Back to Journals » Drug Design, Development and Therapy » Volume 17
Feasibility and Safety of Remazolam versus Propofol When Inserting Laryngeal Masks Without Muscle Relaxants During Hysteroscopy
Authors Tang S, Lu J, Xu C, Wei L, Mei S, Chen R, Meng QT
Received 14 February 2023
Accepted for publication 16 April 2023
Published 1 May 2023 Volume 2023:17 Pages 1313—1322
DOI https://doi.org/10.2147/DDDT.S408584
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Shan Tang,1 Jingxiao Lu,1 Cheng Xu,2 Lu Wei,1 Shenglan Mei,1 Rong Chen,1,2 Qing-Tao Meng1,2
1Department of Anesthesiology, East Hospital, Renmin Hospital of Wuhan University, Wuhan, Hubei, People’s Republic of China; 2Department of Anesthesiology, Renmin Hospital of Wuhan University, Wuhan, Hubei, People’s Republic of China
Correspondence: Qing-Tao Meng, Tel +8615178857650, Email [email protected]
Purpose: This study aimed to evaluate the efficacy and safety of remazolam compared with propofol in patients who underwent laryngeal mask airway (LMA) insertion without the use of muscle relaxant agents during hysteroscopic surgery.
Patients and Methods: A total of 72 patients undergoing hysteroscopy with LMA insertion were assigned to two groups. The patients in the remazolam group received 0.3 μg/kg sufentanil, 0.3 mg/kg remazolam and 1.2 mg/kg remifentanil, whereas the patients in the propofol group received 0.3 μg/kg sufentanil, 2.0 mg/kg propofol and 1.2 mg/kg remifentanil for insertion of the LMA. The primary endpoint was the summed score of the insertion conditions. The secondary endpoints included hemodynamics, the duration of induction, the duration of insertion, tidal volume, plateau pressure and adverse events.
Results: No difference was identified between the propofol group and remazolam group in the median summed score [18.0 (18.0, 18.0), 18.0 (17.0, 18.0), respectively, P > 0.05]. The induction duration was significantly longer (P < 0.05) in the remazolam group than propofol group. The cost of dopamine (P < 0.05) was significantly lower in the remazolam group compared with the patients in the propofol group, while the plateau pressure (P < 0.05) and the incidence of transient mild laryngospasm (P < 0.05) were significantly higher in the remazolam group. No differences were identified between the two groups in terms of heart rate, tidal volume, injection pain or hiccups (P > 0.05).
Conclusion: Remazolam provided similar insertion conditions and better hemodynamic stability than propofol during LMA insertion without the use of muscle relaxant agents. However, a higher incidence of transient mild laryngospasm was found in the remazolam group, which should be considered.
Keywords: remazolam, propofol, laryngeal mask airway, muscle relaxants
Introduction
Hysteroscopy is one of the most common minimally invasive techniques to detect and treat intrauterine diseases.1 Due to the severe discomfort, the majority of patients require anesthesia.2 However, sedation for endoscopy may cause severe morbidity,3 especially respiratory depression.4 Therefore, airway management is important.
The laryngeal mask airway (LMA) is an effective ventilatory tool. Laryngeal mask airway insertion is more reliable than mask oxygen delivery and less damaging than tracheal intubation in hysteroscopy.5 Without the use of neuromuscular blocking agents, the patient can wake up without their effects. However, the insertion of LMA requires a sufficient depth of anesthesia to suppress the upper airway reflexes when induced without muscle relaxant.6
Propofol, which has excellent sedative properties, is commonly used as an induction agent. Studies have shown that propofol can provide suitable conditions for LMA insertion compared to other intravenous induction agents.7 However, when propofol is used alone, undesirable events such as gagging, coughing, and head or limb movements occur frequently.7 In addition, the success rate of insertion decreases when using propofol alone. Therefore, opioids have been used together with propofol to improve the insertion conditions and success rate of inserting an LMA.8 Nevertheless, propofol has some unfavorable adverse effects, including pain noted during intravenous injection and dose-related cardiovascular depression. These side effects have led to the evaluation of new sedatives.
Remazolam, an ultrashort-acting benzodiazepine hypnotic, appears to be an effective and safe sedative for induction. Remazolam has a short half-life, which results in quick onset and recovery. Most importantly, the cardiovascular system is least negatively affected by remazolam, which is associated with less injection pain.9–11 However, there is a scarcity of data for the efficacy and safety of remazolam for LMA insertion without the use of muscle relaxants. Given these advantages of remazolam, we decided to perform this trial by evaluating patients undergoing hysteroscopy surgery that required LMA insertion in our hospital.
Materials and Methods
Trial Design
The study protocol was approved by the Ethics Committee of Renmin Hospital of Wuhan University (WDRY2022-K111) and was registered in the Chinese Clinical Trial Registry (ChiCTR2100052136). This single-center, prospective, randomized study was conducted from July to December 2022. Signed informed consent was obtained from all participants before enrollment. This study complied with the Declaration of Helsinki.
Patients
In the East Hospital, Renmin Hospital of Wuhan University, Wuhan, China, 80 patients who were scheduled for elective hysteroscopic surgery under general anesthesia were recruited into this study. The inclusion criteria of the patients were age between 18 and 60 years, American Society of Anesthesiologists (ASA) score of I or II and body mass index (BMI) between 19 and 30 kg/m2. Patients were excluded if they abused alcohol or drugs; had hypertension; had a known sensitivity to general anesthetic drugs; or had clinically significant cardiovascular, respiratory, or liver disease.
Randomization and Intervention
The eligible participants were randomized into the remazolam group or the propofol group by a computer-generated coding system. The group assignment was placed in an opaque envelope by a masked investigator. This is a single-blind trial. Because the appearance and volume of the drug made double-blind difficult. The grouping allocation was unblinded to the anesthesiologists who induce the general anesthesia and insert the laryngeal mask. The one who inserted the LMA collected the data. The data analysis was completed by another anesthesiologist who had no knowledge of the grouping. All surgeons and patients were blinded to group allocation.
In the operation room, vital signs (noninvasive blood pressure, heart rate and pulse oxygen saturation) were monitored. Remazolam (remazolam Tosilate for Injection, 36 mg, SFDA No. 20190034, Jiangsu Hengrui Medicine Co., Ltd., LianYungang, JiangSu) was diluted with 36 mL of normal saline, and propofol (propofol injectable emulsion, 0.2 g: 20 mL, SFDA No. 19990282, Xi’an Libang Pharmaceutical Co., Ltd., XiAn, ShanXi, CHN) was drawn into a 20 mL syringe. Sufentanil was diluted to 0.25 μg/mL, and remifentanil was diluted to 10 μg/mL.
All patients received oxygen delivered via a mask at a rate of 6 L/min. The patients in the remazolam group received half of the 0.3 μg/kg sufentanil, half of the 0.3 mg/kg remazolam, and half of the 0.15 mg/kg remifentanil in sequence, and then the remaining halves of the drugs were administered in that order for induction. The induction time was finished within 85–95 s, and the drugs were given via the dorsum hand vein. The propofol group received a similar induction sequence, but 2.0 mg/kg of propofol was administered instead of 0.3 mg/kg of remazolam in these patients.
In all the patients, 3-size laryngeal masks were inserted by the same attending anesthesiologist. The LMA insertion conditions were evaluated according to mouth opening (3 = full, 2 = partial, 1 = none), swallowing (3 = nil, 2 = mild, 1 = severe), coughing (3 = nil, 2 = mild, 1 = severe), head or body motion (3 = nil, 2 = mild, 1 = severe), laryngospasm (3 = none, 2 = mild, 1 = severe), and ease of LMA insertion (3 = easy, 2 = difficult, 1 = impossible).12 The scores were summed. The insertion condition was considered poor if the total sum was 16 or less, satisfactory if 17, and excellent if 18. However, only a total score of 17 or 18 is considered clinically feasible for LMA insertion.
Also, 0.5 mg/kg of propofol or 0.1 mg/kg of cisatracurium was then added before the next attempt if any severe involuntary movements or severe laryngospasm appeared during LMA insertion. These patients were excluded.
Two minutes after successful LMA insertion, anesthesia was maintained by sevoflurane 1–1.5% and remifentanil 0.08–0.15 μg/(kg.min). Mechanical ventilation and volume control mode (7 mL/kg) were used after LMA insertion. The end-tidal carbon dioxide concentration was maintained at 35–45 mmHg by adjusting the ventilatory frequency.
Noninvasive blood pressure (systolic blood pressure (SBP), diastolic blood pressure (DBP)) and heart rate (HR) were recorded after the patient had entered the operating room (T1), before induction (T2), before LMA insertion (T3), immediately after LMA insertion (T4), and the first (T5), second (T6) and third minute (T7) after LMA insertion.
Outcomes
Primary Outcome
The primary outcome of this study was the summed scores of the LMA insertion conditions.
Secondary Outcome
The patients’ vital sign data fluctuations, including SBP, DBP and HR, were recorded. Complications such as injection pain, muscle rigidity or hiccups were noted. Bradycardia (HR < 50 beats)13 was treated with 0.3 mg of atropine each time. Considering that infusion of remifentanil may cause post-induction bradycardia,14 2 mg of dopamine was chosen to treat post-induction hypotension (SBP < 95 mmHg) in our study. The dose of atropine or dopamine was recorded. The induction duration (time from administration to insertion of LMA) and the number of successful insertion attempts were recorded. The tidal volume and airway pressure were recorded immediately after LMA insertion.
Statistical Analysis
Statistical analyses were performed using IBM SPSS Statistics software, version 25. Shapiro‒Wilk tests were used to assess the data distribution. Normally distributed continuous variables were reported as the means ± standard deviations (SD) and analyzed using Student’s t-test. Nonnormally distributed continuous variables were expressed as the medians and interquartile ranges and analyzed using the Mann‒Whitney U-test. Qualitative data are presented as frequencies and percentages. Between-group comparisons of categorical data were analyzed using the chi-square test or Fisher’s exact test. P < 0.05 was considered to indicate statistical significance.
Upon reviewing the literature, the previous success rate for LMA placement with 2.0 mg/kg propofol combined with remifentanil has been reported to be 100%.7 We assumed a success rate of 80% for remifentanil, which could be counted as a high feasibility. If α=0.05 and β=0.2, using the two-sample rate comparison formula, at least 36 patients would be needed in each group.
Results
A total of 80 patients were screened for eligibility, and 72 patients were finally included in this study (Figure 1). No patient was excluded from our study due to a severe adverse event. There were no significant differences in age, BMI or ASA between the two groups (Table 1).
 |
Table 1 Demographic Characteristics and Clinical Data of the Patients |
 |
Figure 1 Patient screening, enrollment and randomization. |
The LAM insertion summed score was 18.0 (18.0, 18.0) in the propofol group and 18.0 (17.0, 18.0) in the remazolam group. These scores were comparable (P > 0.05) (Table 2). Thirty-six (100%) patients had a satisfactory or an excellent score in the propofol group compared with thirty-four (94.4%) patients that had these scores in the remazolam group. The difference was also not significant (P > 0.05) (Table 2). The incidence of transient mild laryngospasm was significantly higher in the remazolam group than in the propofol group (19.4% vs 0.0%, P < 0.05) (Table 3). However, neither group required additional propofol or cisatracurium and a second attempt for LAM insertion. There were no significant differences in the incidence of mouth opening, swallowing, coughing, head or body motion or in the ease of LMA insertion between the two groups (Table 3).
 |
Table 2 Difference in Summed Score, Satisfactory or Excellent Rate and Secondary Outcomes Between the Two Groups |
 |
Table 3 Grading Conditions for Laryngeal Mask Airway Insertion Between the Two Groups |
The insertion durations were similar in the two groups (Table 2). However, the time from induction to successful LMA insertion in the remazolam group (250.0 (223.0, 262.3) s) was significantly longer than that in the propofol group (189.5.0 (169.0, 224.0) s) (P < 0.05) (Table 2). The plateau pressure in the remazolam group (15.5 (14.0, 21.8) mmHg) was significantly higher than that in the propofol group (14.0 (12.0, 15.8) mmHg) (Table 2). However, the tidal volumes were similar in both groups (Table 2).
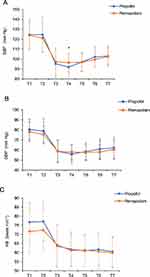
Figure 2 shows the trends of SBP, DBP and HR. There was a significant decrease in SBP (P < 0.05) but only immediately after the insertion of the laryngeal mask in the propofol group compared with the remazolam group. The patients in both groups had similar DBP and HR at all time points (P > 0.05). The cost of dopamine was significantly lower in the 0.0 (0.0, 2.0) mg remazolam group than in the 2.0 (2.0, 4.0) mg propofol group (P < 0.05) (Table 2).
A greater number of patients had injection pain in the propofol group (8.0 (22.2%)) than in the remazolam group (3.0 (8.3%)), although this difference did not reach statistical significance (P > 0.05) (Table 4). Three patients in the remazolam group had hiccups compared with none in the propofol group (Table 4).
 |
Table 4 Difference in Adverse Events Between the Two Groups |
Discussion
This research evaluated the feasibility and safety of LMA insertion by remazolam without using a neuromuscular blocking agent in patients undergoing hysteroscopic surgery. The described methods of inserting an LMA are particularly useful for patients with short-term surgery and who have contraindications for the use of neuromuscular blocking agents. There are two important findings in our study. First, 0.3 mg/kg of remazolam is comparable to 2.0 mg/kg of propofol in performing LMA placement without the use of a neuromuscular blocking agent. However, there was slightly poorer inhibition of airway reflexes in the remazolam group. Second, remazolam has a lower incidence of hypotension and a slower onset of action.
Remazolam is a rapidly metabolized benzodiazepine (BZD) that has been indicated to be an effective sedative hypnotic during induction for general anesthesia.15,16 Previous study reported that a supraglottic airway could be successfully intubated with the use of remazolam and remifentanil without a neuromuscular blocking agent.17 Our induction also proved this. The LMA insertion conditions in the remazolam group were noninferior to those in the propofol group in our study. Furthermore, in 34 patients (94.4%) in the remazolam group, excellent or satisfactory conditions were achieved, while in 36 patients (100%) in the propofol group, excellent or satisfactory conditions were achieved. However, the induction time was significantly slower and transiently elevated plateau pressure and laryngospasm occurred more frequently in the remazolam group. These factors did not affect our procedures. Moreover, the induction duration was at a median of 250.0 (223.0, 262.3) seconds in the remazolam group compared to 189.5 (169.0, 224.0) seconds in the propofol group. The length of induction for both groups was statistically significant. However, it was less clinically significant.
Some studies highlighted a lower incidence of hypotension in the remazolam group than in the propofol group.18,19 Our experiment also confirmed this result. Although a significant difference in SBP only occurred after immediate LMA insertion in our study, additional dopamine was required significantly more frequently to stabilize the blood pressure in the patients treated with sufentanil-propofol-remifentanil. Apparently, remazolam was associated with better hemodynamic stability than propofol. The heart rate between both groups was not statistically significant. However, there was a clinical decrease in the anesthetized subjects compared with the conscious subjects regarding blood pressure and heart rate in both groups. In summary, remazolam may be more acceptable in elderly individuals or in patients with cardiovascular disease to prevent fluctuations in blood pressure.
Many studies have shown a higher incidence of injection pain with propofol than with remazolam.18,19 However, the incidence of injection pain was not significantly different in our groups. This might be related to the first administration of sufentanil during our induction, which, as previously reported, helps to reduce propofol injection pain.20,21 Injection pain still occurred in the remazolam group in our study, but there were fewer patients that had injection pain in this group than in the propofol group. The reasons for this may be that, first, sufentanil did not reduce the injection pain of remazolam. Second, the bolus administration of remazolam resulted in a higher incidence of injection pain. Some studies have shown that an infusion of remazolam did not cause injection pain either when opioids were given in advance or during induction.16,17 The possible reasons for this discrepancy may be the differences in the rate, concentration, or method of administration of remazolam or even could be due to the differences in the definition of injection pain across studies. Accordingly, the detailed reasons could be explored.
A recent study suggested that hiccups occurred “frequently” when receiving remazolam at a 0.4 mg/kg bolus in 1 minus.22 Remazolam was reported to have a low incidence of hiccups in a continuous infusion study.23,24 Hiccups were observed in our study. We also induced with bolus injections. Therefore, the incidence of hiccups may also be related to the rate or the method of remazolam administration during induction. Another possible reason for hiccups may be that remazolam is a benzodiazepine.25 Benzodiazepines and corticosteroids are suspected to be the most frequent agents to induce hiccups.26,27 Some studies have shown that midazolam also causes hiccups and can be reversed by flumazenil.28,29 However, the mechanism of hiccups induced by benzodiazepines has not been clearly explained. Hiccups may be associated with GABA neurotransmitters, and benzodiazepines can stimulate GABA neurotransmitters to produce various effects. Notably, the abrupt onset of hiccups may cause unexpected pulmonary aspiration or hamper endoscopic investigations when patients are unconscious. However, the symptoms were self-limiting within several minutes and did not affect our operations.
In addition, seven patients in the remazolam group presented with decreased pulmonary compliance, elevated plateau pressures and slightly decreased tidal volumes during induction, which are results that are reported first in this study. These symptoms were short-lasting and recovered spontaneously in a few minutes. We excluded opioid-induced chest wall rigidity because of the lack of generalized muscle stiffness, and naloxone was not needed.30 One consideration may be transient mild laryngospasm because pulmonary croup was also not heard by a stethoscope. Nevertheless, no studies have been reported about upper airway reflexes when using remazolam.
Some studies have reported that opioids may cause laryngospasm.31,32 Therefore, we assumed that mild upper airway reflexes might be induced by sufentanil and reversed by propofol in the propofol group.33 When mild upper airway reflexes occurred in the remazolam group, they could be resolved by continuous positive mask airway pressure during induction rather than disappearing on their own. However, Zhao and Liu did not find laryngospasm when using opioids first.19,34 Moreover, sufentanil is commonly used first in clinical practice, with less laryngospasm reported and more coughing risk.35 Consequently, we presumed that laryngospasm was more likely to be induced by remazolam. In addition, Davis reported midazolam-induced laryngospasm,28 and midazolam is also a benzodiazepine.
Since laryngospasm has not been reported when using remazolam. We speculate that the laryngospasm is related to the mode of infusion, the low concentration of administration,17,36 or the use of a neuromuscular blocking agent during general anesthesia.37 Because our study used a bolus of 0.3 mg/kg remazolam within 95 seconds.
Therefore, whether sufentanil, remazolam or their combination caused upper airway reactions in our study are unclear. The use of flumazenil, the use of naloxone or changes in the method and concentration of administration of remazolam may lead to different conclusions. Consequently, further investigation is urged.
There are some limitations. First, this study included only women who underwent a hysteroscopy. A study including men is necessary. Second, because of the physical appearance of propofol, the anesthesiologist who collected data was unblinded to the patient’s assignment, and some potentially confounding factors may not have been excluded. Third, different methods or dosages of administration were warranted due to the similar satisfactory rate with propofol but the higher incidence of laryngospasm. Last, this was a single-center investigation with a relatively small sample size. Multicenter, large sample-size studies are needed to improve our understanding of the findings. Thus, our conclusion should be interpreted as conservative.
Conclusion
In summary, remazolam had similar sedative efficacy to propofol at the time of LAM insertion without neuromuscular blocking agents. Moreover, remazolam provides a more stable arterial pressure. However, self-limiting laryngospasms and hiccups should be considered when using remazolam without muscular blocking agents. Further investigation is needed.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of Renmin Hospital of Wuhan University (WDRY2022-K111).
Acknowledgments
We thank all authors for their substantial contributions to this trial. We are also grateful to Panheng Li and Haozhi Dai for their assistance.
Funding
This work was supported by Beijing Medical Award Foundation (YXJL-2021-0307-0680).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sutton C. Hysteroscopic surgery. Best Pract Res Clin Obstet Gynaecol. 2006;20(1):105–137. doi:10.1016/j.bpobgyn.2005.10.002
2. Yu J, Xiang B, Song Y, Chen H, Li Y, Liu C. ED50 of propofol in combination with low-dose sufentanil for intravenous anaesthesia in hysteroscopy. Basic Clin Pharmacol Toxicol. 2019;125(5):460–465. doi:10.1111/bcpt.13280
3. Wernli KJ, Brenner AT, Rutter CM, Inadomi JM. Risks associated with anesthesia services during colonoscopy. Available from: https://pubmed.ncbi.nlm.nih.gov/26709032/.
4. Razpotnik M, Bota S, Essler G, Weber-Eibel J, Peck-Radosavljevic M. Impact of endoscopist experience, patient age and comorbidities on dose of sedation and sedation-related complications by endoscopic ultrasound. Eur J Gastroenterol Hepatol. 2022;34(2):177–183. doi:10.1097/MEG.0000000000002084
5. Peirovifar A, Eydi M, Mirinejhad MM, Mahmoodpoor A, Mohammadi A, Golzari SE. Comparison of postoperative complication between Laryngeal Mask Airway and endotracheal tube during low-flow anesthesia with controlled ventilation. Pak J Med Sci. 2013;29(2):601–605. doi:10.12669/pjms.292.2980
6. Driver I, Wilson C, Wiltshire S, Mills P, Howard-Griffin R. Co-induction and laryngeal mask insertion. A comparison of thiopentone versus propofol. Anaesthesia. 1997;52(7):698–700. doi:10.1111/j.1365-2044.1997.az0130b.x
7. Scanlon P, Carey M, Power M, Kirby F. Patient response to laryngeal mask insertion after induction of anaesthesia with propofol or thiopentone. Can J Anaesth. 1993;40(9):816–818. doi:10.1007/BF03009250
8. Sivalingam P, Kandasamy R, Madhavan G, Dhakshinamoorthi P. Conditions for laryngeal mask insertion. A comparison of propofol versus sevoflurane with or without alfentanil. Anaesthesia. 1999;54(3):271–276. doi:10.1046/j.1365-2044.1999.00663.x
9. Wiltshire HR, Kilpatrick GJ, Tilbrook GS, Borkett KM. A placebo- and midazolam-controlled Phase I single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): part II. Population pharmacokinetic and pharmacodynamic modeling and simulation. Anesth Analg. 2012;115(2):284–296. doi:10.1213/ANE.0b013e318241f68a
10. Rex DK, Bhandari R, Desta T, et al. A Phase III study evaluating the efficacy and safety of remimazolam (CNS 7056) compared with placebo and midazolam in patients undergoing colonoscopy. Gastrointest Endosc. 2018;88(3):427–437.e6. doi:10.1016/j.gie.2018.04.2351
11. Zhou Y, Hu P, Huang Y, et al. Population pharmacokinetic/pharmacodynamic model-guided dosing optimization of a novel sedative HR7056 in Chinese healthy subjects. Front Pharmacol. 2018;9:1316. doi:10.3389/fphar.2018.01316
12. Ganatra SB, D’Mello J, Butani M, Jhamnani P. Conditions for insertion of the laryngeal mask airway: comparisons between sevoflurane and propofol using fentanyl as a co-induction agent. A pilot study. EJA. 2002;19(05):371. doi:10.1017/S0265021502000601
13. Kusumoto FM, Schoenfeld MH, Barrett C, et al. 2018 ACC/AHA/HRS guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the heart Rhythm society. Circulation. 2019;140(8):e382–e482. doi:10.1161/CIR.0000000000000628
14. DeSouza G, Lewis MC, TerRiet MF. Severe bradycardia after remifentanil. Anesthesiology. 1997;87(4):1019–1020. doi:10.1097/00000542-199710000-00061
15. Chae D, Kim HC, Song Y, Choi YS, Han DW. Pharmacodynamic analysis of intravenous bolus remimazolam for loss of consciousness in patients undergoing general anaesthesia: a randomised, prospective, double-blind study. Br J Anaesth. 2022;129(1):49–57. doi:10.1016/j.bja.2022.02.040
16. Doi M, Morita K, Takeda J, Sakamoto A, Yamakage M, Suzuki T. Efficacy and safety of remimazolam versus propofol for general anesthesia: a multicenter, single-blind, randomized, parallel-group, phase IIb/III trial. J Anesth. 2020;34(4):543–553. doi:10.1007/s00540-020-02788-6
17. Park I, Cho M, Nam SW, Hwang JW, Do SH, Na HS. Total intravenous anesthesia induced and maintained by a combination of remimazolam and remifentanil without a neuromuscular blocking agent: a prospective, observational pilot study. BMC Anesthesiol. 2022;22(1):237. doi:10.1186/s12871-022-01779-2
18. Qiu Y, Gu W, Zhao M, Zhang Y, Wu J. The hemodynamic stability of remimazolam compared with propofol in patients undergoing endoscopic submucosal dissection: a randomized trial. Front Med. 2022;9:938940. doi:10.3389/fmed.2022.938940
19. Zhao N, Zeng J, Fan L, et al. Moderate sedation by total intravenous remimazolam-alfentanil vs. propofol-alfentanil for third molar extraction: a prospective randomized controlled trial. Front Med. 2022;9:950564. doi:10.3389/fmed.2022.950564
20. Nathanson MH, Gajraj NM, Russell JA. Prevention of pain on injection of propofol: a comparison of lidocaine with alfentanil. Anesth Analg. 1996;82(3):469–471. doi:10.1097/00000539-199603000-00006
21. Honarmand A, Safavi M. Prevention of propofol-induced injection pain by sufentanil: a placebo-controlled comparison with remifentanil. Clin Drug Investig. 2008;28(1):27–35. doi:10.2165/00044011-200828010-00004
22. Chen X, Sang N, Song K, et al. Psychomotor recovery following remimazolam-induced sedation and the effectiveness of flumazenil as an antidote. Clin Ther. 2020;42(4):614–624. doi:10.1016/j.clinthera.2020.02.006
23. Schüttler J, Eisenried A, Lerch M, Fechner J, Jeleazcov C, Ihmsen H. Pharmacokinetics and pharmacodynamics of remimazolam (CNS 7056) after continuous infusion in healthy male volunteers: Part I. pharmacokinetics and clinical pharmacodynamics. Anesthesiology. 2020;132(4):636–651. doi:10.1097/ALN.0000000000003103
24. Sheng X, Liang Y, Yang X, et al. Safety, pharmacokinetic and pharmacodynamic properties of single ascending dose and continuous infusion of remimazolam besylate in healthy Chinese volunteers. Eur J Clin Pharmacol. 2020;76(3):383–391. doi:10.1007/s00228-019-02800-3
25. Micallef J, Tardieu S, Pradel V, Blin O. Benzodiazépines et hoquet : à propos de trois cas [Benzodiazepine and hiccup: three case reports]. Therapie. 2005;60(1):57–60. French. doi:10.2515/therapie:2005007
26. Thompson DF, Landry JP. Drug-induced hiccups. Ann Pharmacother. 1997;31(3):367–369. doi:10.1177/106002809703100318
27. Bor S, Mandiracioglu A, Kitapcioglu G, Caymaz-Bor C, Gilbert RJ. Gastroesophageal reflux disease in a low-income region in Turkey. Am J Gastroenterol. 2005;100(4):759–765. doi:10.1111/j.1572-0241.2005.41065.x
28. Davis DP, Hamilton RS, Webster TH. Reversal of midazolam-induced laryngospasm with flumazenil. Ann Emerg Med. 1998;32(2):263–265. doi:10.1016/S0196-0644(98)70148-9
29. Arroyo-Cózar M, Grau Delgado J, Gabaldón Conejos T. Hiccups induced by midazolam during sedation in flexible bronchoscopy. Archivos de Bronconeumología. 2012;48(3):103. doi:10.1016/j.arbr.2011.11.007
30. Roan JP, Bajaj N, Davis FA, Kandinata N. Opioids and chest wall rigidity during mechanical ventilation. Ann Intern Med. 2018;168(9):678. doi:10.7326/L17-0612
31. Fahnenstich H, Steffan J, Kau N, Bartmann P. Fentanyl-induced chest wall rigidity and laryngospasm in preterm and term infants. Crit Care Med. 2000;28(3):836–839. doi:10.1097/00003246-200003000-00037
32. Miner NB, Schutzer WE, Zarnegarnia Y, Janowsky A, Torralva R. Fentanyl causes naloxone-resistant vocal cord closure: a platform for testing opioid overdose treatments. Drug Alcohol Depend. 2021;227:108974. doi:10.1016/j.drugalcdep.2021.108974
33. Nawfal M, Baraka A. Propofol for relief of extubation laryngospasm. Anaesthesia. 2002;57(10):1036. doi:10.1046/j.1365-2044.2002.283810.x
34. Liu X, Ding B, Shi F, et al. The efficacy and safety of remimazolam tosilate versus etomidate-propofol in elderly outpatients undergoing colonoscopy: a prospective, randomized, single-blind, non-inferiority trial. Drug Des Devel Ther. 2021;15:4675–4685. doi:10.2147/DDDT.S339535
35. Agarwal A, Gautam S, Nath SS, Gupta D, Singh U. Comparison of the incidence and severity of cough induced by sufentanil and fentanyl: a prospective, randomised, double-blind study. Anaesthesia. 2007;62(12):1230–1232. doi:10.1111/j.1365-2044.2007.05249.x
36. Borkett KM, Riff DS, Schwartz HI, et al. A Phase IIa, randomized, double-blind study of remimazolam (CNS 7056) versus midazolam for sedation in upper gastrointestinal endoscopy. Anesth Analg. 2015;120(4):771–780. doi:10.1213/ANE.0000000000000548
37. Mao Y, Guo J, Yuan J, Zhao E, Yang J. Quality of recovery after general anesthesia with remimazolam in patients’ undergoing urologic surgery: a randomized controlled trial comparing remimazolam with propofol. DDDT. 2022;16:1199–1209. doi:10.2147/DDDT.S359496
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.