Back to Journals » Infection and Drug Resistance » Volume 13
Fatal Prosthetic Valve Endocarditis Due to Aspergillus flavus in a Diabetic Patient
Authors Jalalian R, Shokohi T , Mirzakhani R, Ghasemian R , Hedayati MT, Ardalani S, Azizi S, Kalhori S, Kermani F , Mayahi S
Received 15 May 2020
Accepted for publication 26 June 2020
Published 10 July 2020 Volume 2020:13 Pages 2245—2250
DOI https://doi.org/10.2147/IDR.S258637
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Rozita Jalalian,1 Tahereh Shokohi,2,3 Roghayeh Mirzakhani,4 Roya Ghasemian,5 Mohammad Taghi Hedayati,2,3 Sirus Ardalani,6 Soheil Azizi,7 Shamsi Kalhori,7 Firoozeh Kermani,4 Sabah Mayahi3
1Department of Cardiology, Mazandaran University of Medical Sciences, Sari, Iran; 2Invasive Fungi Research Centre (IFRC), Mazandaran University of Medical Sciences, Sari, Iran; 3Department of Medical Mycology, School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran; 4Student Research Committee, School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran; 5Antimicrobial Resistance Research Center, and Department of Infectious Diseases, Mazandaran University of Medical Sciences, Sari, Iran; 6Department of Cardiac Surgery, Cardiovascular Research Center of Mazandaran Heart Center, Mazandaran University of Medical Sciences, Sari, Iran; 7Department of Pathology, Mazandaran Heart Center, Mazandaran University of Medical Sciences, Sari, Iran
Correspondence: Tahereh Shokohi
Department of Medical Mycology, School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran
Tel +98 11 3354-3087
Fax +98 11 3354-3248
Email [email protected]
Abstract: Aspergillus endocarditis (AE) accounts for a-quarter of all fungal endocarditis, mainly in immunocompromised hosts prior to heart-valve surgery with high mortality, even with treatment. Herein, we report a rare case of AE in a diabetic 60-year-old woman with a history of redo mitral valve prosthesis suspecious of acute endocarditis. She underwent second redo surgical mitral valve replacement in combination with mechanical aortic valve replacement. Blood cultures were negative. The explanted valve and vegetation were subjected to identification. Grown colonies were identified as Aspergillus flavus, based on conventional and molecular methods. Despite the administration of liposomal amphotericin B and improvement in her general condition shortly after initiation of therapy, the patient passed away. As AE is a late consequence of redo prosthetic valve replacement, extended follow-up, early diagnosis, repeating valve-replacement surgeries, and timely selective antifungal treatments are warranted.
Keywords: fungal endocarditis, Aspergillus, antifungal, prosthetic valve replacement
Introduction
Fungal endocarditis is an uncommon infection, representing less than 2% of all cases of endocarditis,1 and is commonly caused by Candida spp.2,3 Aspergillus endocarditis (AE) accounts for a-quarter of all fungal endocarditis, mainly in immunocompromised hosts with high mortality, even with treatment.4 In most AE cases, a predisposing factor is present. About 50% of cases have heart-valve surgery in their history,5 even without any classic risk factors or typical echo findings being reported.6 The most common sites of involvement are mitral and aortic valves.7 Due to negative blood cultures and aspecific clinical presentations, early diagnosis of such infections needs a high level of clinical suspicion. We report a rare case of AE in a diabetic patient with two redo operations for prosthetic valve dysfunction.
Case Report
On February 18, 2019, a 60-year-old woman with severe dyspnea, fine rales in the lung, tachycardia, and tachypnea was admitted at the emergency department of Heart Center of Mazandaran University of Medical Sciences, Sari, northern Iran. She suffered from multiple comorbidities, including diabetes mellitus, obesity (BMI 36.1 kg/m2), hypertension, and hypercholesterolemia. Her medical history revealed that she had been operated on 5 years ago for mitral valve (MV) replacement due to rheumatic heart disease. Laboratory data showed prothrombin time 18 seconds, international normalised ratio 2, white blood cells 4,000 /µL, and hemoglobin 12g/dL. Fluoroscopy evaluation showed that the motion of one leaflet of the prosthetic MV was fixed, while the other had severely restricted motion (Figure 1A). Transesophageal echocardiography demonstrated a mechanical prosthetic bileaflet MV, fixed medial leaflet MV, elevated MV mean pressure gradient (22 mmHg), pressure half-time (225 mL/s), MV left ventricular–outflow tract velocity time integral(48%), and a large obstructive mass (1.5×2 cm) on the prosthetic MV medial leaflet, which was highly suggestive of a large thrombus. Severe tricuspid regurgitation, mild–moderate aortic insufficiency, and severe pulmonary hypertension (pulmonary artery pressure 80 mmHg) was also present. Coronary angiography revealed normal arteries. Based on the hemodynamic condition of the patient (functional class IV) and large thrombus, urgent cardiothoracic surgery consultation took place, and she was a candidate for surgery. The thrombotic prosthetic MV was removed and replaced with another mechanical prosthetic MV (21 mm; CarboMedics, Austin, TX, USA) and tricuspid valve repair performed. Gross pathology showed a severe thick pannus formation with large thrombus on the prosthetic MV that caused malfunction (Figure 1B). Operation accomplished successfully and patient was discharged from the hospital with good condition.
On April 13, 2019, about 2 months after discharge, she went back to the emergency department with acute pulmonary edema refractory to vasodilator and diuretic therapy (Figure 1C). She also complained of fever and weakness for 2 weeks. Fluoroscopy showed normal leaflet motion. Chest X-ray revealed severe pulmonary venous congestion throughout the lungs, alveolar edema, and consolidation. She underwent transesophageal echocardiography (TEE). Upon reaching the TEE room, she complained of sudden vision loss in her right eye. TEE was done, and data revealed severe left ventricular systolic dysfunction (ejection fraction 30%), a bileaflet mechanical prosthetic MV, normal leaflet motion, a small mobile mass on the anteromedial swing ring, and severe aortic insufficiency. Due to destruction of the left coronary cusp of the aorta, the large size mobile mass (2.2×2 cm) protruded to the left ventricular outflow tract (vegetation mostly). Periaortic thickening with echo-free space around aorta (abscess) extended to the intervalvular fibrosa (Figure 2A and B). Noncontrast brain computed tomography showed hyperdense intracranial hemorrhage in the left parietal lobe. On the second day of admission (April 15, 2019), due to prosthetic MV and native aortic valve infective endocarditis with decompensated heart failure and after consultation with a neurologist, she underwent second emergency redo surgical MV repair in combination with mechanical aortic valve replacement and aortoplasties.
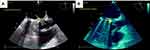 |
Figure 2 (A, B) Transesophageal echocardiography showed large vegetation (arrow) of aorta. |
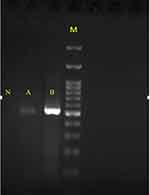
The explanted valve and vegetation were sent to the laboratory for further evaluation. Direct microscopic examination (KOH preparation, 20%) of tissue biopsies showed a mass of dichotomous branching hyphae (Figure 3A), which was confirmed by histopathology (periodic acid–Schiff staining, Figure 3B). Culture of biopsied tissue onto Sabouraud dextrose agar medium after a 24-hour incubation at 27°C yielded Aspergillus spp. On slide culture, the grown Aspergillus showed echinulate and hyaline conidiophores with globose to subglobose vesicles and uniseriate phialides. Conidia were globose to subglobose and echinulate (Figure 3C). On the basis of microscopic and macroscopic characteristics, the fungus was identified as Aspergillus section Flavi. Accurate identification of grown Aspergillus colonies was performed by polymerase chain reaction (PCR) assay using the two universal fungal primers Bt2a and Bt2b8 targeting the β-tubulin gene (Figure 4). Amplicons were sequenced and compared with the GenBank database (https://blast.ncbi.nlm.nih.gov/Blast.cgi) for final identification at species level. The isolate was assigned to A. flavus based on the complete identity with corresponding sequences of the A. flavus strain CBS 816.96. Then, they were submitted to GenBank and received the accession number MN097724. Prior to antifungal therapy, three consecutive blood samples were taken and inoculated with blood cultures containing brain–heart infusion (BHI) broth and BHI agar and processed. The blood cultures had no microbial growth after 2 weeks of incubation at 37°C. DNA was extracted from negative blood culture bottles using a QIAmp DNA blood minikit and subjected to β-tubulin gene amplification. The β-tubulin amplicon from the negative blood culture sequenced and its DNA-sequence data were in complete agreement with the DNA sequences of A. flavus strain CBS 816.96, similar to the aforementioned finding regarding the colony grown from the explanted valve (Figure 4). Liposomal amphotericin B at a dose of 5 mg/kg body weight/day (350 mg) together with broad-spectrum antibiotics (ciprofloxacin, meropenem, and vancomycin) were administered via a peripherally inserted venous catheter. On day 11 of admission, after 7 days’ antifungal therapy, the patient expired from presumed severe sepsis, probably related to progressive AE.
In our institution, antifungal susceptibility testing is not a routine workup; however, in vitro antifungal-susceptibility testing was performed according to document M38-A2 of the Clinical and Laboratory Standards Institute (CLSI)9 on Aspergillus isolates after the patient had died. Minimum inhibitory concentrations were obtained for amphotericin B(2 μg/mL), fluconazole (16 μg/mL), itraconazole (0.016 μg/mL), voriconazole (0.25 μg/mL), posaconazole (0.032 μg/mL), and minimum effective concentrations for caspofungin; (0.008 μg/mL, anidulafungin; (0.008 μg/mL and micafungin (0.008 μg/mL).This case report was performed in compliance with the Declaration of Helsinki, and written informed consent was obtained from the legal guardian for its details to be included in the manuscript and for publication. No institutional approval was required to publish the case details.
Discussion
Fungal endocarditis is a rare clinical entity. AE occurs in approximately 20%–30% of all fungal endocarditis cases, with an 80%–96% mortality rate.10−12 In a large literature review of fungal endocarditis spanning 1965– 1995, 97% of patients had at least one known risk factor, 54% of whom had had prior valve surgery as a key risk factor.11 In another case series, 74% of patients had history of cardiac valve surgery.13 In our patient, preexisting rheumatic heart disease and redo prosthetic valve replacement were the major predisposing factors. Other risk factors that have been suggested are use of broad-spectrum antibiotics, indwelling central venous catheters, and intravenous drug use.10,13 As in our patient, the aortic and MVs with large, brittle vegetation and higher risk of embolization are the most common sites of involvement.10 TEE can be a useful modality in detecting 77% of fungal endocarditis and 89% of AE.14 Due to the high mortality rate, empirical treatment and surgical intervention should be started immediately if there is clinical suspicion.
Aspergillus spp. are a ubiquitous saprophytic fungi found in outdoor and indoor environments, including hospitals, and can be a major cause of life-threatening invasive aspergillosis. The most common causative agent of AE has been reported as A. fumigatus (60%–90%).10 In a literature review covering 1995– 2000, 28 AE cases were found, which were mostly caused by A. fumigatus (54%), followed by A. terreus (18%), A. niger, and A. flavus (both 7%).10 Still, a comprehensive review indicated A. flavus as the prevalent Aspergillus spp. in invasive aspergillosis and even in environmental isolates from Iran.15
Since about 93% of blood cultures of AE are never positive,16 Aspergillus PCR of blood may help in rapid diagnosis.17 Due to uncertainty and lack of standardization, recommendations of PCR for routine use in clinical practice of patients with AE remains ambiguous.7 In cases of endocarditis with negative blood culture, for accurate and early diagnosis and ruling out fungal etiology, 1,3-β-D-glucans (BDG) and/or galactomannan (GM) are promising approaches for AE to initiate antifungal therapy prior to vulvar dysfunction, heart failure, and/or embolic phenomena.18 As a cell-wall biomarker of Aspergillus, GM has been widely used to diagnose invasive aspergillosis in neutropenic patients. Since the GM antigen is cleared by neutrophils, its usage in non-neutropenic patients is limited, due to low diagnostic value and high likelihood of false-negative results.19 In some immunocompetent cases, despite progressive aspergillosis and blood-vessel invasion, GM assays are negative.20 In a review of 20 AE cases, PCR was positive in 100%, serum BDG in 85.7% and GM in 62.5%, while in 20% of cases all these three tests were negative.21 However, in this study, these tests were not available.
In our case, accurate identification at the species level and antifungal-susceptibility testing were achieved. Due to rising azole-resistant infections and differences in antifungal-susceptibility profiles among strains in types and species of Aspergillus, performing this test is crucial for patient management.22 It should be noted that the CLSI has not proposed breakpoints for Aspergillus spp.9 However, minimum inhibitory concentrations for all antifungals were lower than the epidemiological cutoff value of 97.5% and classified as wild type according to CLSI M59.23 For treatment of AE, voriconazole 4 mg/kg twice daily as the first line is also recommended,7 and liposomal amphotericin B and posaconazole are the second- and third-line antifungals, respectively.24 Recently, combination-salvage therapies, such as voriconazole–anidulafungin, have been suggested for invasive aspergillosis.4 In our case, voriconazole was not available, due to availability and less toxicity (in comparison to the deoxycholate one), and liposomal amphotericin B had been started. Nonetheless, she expired after 7 days of antifungal treatment, despite the fact that her general condition was steadily improving shortly after initiation of therapy.
Conclusion
The present report highlights the occurrence of AE as a rare clinical entity usually related to a prosthetic valve and uncontrolled diabetes. This should bring the attention of physicians to the possibility of endocarditis in patients with recent cardiac surgery who present early with acute heart failure. In such a patient, echocardiographic assessment, prompt valve-replacement surgery (if feasible), PCR and molecular-based diagnostics, and early administration of appropriate antifungal treatment should improve gloomy prognoses.
Disclosure
The authors have no conflicts of interest to declare.
References
1. Antinori S, Ferraris L, Orlando G, et al. Fungal endocarditis observed over an 8-year period and a review of the literature. Mycopathologia. 2014;178(1–2):37–51. doi:10.1007/s11046-014-9754-4
2. Shokohi T, Nouraei SM, Afsarian MH, Najafi N, Mehdipour S. Fungal prosthetic valve endocarditis by Candida parapsilosis: a case report. Jundishapur. J Microbiol. 2014;7(3):e9428.
3. Kermani F, Shokohi T, Abastabar M, et al. Prosthetic valve endocarditis caused by multidrug-resistant Candida albicans in a patient with myelodysplasia syndrome: a case report and literature review. Curr Med Mycol. 2018;4(3):23–27. doi:10.18502/cmm.4.3.171
4. Sulik-Tyszka B, Kacprzyk P, Mądry K, Ziarkiewicz-Wróblewska B, Jędrzejczak W, Wróblewska M. Aspergillosis of the heart and lung and review of published reports on fungal endocarditis. Mycopathologia. 2016;181(7–8):583–588. doi:10.1007/s11046-016-0012-9
5. McCormack J, Pollard J. Aspergillus endocarditis 2003–2009. Med Mycol. 2011;49(Suppl.1):S30–S34.
6. Marinelli T, Kidd S, Shaw D, Rowe E. Aspergillus fumigatus endocarditis in an indigenous Australian male without classic risk factors. Med Mycol Case Rep. 2018;22:61–64. doi:10.1016/j.mmcr.2018.09.005
7. Patterson TF, Thompson GR, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the infectious diseases Society of America. Clin Infect Dis. 2016;63(4):e1–e60. doi:10.1093/cid/ciw326
8. Nasri T, Hedayati MT, Abastabar M, et al. PCR-RFLP on β-tubulin gene for rapid identification of the most clinically important species of Aspergillus. J Microbiol Methods. 2015;117:144–147. doi:10.1016/j.mimet.2015.08.007
9. Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi. 3rd Ed. CLSI Standard M38. Wayne, PA: Clinical and Laboratory Standards Institute; 2017.
10. Pierotti LC, Baddour LM. Fungal endocarditis, 1995–2000. Chest. 2002;122(1):302–310. doi:10.1378/chest.122.1.302
11. Ellis ME, Al-Abdely H, Sandridge A, Greer W, Ventura W. Fungal endocarditis: evidence in the world literature, 1965–1995. Clin Infect Dis. 2001;32(1):50–62. doi:10.1086/317550
12. Meshaal MS, Labib D, Said K, et al. Aspergillus endocarditis: diagnostic criteria and predictors of outcome, a retrospective cohort study. PLoS One. 2018;13(8):e0201459. doi:10.1371/journal.pone.0201459
13. McCormack J, Pollard J. Aspergillus endocarditis 2003–2009. Med Mycol. 2011;49(Suppl 1):S30–4.
14. Yuan SM. Fungal endocarditis. Braz J Cardiovasc Surg. 2016;31:252–255. doi:10.5935/1678-9741.20160026
15. Tavakoli M, Yazdani Charati J, Hedayati MT, et al. National trends in incidence, prevalence and disability-adjusted life years of invasive aspergillosis in Iran: a systematic review and meta-analysis. Expert Rev Respir Med. 2019;13(11):1121–1134. doi:10.1080/17476348.2019.1657835
16. Pasqualotto AC, Denning DW. Post-operative aspergillosis. Clin Microbiol Infect. 2006;12(11):1060–1076. doi:10.1111/j.1469-0691.2006.01512.x
17. Nabili M, Shokohi T, Janbabaie G, Hashemi-Soteh MB, Ali-Moghaddam K, Aghili SR. Detection of invasive aspergillosis in bone marrow transplant recipients using real-time PCR. J Glob Infect Dis. 2013;5(2):68–75. doi:10.4103/0974-777X.112296
18. Hatlen TJ, Filler SG, Bayer A, Shah S, Shodhan S, Van TT. Aspergillus endocarditis diagnosed by fungemia plus serum antigen testing. Med Mycol Case Rep. 2018;23:1–3. doi:10.1016/j.mmcr.2018.10.004
19. He H, Ding L, Chang S, Li F, Zhan Q. Value of consecutive galactomannan determinations for the diagnosis and prognosis of invasive pulmonary aspergillosis in critically ill chronic obstructive pulmonary disease. Med Mycol. 2011;49(4):345–351. doi:10.3109/13693786.2010.521523
20. Bajaj N, Chadha D, Hasija P, et al. Invasive aspergillosis presenting as an intracardiac mass in an immunocompetent host. Case Rep. 2016. doi:10.1136/bcr-2015-213205
21. Imbert S, Gauthier L, Joly I, et al. Aspergillus PCR in serum for the diagnosis, follow-up and prognosis of invasive aspergillosis in neutropenic and nonneutropenic patients. Clin Microbiol Infect. 2016;22(6):562–e1. doi:10.1016/j.cmi.2016.01.027
22. Taghizadeh-Armaki M, Hedayati MT, Ansari S, et al. Genetic diversity and in vitro antifungal susceptibility of 200 clinical and environmental Aspergillus flavus isolates. Antimicrob Agents Chemother. 2017;61(5):e00004–17. doi:10.1128/AAC.00004-17
23. Clinical and Laboratory Standards Institute (CLSI). Epidemiological Cutoff Values for Antifungal Susceptibility Testing. 2nd Ed. CLSI Supplement M59. Wayne, Pennsylvania: Clinical and Laboratory Standards Institute; 2018.
24. Pasha AK, Lee JZ, Low S-W, et al. Fungal endocarditis: update on diagnosis and management. Am J Med. 2016;129(10):1037–1043. doi:10.1016/j.amjmed.2016.05.012
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.