Back to Journals » Pharmacogenomics and Personalized Medicine » Volume 17
FBLN5 as One Presumably Prognostic Gene Potentially Modulating Tumor Immune Microenvironment for Renal Clear Cell Carcinoma in Children and Young Adults
Authors Zhang M, Chen F, Feng S, Liu X, Wang Z, Shen N, Meng L, Zhu D, Zhu Z
Received 1 November 2023
Accepted for publication 13 January 2024
Published 19 January 2024 Volume 2024:17 Pages 27—40
DOI https://doi.org/10.2147/PGPM.S442803
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Ming Zhang,1,* Feng Chen,2,* Shaoguang Feng,3,* Xu Liu,4 Zhen Wang,5 Nan Shen,6 Lingjian Meng,7 Dongsheng Zhu,1 Zhitao Zhu8
1Department of Pediatric Surgery, The First People’s Hospital of Lianyungang, Lianyungang, People’s Republic of China; 2Department of Pediatric, Luodian Hospital, Shanghai, People’s Republic of China; 3Department of Pediatric Surgery, The Children’s Hospital of Hangzhou, Hangzhou, People’s Republic of China; 4Department of Pediatric Surgery, The Children’s Hospital of Xuzhou, Xuzhou, People’s Republic of China; 5Department of Pediatric, the Maternal and Child Health Hospital of Zibo, Zibo, People’s Republic of China; 6Department of Pediatrics, Suqian Hospital Affiliated to Xuzhou Medical University, Suqian, People’s Republic of China; 7Department of Pediatrics, Hospital Affiliated to Xuzhou Medical University, Xuzhou, People’s Republic of China; 8Department of Radiology, The Second People’s Hospital of Lianyungang, Lianyungang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhitao Zhu; Dongsheng Zhu, Email [email protected]; [email protected]
Objective: To investigate the role of FBLN5in renal clear cell carcinoma (KIRC), in particular on the tumor’s immune microenvironment, including children and young adults.
Methods: FBLN5 expression in tumor and normal samples was explored using SangerBox, TIMER2.0, GEPIA, UALCAN, HPA databases. The Linkedomics database was used to obtain FBLN5 co-expressed genes in KIRC tissue. SangerBox was also used to estimate immune infiltration of FBLN5 in KIRC. The Kaplan-Meier plotter was used to investigate the survival effects of FBLN5 expression in the presence of immune infiltration. We then collected 48 cases from 7 hospitals over a-20 year period to calculate the impact of FBLN5 on the prognosis of children and young adults with KIRC.
Results: FBLN5 expression was significantly reduced in KIRC tissue compared to normal adjacent tissue. FBLN5 was potentially involved in the immune-related biological processes. In addition, FBLN5 expression has been linked to a number of immune checkpoints, cytokines, chemokines and chemokine receptors in KIRC. At the same time, the expression of FBLN5 affected the survival rates differently in KIRC patients with high or low levels of immune infiltration. High expression of FBLN5 in children and young adults with KIRC was associated with a favorable prognosis.
Conclusion: This study shed light on the potential of FBLN5 as a prognostic marker in children and young adults with KIRC and as an immune-related target for clinical treatment.
Keywords: FBLN5, immune microenvironment, prognostic biomarker, kidney cancer, children
Introduction
Renal cancer is the third most frequent urological malignancy, with 77,410 new cases and 46,345 deaths reported in 2022 by the Chinese Cancer Observatory.1 Renal clear cell carcinoma (KIRC) is the most frequent subtype of kidney cancer and accounts for more than 70% of all cases.2 However, the most common neoplasm of the kidney is nephroblastoma in childhood, and KIRC is rare in children and young adults.3 Fewer than 4% of renal tumors in children are KIRC.4 Early diagnosis of KIRC is crucial, as it can make treatment challenging and increase the risk of recurrence when diagnosed at an advanced stage.5 Patients with metastatic KIRC have a 5-year survival rate of only 10%.6
KIRC has long been known to be sensitive to immunotherapies, and interleukin-2 (IL-2) has been approved for treatment of KIRC since 1992.7 Some scholars have even suggested that combination immunotherapies have the potential to become a first-line treatment option in KIRC.8 So far, many KIRC patients have benefited from immunotherapy. Immune cells, consisting of B cells, T cells, natural killer cells, etc., are critical for carcinogenesis and immunotherapeutic responses.9 Immune checkpoint blockade therapy has also been successful in multiple cancers, including KIRC, and many patients may benefit.10 From this, it can be seen that immunotherapy plays a role in the treatment of KIRC and deserves further investigation.
The tumor microenvironment (TME), which is critical for carcinogenesis and therapeutic responses, is composed of immune cells, fibroblasts, endothelial cells and various biological molecules.11 Recent studies have found that fibroblasts play a role in promoting angiogenesis in TME, which then promotes tumor growth.12 Fibroblasts can activate the transforming growth factor-beta (TGF-β) signaling pathway, causing tumor cells to acquire interstitial morphology and weakening adhesion between tumor cells, which then lead to distant metastasis.13 In addition, fibroblasts may also weaken the immune response to tumors by modulating the infiltration and percolation distribution of immune cells in the TME through various factors such as CXCL12, CXCL16, IL6, IL8, PD-1 and PD-2, which then enable tumor cells to acquire metastasis capacity.14
The fibulin (FBLN) family is widely found in the extracellular matrix (ECM) and plays a role in the formation and stabilization of basement membranes and elastic fibers.15 FBLN5, a key member of the FBLN family, has been reported to be involved in cell proliferation and cell motility.16 In ovarian cancer, FBLN5 was found can inhibit tumor progression as a target of TGF-β in fibroblasts.17 However, according to previous literatures, the role of FBLN5 was different and it was an inhibitor or promoter of tumor cells depending on the type of cancer and environment. In bladder cancer18 and lung cancer,19 cancer suppression by FBLN5 has been observed. Conversely, FBLN5 has been found to promote cell growth and metastasis in breast and pancreatic cancers.20,21 However, the role of FBLN5 in KIRC is unclear. In view of this, the clinical significance and biological role of FBLN5 was assessed in this study, particularly in children and young adults.
Materials and Methods
Patients
We retrospectively collected renal cancer data from seven hospitals in China between 2001 and 2021. Patient inclusion criteria: younger than 23 years, a cut-off of 23 years chosen based on similar criteria in other research;22 the pathologic classification was KIRC; tumor samples and paired normal nearby tumor samples were available; neither chemotherapy nor radiotherapy was administered; written informed consent was obtained from the participants. Patient exclusion criteria: excluded from all the other cases mentioned above.
Ethics Statement
The study involving human participants was reviewed and approved by The Ethics Committee of Lianyungang First Hospital (20220215) and conducted in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from the participants or guardians of patients younger than 18 years of age included in the present study.
Bioinformatics Analysis
FBLN5 expression in tumor and normal samples was explored using SangerBox (http://www.sangerbox.com/), TIMER2.0 (http://timer.comp-genomics.org/timer/), GEPIA (http://gepia.cancer-pku.cn/), UALCAN (https://ualcan.path.uab.edu/), and HPA (https://www.proteinatlas.org/) databases. In the above databases, “FBLN5” was put into the “search” module and then the expression of FBLN5 was obtained, respectively. The Linkedomics database (https://www.linkedomics.org/login.php) was used to obtain FBLN5 co-expressed genes in KIRC. In the Linkedomics database, we first select the KIRC cancer cohort and then put “FBLN5” in the “Search Attribute” module, after which we obtain the FBLN5 co-expressed genes. SangerBox was also used to estimate 111 infiltration of FBLN5 in KIRC. “Immune genes” and “immune checkpoint” modules for FBLN5 were selected to estimate immune infiltration in SangerBox. The Kaplan-Meier plotter (http://kmplot.com/analysis/) was used to investigate the survival effects of FBLN5 expression in the presence of immune infiltration.
FBLN5‑related Genes Enrichment Analysis
After obtaining FBLN5‑related genes from the Linkedomics database, Kyoto Encyclopedia of Genes and Genomes (KEGG) (https://www.genome.jp/kegg/) was used for pathway analysis, using the threshold of a count >3, and P<0.05. The enriched pathways were visualized using the “ggplot2” R packages.
Cell Lines
The ACHN cell line was chosen for this research because it was derived from a 22-year-old male with KIRC according to precious literatures.23,24 HK2 (catalog no. MZ-0086) and ACHN (catalog no. MZ-0018) cell lines were purchased from Mingzhou Biotechnology Co., Ltd (Ningbo, China). A DMEM (Invitrogen; Thermo Fisher Scientific, Inc.) containing 10% FBS (Gibco; Thermo Fisher Scientific, Inc.), streptomycin (100 μg/mL), and penicillin (100 U/mL) was used for cell culture. The cells were incubated in a humidified incubator containing 5% CO2 at 37 °C.
RNA Isolation and Reverse Transcription‑Quantitative PCR (RT‑qPCR)
RNA isolation and RT‑qPCR were followed as described previously.25 β‑actin expression level was used to normalize the relative expression level of FBLN5. Experiment primers were listed in Table 1. All experiments were performed in a triplet.
 |
Table 1 Primer Sequences Used for qPCR |
Western Blot Assay
Western blotting analysis was performed as we previously reported.26 Total proteins were extracted from HK2 and ACHN cells using RIPA buffer (Biosharp Life Sciences, China) and protein quantitation using Bradford protein assay ((Bio-Rad Laboratories, Inc.). Electrophoresis was performed using a 10% SDS-PAGE gel, which was then transferred to a PVDF membrane, after that 5% fat-free milk was blocked at room temperature for 2 h. The membranes were cropped and incubated with primary antibodies at 4 °C overnight and with a secondary antibody for 1 h at room temperature. Pierce™ ECL Western Blotting Substrate (cat. no. 32109; Thermo Fisher Scientific, Inc.) was used to visualize the bound antibodies. The antibodies used were listed in Table 2.
 |
Table 2 The List of the Antibodies Used for Western Blotting |
Statistical Analyses
Statistical analysis was performed using SPSS 25, GraphPad Prism 8.0, and R 4.2.2. The significance of the differences between the two groups was assessed using the Mann–Whitney U-test. The log-rank method was used to determine the significance of the expression of FBLN5 on patient survival. Multivariate Cox regression analysis was also applied to the overall survival risk factors. The diagnostic and prognostic values of FBLN5 for KIRC in children and young adults were analyzed using receiver operating characteristic (ROC) curve. Nomogram was used to predict the prognosis of FBLN5 expression in KIRC, and calibration curves were used to compare nomogram predictions with actual observed survival outcomes. The decision curve analysis (DCA) diagram was drawn to analysis the benefit of FBLN5 expression for prognosis. “timeROC”, “rmda”, “pROC”, “survival”, “rms”, “clusterProfiler” and “ggplot2” R packages were used for analyses. A p-value below 0.05 was considered significant.
Results
FBLN5 Expression Was Down-Regulated in KIRC
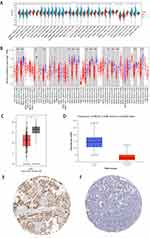
We found that FBLN5 is significantly less r expressed in tumor tissue compared to normal tissue in KRIC using SangerBox (Figure 1A), TIMER2.0 (Figure 1B), GEPIA (Figure 1C), UALCAN (Figure 1D), and HPA (Figure 1E and F) databases, respectively.
FBLN5 Expression in Different KIRC Groups
The UALCAN database was used to evaluate the expression of FBLN5 in different clinical subgroups of KIRC. It was found that the expression of FBLN5 was lowest in the age group older than 80 years, with a significant difference when compared to the 41–60 year group (Figure 2A). There were no significant differences in sex (Figure 2B), race (Figure 2C), tumor grade (Figure 2D), or N stage (Figure 2E). When it came to tumor subtypes, FBLN5 expression was significantly lower in the ccA subtype than in the ccB subtype, and both subtypes had lower FBLN5 expression than the normal sample (Figure 2F). Regarding the KIRC stage, we found that the expression of FBLN5 also does not show a trend of correlation with increasing tumor grade increased, with stage 2 having the lowest expression and being significant different from the others (Figure 2G).
FBLN5 May Play a Role in KIRC’s TME
We investigated the mechanism of FBLN5 in KIRC using the Linkedomics database, where the co-expressed mRNAs of FBLN5 in KIRC patients were retrieved and the results are shown as a volcano plot (Figure 3A). The top 50 positive genes associated with FBLN5 were shown in Figure 3B, and the top 50 negative genes associated with FBLN5 were shown in Figure 3C. Gene Ontology analyses of these co-expressed genes indicated that FBLN5 may be involved in the biological process and muscle tissue development; the cellular component of FBLN5 might be involved in fiber and collagen-containing extracellular matrix formation; and the molecular function of FBLN5 might be involved in extracellular matrix structural constituent (Figure 3D). In terms of the results of the KEGG analysis result, it was found that FBLN5 is related to the ECM receptor interaction (Figure 3E). All the above analysis suggested that FBLN5 may be related to the ECM and fiber information in KIRC. ECM, immune cells and fibroblasts are the important components of TME, and they are closely related and interact with each other.27,28 Hence, we speculate that FBLN5 may play a role in the immune microenvironment.
FBLN5 May Play a Role in Immune Microenvironment Modulation in KIRC
To analyze whether there is a link between FBLN5 expression and immune regulation, we explored the relationship between FBLN5 expression and numerous immune genes in KIRC to confirm the immunological function of FBLN5 using Sangerbox. FBLN5 was found to have a significant positive expression associated with the traditional immunological checkpoints CD27, CD28, HMGB1, TLR4, IL1B, and IL2 (Figure 4A). For immunological chemokines, we found that FNLN5 is co-expressed with CXCL12, CCL14, CCL19 et al; for immunological receptor, it was found FBNL5 is co-expression with CCR1-10; and FBLN5 was also co-expressed with a large number of immunostimulators, including CD40, CD28, CD48, IL-2 et al (Figure 4B). The abundance of tumor infiltrating immune cells in KIRC was then evaluated using the CIBERSORT algorithm (Figure 5A), and it was found that T cell CD8+, B cell, macrophage, endothelial cell, NK cell, and T cell CD4+ were all correlated with FBLN5 expression (Figure 5B and C). Following this, we investigated the relationship between FBLN5 expression and the infiltration, and the results showed a positive correlation between stromal, immune and ESTIMATE scores and FBLN5 (Figure 5D–F).
 |
Figure 4 Relationships between FBLN5 and tumor immune microenvironment in KIRC; (A) immune genes; (B) immune checkpoint.*p < 0.05. |
FBLN5 Expression with Different Level of Immune Cell Infiltration Affects the Prognosis of KIRC
SangerBox found that FBLN5 levels did not affect the survival time in KIRC patients (Figure 6A). Kaplan-Meier Plotter database (Figure 6B) and GEPIA database (Figure 6C) showed similar findings. The Kaplan-Meier Plotter database was then used to explore the effects of FBLN5 expression on the KIRC patients through the control of the immune microenvironment. The results revealed a poor prognosis for patients with high FBLN5 expression in their reduced basophils (Figure 6D); however, a preferred prognosis was seen in patients with enriched basophils (Figure 6E), and similar results were seen in patients with reduced CD4+ memory T cells (Figure 6I) and enriched natural killer cells (Figure 6R). In addition to the results noted above, the expression of FBLN5 had no effect on the overall survival of patients with KIRC (Figure 6F–H, J–Q and S–W). As a result of the previous findings, it appears that FBLN5 regulates carcinogenesis by influencing the immune system.
Expression of FBLN5 and Children and Young Adults’ Prognosis
Given the differences between the immune systems of children and adult, the immune systems of children and young adults are relatively underdeveloped.29 We further explored the role of FBLN5 in the KIRC of children and young adults. First, RT-qPCR was measured for FBLN5 in the normal renal cell line HK2 and in the KIRC cell line ACHN, where it was found that FBLN5 expression was lower in ACHN compared to HK2 (Figure 7A). The western bolting results gave similar finding at the protein level (Figure 7B). Tumor tissue and paired normal nearby tumor tissue were also explored, and it was found that FBLN5 expression is reduced in tumor tissue (Figure 7C). A ROC curve was then calculated to determine the area under the curve (AUC) for FBLN5 in KIRC. It was found that FBLN5 had a sensitivity of 0.729 for diagnostic KIRC, a specificity of 0.937, and an AUC of 0.825 (Figure 7D). The survival time and the relative expression of FBLN5 for the 48 patients were shown in Figure 7E. And patients in the high-risk group had longer survival times (Figure 7F). The time dependent ROC results suggested that FBLN5 has a good value in predicting the prognosis of KIRC in children and young adults (Figure 7G). After that, we calculated the score of each patient using the nomogram and found that FBLN5 has an important score (Figure 7H). Furthermore, the calibration plots showed that the predict risk is very close to the ideal curve (Figure 7I). Moreover, the DCA plots also clearly showed that FBLN5 may be a reflection of patient prognosis (Figure 7J and K). To further assess the relationship between FBLN5 expression levels and prognosis in children and young adults with KIRC, we performed univariate and multivariate Cox analyses and found an independent correlation between FBLN5 expression and patient prognosis (Table 3).
 |
Table 3 Cox Univariate and Multivariate Analyses of FBLN5 Gene Expression |
Discussion
Extensive evidence has shown that the occurrence and development of KIRC is closely related to genes.30 Genes may play the role of prognostic biomarkers to develop a tailored treatment plans for individuals. In this study, we explore the biological function of FBLN5 in KIRC using public databases. We determined the FBLN5 mRNA and protein levels and analyzed the relationship between FBLN5 expression levels and patient outcome. We found that FBLN5 played an important role in the TME.
FBLNs are a family of seven ECM proteins involved in complex biological processes, particularly associated with elastic and fibrous tissue, and play important roles in the ECM, especially affecting fibroblasts.31 FBLN5, a member of the family of FBLNs, is a fibroblast-derived ECM protein that promotes endothelial cell adhesion and plays an essential role in the formation of elastic fibers.16 ECM, immune cells and fibroblasts are important components of TME, and they are closely related and interact with each other.28 The above information suggests that FBLN5 is closely related to the TME. In recent years, there has been a significant increase in the number of studies on the roles of FBLN5 in tumor processes.18–20 However, the significance of FBLN5 in KIRC has not been investigated so far. Hence, we wanted to explore the clinical implications of FBLN5 in KIRC.
TME, which is composed of tumor cells, immune cells, cytokines, and other factors, has a significant impact on tumor initiation and progression.11,27 KIRC has been shown not to be susceptible to radiotherapy or chemotherapy; while immunotherapy has played a role in the diagnosis and treatment of KIRC, cytokines such as IL-2 and interferon were mostly used for treatment in the 1990s.7 In this study, we elucidated that FBLN5 expression was correlated with the infiltration levels of various immune cells, as well as the expression of immune checkpoints, cytokines, chemokines, and chemokine receptors in KIRC, suggesting that FBLN5 might be used as an immune target in combination with the typical targets in KIRC.
The results of GO analysis of our study indicated that FBLN5 was involved in the information of the fibroblasts, which might indicate that the fibroblasts could be regulated by the expression of FBLN5. The discovery of KEGG pathway suggested a link between FBLN5 and ECM receptor interaction, which might indicate that the ECM could be regulated by the expression of FBLN5. It has been reported that cancer associated fibroblasts and ECM acted as “stromal” that could effectively activate immune cells and participate in tumorigenesis, progression, metastasis.14 However, the level of FBLN5 did not affect the survival time of KIRC adult patients using the publicly available database. We therefore further explored the impact of FBLN5 expression on the survival of KIRC patients with high or low immune cell infiltration, and the results revealed a preferred prognosis in patients with enriched basophils, CD4+ memory T cells, natural killer cells had a favorite prognosis. This indicated that FBLN5 is involved in the immune microenvironment in KIRC. Given the differences between the immune systems of children and adults, the immune systems of children and young adults are relatively underdeveloped. Therefore, it is necessary to investigate its relationship with the immune microenvironment and KIRC in children and young adults. Finally, we demonstrated that in children and young adults, FBLN5 was independently correlated with patient outcome. We found that FBLN5 expression affected the survival in children and young adults with KIRC, and that it might play a role in controlling the immune microenvironment in this study. To the best of our knowledge, the immunomodulatory role of FBLN5 has been first identified in children and young adults with KIRC.
Overall, FBLN5 may be useful as a biomarker for the diagnosis and prognosis of children and young adults with KIRC and is expected to provide novel targets for the treatment of this disease in the future. However, the present study has some limitations. First, the clinical sample sizes used were relatively small. Second, it should be performed in vitro and in vivo experiments to verify this. Finally, due to the long span of time and different treatment regimens, clinical data on immunotherapy have not been collected.
Conclusion
This study revealed a significantly reduction in FBLN5 expression in KIRC tissue and identified FBLN5 as a potential independent prognostic biomarker of KIRC in children and young adults. As a result, FBLN5 may be employed as a prognostic factor in clinical diagnosis and treatment of these cases.
Data Sharing Statement
Inquiries can be directed to the corresponding author: Dongsheng Zhu, email: [email protected].
Ethics Statement
The study involving human participants was reviewed and approved by The Ethics Committee of Lianyungang First Hospital approved this study (20220215). Written informed consent was obtained from the participants or guardians of patients younger than 18 years of age. And this study complies with all the rules.
Acknowledgments
Ming Zhang, Feng Chen and Shaoguang Feng are co-first authors and contributed equally to this work.
To whom give me a powerful spiritual support, and may lovers eventually become spouses.
Funding
This study was supported by Department of Health of Zhejiang Province (grant number 2022RC234) and Annual Scientific Project Fund of Xuzhou Children’s hospital (grant number 22040406).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Xia C, Dong X, Li H, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chinese Med J. 2022;135(5):584–590. doi:10.1097/CM9.0000000000002108
2. Xu X, Zhu D. Prognostic significance of subclassifying stage pT3a renal tumors with fat invasion: a retrospective study of 99 patients. J Int Med Res. 2021;49(8):3000605211033178. doi:10.1177/03000605211033178
3. Aldrink JH, Heaton TE, Dasgupta R, et al. Update on Wilms tumor. J Pediatr Surg. 2019;54(3):390–397. doi:10.1016/j.jpedsurg.2018.09.005
4. Muir TE, Cheville JC, Lager DJ. Metanephric adenoma, nephrogenic rests, and Wilms’ tumor: a histologic and immunophenotypic comparison. Am J Surg Pathol. 2001;25(10):1290–1296. doi:10.1097/00000478-200110000-00010
5. Capitanio U, Bensalah K, Bex A, et al. Epidemiology of Renal Cell Carcinoma. Europ urol. 2019;75(1):74–84. doi:10.1016/j.eururo.2018.08.036
6. Turajlic S, Swanton C, Boshoff C. Kidney cancer: the next decade. J Exp Med. 2018;215(10):2477–2479. doi:10.1084/jem.20181617
7. Motzer RJ. New perspectives on the treatment of metastatic renal cell carcinoma: an introduction and historical overview. oncologist. 2011;16(Suppl S2):1–3. doi:10.1634/theoncologist.2011-S2-01
8. Hammers H. Immunotherapy in kidney cancer: the past, present, and future. Curr Opinion Urol. 2016;26(6):543–547. doi:10.1097/MOU.0000000000000338
9. Schwartz M, Zhang Y, Rosenblatt JD. B cell regulation of the anti-tumor response and role in carcinogenesis. J Immunother Cancer. 2016;4(1):40. doi:10.1186/s40425-016-0145-x
10. Klümper N, Ralser DJ, Bawden EG, et al. LAG3 (LAG-3, CD223) DNA methylation correlates with LAG3 expression by tumor and immune cells, immune cell infiltration, and overall survival in clear cell renal cell carcinoma. J Immunother Cancer. 2020;8(1):e000552. doi:10.1136/jitc-2020-000552
11. Bruni D, Angell HK, Galon J. The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nat Rev Cancer. 2020;20(11):662–680. doi:10.1038/s41568-020-0285-7
12. Unterleuthner D, Neuhold P, Schwarz K, et al. Cancer-associated fibroblast-derived WNT2 increases tumor angiogenesis in colon cancer. Angiogenesis. 2020;23(2):159–177. doi:10.1007/s10456-019-09688-8
13. Zhuang J, Lu Q, Shen B, et al. TGFβ1 secreted by cancer-associated fibroblasts induces epithelial-mesenchymal transition of bladder cancer cells through lncRNA-ZEB2NAT. Sci Rep. 2015;5(1):11924. doi:10.1038/srep11924
14. Barrett RL, Puré E. Puré E: cancer-associated fibroblasts and their influence on tumor immunity and immunotherapy. eLife. 2020;9. doi:10.7554/eLife.57243
15. Argraves WS, Greene LM, Cooley MA, Gallagher WM. Fibulins: physiological and disease perspectives. EMBO Rep. 2003;4(12):1127–1131. doi:10.1038/sj.embor.7400033
16. Albig AR, Schiemann WP. Fibulin-5 antagonizes vascular endothelial growth factor (VEGF) signaling and angiogenic sprouting by endothelial cells. DNA Cell Biol. 2004;23(6):367–379. doi:10.1089/104454904323145254
17. Heo JH, Song JY, Jeong JY, et al. Fibulin-5 is a tumour suppressor inhibiting cell migration and invasion in ovarian cancer. J Clin Pathol. 2016;69(2):109–116. doi:10.1136/jclinpath-2015-203129
18. Hu Z, Ai Q, Xu H, et al. Fibulin-5 is down-regulated in urothelial carcinoma of bladder and inhibits growth and invasion of human bladder cancer cell line 5637. Urol Oncol. 2011;29(4):430–435. doi:10.1016/j.urolonc.2009.06.004
19. Chen X, Song X, Yue W, et al. Fibulin-5 inhibits Wnt/β-catenin signaling in lung cancer. Oncotarget. 2015;6(17):15022–15034. doi:10.18632/oncotarget.3609
20. Lee YH, Albig AR, Regner M, Schiemann BJ, Schiemann WP. Fibulin-5 initiates epithelial-mesenchymal transition (EMT) and enhances EMT induced by TGF-beta in mammary epithelial cells via a MMP-dependent mechanism. Carcinogenesis. 2008;29(12):2243–2251. doi:10.1093/carcin/bgn199
21. Topalovski M, Hagopian M, Wang M, Brekken RA. Hypoxia and transforming growth factor β cooperate to induce fibulin-5 expression in pancreatic cancer. J Biol Chem. 2016;291(42):22244–22252. doi:10.1074/jbc.M116.730945
22. Wu A, Kunju LP, Cheng L, Shah RB. Renal cell carcinoma in children and young adults: analysis of clinicopathological, immunohistochemical and molecular characteristics with an emphasis on the spectrum of Xp11.2 translocation-associated and unusual clear cell subtypes. Histopathology. 2008;53(5):533–544. doi:10.1111/j.1365-2559.2008.03151.x
23. Furge KA, Dykema K, Petillo D, et al. Combining differential expression, chromosomal and pathway analyses for the molecular characterization of renal cell carcinoma. Can Urol Assoc J. 2007;1(2 Suppl):S21–S27. doi:10.5489/cuaj.64
24. Kochevar J. A renal cell carcinoma neoplastic antigen detectable by immunohistochemistry is defined by a murine monoclonal antibody. Cancer. 1987;59(12):2031–2036. doi:10.1002/1097-0142(19870615)59:12<2031::AID-CNCR2820591211>3.0.CO;2-0
25. Zhu D, Xu X, Zhang M, Wang T. TPX2 regulated by miR-29c-3p induces cell proliferation in osteosarcoma via the AKT signaling pathway. Oncol Lett. 2022;23(5):143. doi:10.3892/ol.2022.13262
26. Zhu D, Zhu Z, Qi H. NEAT1/microRNA 339-5p/SPI1 axis feedback loop contributes to osteogenic differentiation in acute suppurative osteomyelitis in children. J Inflamm Res. 2023;16:2675–2687. doi:10.2147/JIR.S410339
27. Rigoglio NN, Rabelo ACS, Borghesi J, et al. The tumor microenvironment: focus on extracellular matrix. Adv Exp Med Biol. 2020;1245:1–38. doi:10.1007/978-3-030-40146-7_1
28. Anderson NM, Simon MC. The tumor microenvironment. Curr Biol. 2020;30(16):R921–R925. doi:10.1016/j.cub.2020.06.081
29. Ashraf R, Shah NP. Immune system stimulation by probiotic microorganisms. Crit Rev Food Sci Nutr. 2014;54(7):938–956. doi:10.1080/10408398.2011.619671
30. Ren Z, He Y, Yang Q, et al. A comprehensive analysis of the glutathione peroxidase 8 (GPX8) in human cancer. Front Oncol. 2022;12:812811. doi:10.3389/fonc.2022.812811
31. Obaya AJ, Rua S, Moncada-Pazos A, Cal S. The dual role of fibulins in tumorigenesis. Cancer Lett. 2012;325(2):132–138. doi:10.1016/j.canlet.2012.06.019
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.