routines under study. Beprevent allows each subject to choose which five objects will be labeled to assess the agreed behavioral routines. Likewise, the information provided by these labels (through the use of labeled objects) allows the programming of reminders based on anomalies in these routines, which will be displayed on the patient’s mobile phone, obtaining information on the patient’s performance or the omission of previously established activities and routines15 (Figure 1).
 |
Figure 1 Beprevent Device. |
A multi-center randomized clinical trial will be carried out to evaluate the feasibility of a home activity control system (Beprevent)15 in the management of patients with type 2 diabetes mellitus, through individualized labeling of objects. Given the characteristics of the intervention, clinicians caring for patients will not be blinded to the intervention. However, both patients and statisticians will be blinded to it.
A priori sample size calculation for the randomized clinical trial indicated that 10 participants per group were required to detect a significant differences in between the intervention and control group (Effect size d = 1.12, alpha = 0.05, beta) based on another study.16
After qualification for study, patients will be stratified by centre. They will then be randomised 1:1 to the intervention or control group (Figure 2).
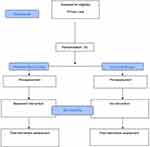 |
Figure 2 Flow chart of the study. |
Participants
Twenty people will be recruited from the health center of Cabra (Córdoba, Spain), aged ≥ 18 years old, with the ability to give informed consent, autonomy for activities of daily living (Barthel = 100), diagnosis of type 2 diabetes mellitus, HbA1C ≥ 9.0%, pharmacological treatment, and no plan to change their address during the time the device is installed in their home through a simple random sampling.
Patient confidentiality will be preserved and will not be made public, in accordance with applicable laws and regulations. Specifically, all actions will comply with Regulation (EU) 2016/679 of the European Parliament and of the Council, of 27 April 2016, regarding the protection of natural persons with regard to the processing of personal and free data.
Informed consent will be obtained from each study participant, and all procedures will be based on the Helsinki declaration. The Research Committee of the Faculty of Health Sciences of the Catholic University of Murcia and the Research Ethics Committee of Cordoba approved this protocol (CE042002/4458).
Procedure
The sample will be randomized and divided into two groups. Of the 20 patients with type 2 diabetes mellitus included in the study, 10 will become part of the intervention group and the other 10 will be included in the control group. The intervention will take place within six months. The assignment of the participants to each of the groups will be carried out within the health center to which each of them is assigned, using a sealed envelope system as a method. All study participants will be evaluated with the outcome variables of the present study before and after the end of the intervention. Patients in the intervention group will have a Beprevent device installed at home, in order to monitor the performance of activities of daily living related to adherence to diabetes mellitus treatment; patients in the control group will also receive a Beprevent device, but it will not be operational.
Intervention
First, a therapeutic agreement will be elaborated in writing, containing a set of activities (and their frequency) that could be of help for improving disease pathology. The document will also specify the periodicity of follow-up visits to the health care center to control disease (at 15, 45, 105 and 180 days) and the expected therapeutic clinical objectives to be reached at the end of the study (6 months). Each participant will be initially evaluated with the scales and questionnaires described below, after explaining the use of the Beprevent system. The assessment will be done in two days, first day will be evaluated five questionnaires (Diabetes Knowledge Questionnaire (DKQ-24), Charlson index, Morisky–Green test, Spanish version of the Summary of Diabetes Self-Care Activities measure (SDSCA-Sp) and International Physical Activity Questionnaire (IPAQ)). The second assessment (Social functioning scale) will be done when the Beprevent device is installed. As part of the installation, five labels with different colours will be attached to selected objects related to behaviors (physical activity, rest, self-care, nutrition and medication). Every time the patient interacts with these objects, the application will register it. The selected objects will be chosen by each patient (We will provide these objects if necessary or if the participants prefer it (sneakers, pill box, toiletry bag …), after discussing with them the purpose. Later, the app will be installed on patient and healthcare worker mobile phones, which will give them information about which objects have been manipulated, when and for how long the activity related to them have been performed. The system will be installed at the participant’s home for six months. Participants will be asked to carry out their life with a normal routine. During this period, the participants will be able to contact the study researchers to solve any issue related to Beprevent use.
At each follow-up visit, the health professionals will review with the patient the adjustment of the agreed behaviors relative to those actually done, based on the data reported by the patient (usual practice). In the patients allocated to the intervention group, these self-reported behaviors will be also matched with those reported by the Beprevent application. The healthcare workers will decide with the patient (based on a patient-centred approach) the necessary adjustments to try to keep the disease controlled. Also, any issues related to the use of the Beprevent system will be discussed. At the end of the follow-up period (6 months), all questionnaires will be repeated, as well as all the investigations carried out at the beginning of the study.
Study Variables
Demographic variables will be recorded, such as age, sex, marital status, educational level, employment situation, and monthly income level; anthropometric variables, such as weight, height, and waist circumference; biochemical parameters, such as HbA1c, total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides; oral antidiabetics, insulin, and drugs for other pathologies; number of visits to primary care and emergency services, number of hospital admissions, tobacco use, consumption of alcohol, and total time dedicated by nursing and medical personnel to patient control. Finally, a series of internationally validated scales and questionnaires will be used to collect information within the different functional areas, as shown in Table 1.
 |
Table 1 Outcome Variables |
Diabetes Knowledge Questionnaire:17 The Diabetes Knowledge Questionnaire-24 (DKQ-24) is the Spanish version of the DKQ-24. This questionnaire is made up of 24 questions about basic knowledge of the disease (10 items), glycaemic control (7 items), and prevention of complications (7 items). The questions are closed, with answer options ‘yes’, “no”, and “I don’t know”. It has a Cronbach alpha reliability index of 0.78, and the content validity was established by a panel of nurses and researchers who are experts in the management of Mexican-American diabetic patients. Construct validity was demonstrated by observing the instrument’s good sensitivity to the intervention.
Charlson Comorbidity Index:18 The Charlson Comorbidity Index is a system of evaluation of life expectancy at 10 years, depending on the age at which it is evaluated, and the comorbidities of the subject. In addition to age, it consists of 19 items, which, if present, have been found to have a specific influence on the life expectancy of the subject. Initially adapted to assess one-year survival, it was eventually adapted in its final form for 10-year survival. It has been used for many other purposes, including the calculation of costs due to suffering from a chronic disease in primary care patients. In general, the absence of comorbidity is considered as 0–1 points and low comorbidity as 2 points and high> 3 points.
Morisky–Green test:19 One of the best known and most widely used questionnaires in both clinical practice and research is the Morisky–Green questionnaire. The first version consists of four dichotomous “yes or no” questions to assess the barriers to correct therapeutic adherence. In the literature, this test is also called the Medication Adherence Questionnaire (MAQ) or the four-item Morisky Medication Adherence Scale (MMAS-4). It has been validated in a wide variety of chronic pathologies and populations, such as hypertension, diabetes, dyslipidaemia, Parkinson’s disease, and cardiovascular disease, as well as in older patients with chronic pathologies. The Spanish version was validated by Jiménez et al in a cohort of hypertensive patients.20 Morisky (1986), using a hypertensive population, reviewed the psychometric properties and tested the concurrent and predictive validity of a self-reported adherence measure structured with four items (alpha reliability = 0.61), which can be easily integrated into the medical visit.21
Summary of Diabetes Self-Care Activities measure (SDSCA-Sp) (Spanish version):22 This 12-item questionnaire addresses different areas of self-care present in people with type 2 diabetes mellitus, such as diet, physical activity, medication, self-analysis of blood glucose, and smoking. It has a response scale from 0 to 7, depending on the number of days that the person has carried out a certain behaviour in the last week. The lower the score, the less adherence the person with diabetes mellitus has to favourable self-care behaviours. The questionnaire does not have a cut-off point, so each item must be assessed individually. It has a moderate–low internal consistency (α-Cronbach = 0.62), and test–retest reliability was evaluated in 198 patients (t = 0.462–0.796, p <0.001), with an overall correlation of 0.764 (p <0.0001).
International Physical Activity Questionnaire (IPAQ):23 This questionnaire consists of seven questions about the frequency, duration, and intensity of the activity (moderate and intense) carried out in the last seven days, as well as walking and sitting time on a working day. It can be applied by direct interview, phone, or self-completed survey, and it was designed to be used in adults between the ages of 18 and 65, with two versions. We will use the short version of the IPAQ that consists of seven items and is most useful when population monitoring is sought in research.24 Regarding psychometric properties, for the long IPAQ, a reliability of around 0.8 (r = 0.81; 95% CI: 0.79–0.82) has been demonstrated, and for the short version, 0.65 (r = 0.76; 95% CI: 0.73–0.77). The validity coefficients observed between the IPAQ forms suggest that both the long and short versions have reasonable agreement (r = 0.67, 95% CI 0.64–0.70).25
System Usability Scale: This is one of the first scales to evaluate the usability of an interface and where it was not necessary for the participant to carry out laboratory tests. It was developed by Brooke (1986) and consists of 10 items (5 positive items and 5 negative items). This scale has two main objectives: the first is that researchers can obtain a measurement of the perception of the usability of a system, and the second is that the scale does not require much time for its application.26 The only measurements that Brooke obtained in relation to the scale showed high levels of correlation among the 10 items that make it up; these ranged from _ + 0.7 to _ + 0.9, a correlation that allowed her to select the items that would make up the final scale, but here neither the reliability nor the validity of the scale was reported.27 One of the first psychometric data reported in an independent study in the HFRG Lucey laboratories (1991) was reliability measurements, with the participation of 77 people; their results showed an alpha coefficient of 0.85.28,29
Social Functioning Scale (SFS):30 The SFS was built specifically to cover those areas of operation that are crucial for the maintenance in the community of people with schizophrenia. These seven areas explored are isolation/social involvement, interpersonal behaviour, prosocial activities, leisure, independence/competition, independence/performance, and employment/occupation. In relation to how to establish the score, the factor analysis of the validation study by Torres et al suggests that it might be appropriate to use an average score from each subscale to obtain a total score.31 A summary of the study tools and variables is presented in Table 1.
Statistical Analyses
Within the descriptive analysis, the quantitative variables will be expressed by calculating their mean (m) and their standard deviation (SD), or median (me) and interquartile range (RIC), with minimum (Min) and maximum (Max) values, in a differentiated way by study groups (control, intervention). Comparisons of the quantitative variables between the two groups will be made using non-parametric tests: Mann–Whitney or Wilcoxon U-tests). Qualitative variables will be expressed through frequency measurements. Intention to treat analyses will be applied if any patient will drop out the study.
The chi-square test for contingency tables will be used to compare proportions of categorical variables, with Yates correction, and if the expected frequency is ≤5, Fisher’s exact test. The association strength estimation will be carried out by calculating the absolute and relative risks and an exploratory differential analysis to describe the intra- and intergroup differences after performing the intervention with the Beprevent tool. All the data will be analysed with the statistical package SPSS 22.0 and R software (version 3.5.0).
Discussion
A clinical trial will be carried out in patients with type 2 diabetes mellitus, using an intelligent system (Beprevent), in order to improve adherence to activities of daily living, physical activity, control of medication, knowledge on diabetes, and biochemical parameters.
This project involves the incorporation of telemedicine in the management of patients with diabetes. Thus, according to the currently published bibliography, the use of smart devices in this population could help to improve the quality of life of these people, decrease medical visits, and improve adherence to patterns of diabetes mellitus care at home.32 There are currently no clinical trials or published protocols that monitor the activities of daily living in patients with diabetes on an individual basis using AI devices; therefore, in our opinion, this would be the first study for this purpose.
Any future efforts to encourage the use of diabetes self-monitoring apps will serve to increase awareness of the existence and potential benefits of using the app, taking into account a large number of medical complications and deaths that are caused by this disease.33,34 In addition to objectively evaluating the benefits, future research needs to identify what drives the use of the application and how to support patients with this pathology who want to adopt this new technology to optimise their diabetes self-management.
Although the high cost of the Beprevent devices 250 euros (device, sensors and installation) and its maintenance and monitoring fee is 6 euros per month. This tool will help the health professional to know the lifestyle and self-care guidelines that the patient maintains at home. It will make it easier for the health professional to design a therapeutic plan, together with the patient, on the type and frequency of activities and care to be carried out by the patient to facilitate the monitoring of said activities by the reference health professionals and the consequences on the patient’s health status, through: their routines (routines as indicators of health status, due to their predictive value in physical, cognitive or psychological capacity), home (orientation towards home care and the care environment the person), family (support for primary caregivers and the family environment to facilitate patient care) and personalization (information service that facilitates interventions based on the characteristics and habits of the patient).
Regarding the limitations of the study, the main risk that is expected is related to the acceptability of the tool by the patient and/or the health personnel that follow it. The patients or their relatives may feel intimidated by the installation in their home of an AI tool to monitor different activities, although it is expected that the degree of acceptance will be high, since it is a non-invasive tool (it does not record images or sounds) and the patient will be the one to decide which objects the activity tracking labels are placed on and who will have access to the application.
Strength and Limitations of This Study
Among the strengths of the study, this type of home activity control system could improve the therapeutic alliance in the management of Diabetes Mellitus, as well as the self-care management of patients with this pathology. With Beprevent devices is it possible to able to control and collect data on adherence to treatment (whether or not he takes the prescribed medication, the day he takes it, time, and times he takes it per day), we will have information on the number of times the patient eats daily, if performs physical activity or not (at what time and for how long), if they carry out daily hygiene (important in diabetics, especially in lower limbs, due to the appearance of ulcers), if they respect their nightly rest (at what time do they go to bed and how long you sleep), etc. With all this information we will know if the patient is correctly following the indications in relation to their therapeutic regimen and we will be able to help them reduce and maintain adequate blood glucose levels, a normal weight and healthy habits (adequate nutrition, regular physical activity, disease control, etc).
Regarding the limitations, the most important thing in our understanding would be the acceptability of the tool by the patient and/or the healthcare personnel who follow it, since patients and/or their families could feel intimidated by the installation at home of an AI tool to monitor different activities. Other limitations could be the handling or use of the device in a way that false positives or false negatives are recorded, since the device could be manually manipulated by the user. Another problem during the use of this device would be in the stickers (sensors) responsible for capturing the performance or not of the activities/occupations. If these stickers (sensors) move (detach from the place or object of choice) they stop capturing data and therefore evaluating the patient. The way to correct it is to make an appointment with the patient as soon as possible to go to the home and restore the functionality of the sticker (sensor).
What does exist is a list prior to the protocol where a study was carried out of the sites and activities where these stickers (sensors) should be placed to capture the activity and collect the performance or omission of certain activities in the best possible way.
For example, to find out whether or not a subject takes their usual daily medication (adherence to treatment), a sticker (sensor) is placed in a toiletry bag (previously provided by the researchers to the patient under study), where the patient is going to keep your regular/daily medication. Every time the patient opens this bag to take their usual/daily medication, the sticker will send the information to Beprevent and it is recorded (day/time). In this way, during the duration of the study, we will be able to know if the subject adequately complies with his therapeutic regimen (if he takes his usual medication, at what time, if he takes the same medication more than once in an erroneous way …).
Abbreviations
AI, Artificial Intelligence; DKQ-24, Diabetes Knowledge Questionnaire-24; DSSs, Decision Support Systems; HbA1c, Glycosylated haemoglobin; HFRG, Human Factors Research Group; IPAQ, International Physical Activity Questionnaire; m, mean; MAQ, Medication Adherence Questionnaire; Max, Maximum; me, median; Min, Minimum; MMAS-4, four-item Morisky Medication Adherence Scale; RIC, Interquartile range; SD, Standard Deviation; SDSCA-Sp, Summary of Diabetes Self-Care Activities measure; SFS, Social Functioning Scale.
Ethics Approval
The Research Committee of the Faculty of Health Sciences of the Catholic University of Murcia approved this protocol (CE042002).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study has received a research grant from the Fundación Progreso y Salud (Andalucía, Spain).
Disclosure
The authors report no conflicts of interest in relation to this work.
References
1. Baena-Díez JM, Peñafiel J, Subirana I, et al. Risk of cause-specific death in individuals with diabetes: a competing risks analysis. Diabetes Care. 2016;39(11):1987–1995. doi:10.2337/dc16-0614
2. Hills AP, Misra A, Gill JMR, et al. Public health and health systems: implications for the prevention and management of type 2 diabetes in south Asia. Lancet Diabetes Endocrinol. 2018;6(12):992–1002. doi:10.1016/S2213-8587(18)30203-1
3. Cho NH, Shaw JE, Karuranga S, et al. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–281. doi:10.1016/j.diabres.2018.02.023
4. Lazarou C, Panagiotakos D, Matalas AL. The role of diet in prevention and management of type 2 diabetes: implications for public health. Crit Rev Food Sci Nutr. 2012;52(5):382–389. doi:10.1080/10408398.2010.500258
5. Dambha-Miller H, Feldman AL, Kinmonth AL, Griffin SJ. Association between primary care practitioner empathy and risk of cardiovascular events and all-cause mortality among patients with type 2 diabetes: a population-based prospective cohort study. Ann Fam Med. 2019;17(4):311–318. doi:10.1370/afm.2421
6. Mueller C, Schauerte I, Martin S, Irrgang V. Evaluation of self-care activities and quality of life in patients with type 2 diabetes treated with metformin using the 2D matrix code of outer drug packages as patient identifier: the depro proof-of-concept observational study. JMIR Diabetes. 2022;7(2):e31832. doi:10.2196/31832
7. Jamal A, Tharkar S, Babaier WS, et al. Blood glucose monitoring and sharing amongst people with diabetes and their facilitators: cross-sectional study of methods and practices. JMIR Diabetes. 2021;6(4):e29178. doi:10.2196/29178
8. Shankaracharya Odedra D, Samanta S, Vidyarthi AS, Vidyarthi AS. Computational intelligence in early diabetes diagnosis: a review. Rev Diabet Stud. 2010;7(4):252–262. doi:10.1900/RDS.2010.7.252
9. Negreiros FD, Da S, Araújo AL, et al. Digital technologies in the care of people with diabetes during the COVID-19 pandemic: a scoping review. Rev Esc Enferm USP. 2021;55:e20210295. doi:10.1590/1980-220x-reeusp-2021-0295
10. Moody L, Wood E, Needham A, Booth A, Jimenez-Aranda A, Tindale W. Identifying individual enablers and barriers to the use of digital technology for the self-management of long-term conditions by older adults. J Med Eng Technol. 2022;1–14. doi:10.1080/03091902.2022.2089249
11. Correia JC, Waqas A, Aujoulat I, et al. Evolution of therapeutic patient education: a systematic scoping review and scientometric analysis. Int J Environ Res Public Health. 2022;19(10):6128. doi:10.3390/ijerph19106128
12. May SG, Huber C, Roach M, et al. Adoption of digital health technologies in the practice of behavioral health: qualitative case study of glucose monitoring technology. J Med Internet Res. 2021;23(2):e18119. doi:10.2196/18119
13. Garg S, Norman GJ. Impact of COVID-19 on health economics and technology of diabetes care: use cases of real-time continuous glucose monitoring to transform health care during a global pandemic. Diabetes Technol Ther. 2021;23(S1):S15–20. doi:10.1089/dia.2020.0656
14. Chan AW, Tetzlaff JM, Gøtzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586–e7586. doi:10.1136/bmj.e7586
15. Beprevent. Beprevent - Conéctate con tu ser querido; 2020. Available from: https://beprevent.es/.
16. Clark TL, Gallo L, Euyoque JA, Philis-Tsimikas A, Fortmann A. Does diabetes distress influence clinical response to an mhealth diabetes self-management education and support intervention? Diabetes Educ. 2020;0145721720913276. doi:10.1177/0145721720913276
17. Garcia AA, Villagomez ET, Brown SA, Kouzekanani K, Hanis CL. The Starr County diabetes education study: development of the Spanish-language diabetes knowledge questionnaire. Diabetes Care. 2001;24(1):16–21. doi:10.2337/diacare.24.1.16
18. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi:10.1016/0021-9681(87)90171-8
19. Nguyen TMU, La Caze A, Cottrell N. What are validated self-report adherence scales really measuring?: a systematic review. Br J Clin Pharmacol. 2014;77(3):427–445. doi:10.1111/bcp.12194
20. Val Jiménez A, Amorós Ballestero G, Martínez Visa P, Fernández Ferré ML, León Sanromà M. Descriptive study of patient compliance in pharmacologic antihypertensive treatment and validation of the Morisky and Green test. Aten Primaria. 1992;10(5):767–770. Spanish.
21. Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. doi:10.1097/00005650-198601000-00007
22. Caro-Bautista J, Morilla-Herrera JC, Villa-Estrada F, Cuevas-Fernández-Gallego M, Lupiáñez-Pérez I, Morales-Asencio JM. Spanish cultural adaptation and psychometric validation of the Summary of Diabetes Self-Care Activities measure (SDSCA) among persons with type 2 diabetes mellitus. Aten Primaria. 2016;48(7):458–467. Spanish. doi:10.1016/j.aprim.2015.08.005
23. Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–1395. doi:10.1249/01.MSS.0000078924.61453.FB
24. Giné-Garriga M, Martin C, Martín C, et al. Referral from primary care to a physical activity programme: establishing long-term adherence? A randomized controlled trial. Rationale and study design. BMC Public Health. 2009;9(1):31. doi:10.1186/1471-2458-9-31
25. Mantilla Toloza SC, Gómez-Conesa A. El Cuestionario Internacional de Actividad Física. Un instrumento adecuado en el seguimiento de la actividad física poblacional. Revista Iberoamericana de Fisioterapia y Kinesiología. 2007;10(1):48–52. doi:10.1016/S1138-6045(07)73665-1
26. Brooke J. SUS: a retrospective. J Usability Stud. 2013;8(2):29–40.
27. Brooke J. SUS - A quick and dirty usability scale. Usability Eval Ind. 1996;189(194):4–7.
28. Lucey NM. More Than Meets the I: User-Satisfaction Of Computer Systems [Unpublished thesis for Diploma in Applied Psychology]. Cork, Ireland: University College Cork; 1991.
29. Kirakowski J. The use of questionnaire methods for usability assessment. Unpublished manuscript Recuperado el; 1994:12.
30. Fernandez OV, Giráldez SL, Sáiz AG, García AO, Sánchez MA, Guttierrez Perez AM. Integrated psychological treatment for schizophrenic patients. Psicothema-Oviedo. 1998;10:459–474.
31. Torres A, Olivares JM. Validation of the Spanish version of the social functioning scale. Actas Esp Psiquiatr. 2005;33(4):216–220.
32. Holt RI. The role of telehealth and diabetes. Diabet Med. 2019;36(5):529–530. doi:10.1111/dme.13958
33. Seng JJB, Kwan YH, Lee VSY, et al. Differential health care use, diabetes-related complications, and mortality among five unique classes of patients with type 2 diabetes in Singapore: a latent class analysis of 71,125 patients. Diabetes Care. 2020;43(5):1048–1056. doi:10.2337/dc19-2519
34. Ahlqvist E, Storm P, Käräjämäki A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018;6(5):361–369. doi:10.1016/S2213-8587(18)30051-2
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
