Back to Journals » Journal of Inflammation Research » Volume 15
Fatal COVID-19 is Associated with Reduced HLA-DR, CD123 or CD11c Expression on Circulating Dendritic Cells
Authors Hasan A , Al-Ozairi E , Hassan NYM, Ali S, Ahmad R , Al-Shatti N, Alshemmari S, Al-Mulla F
Received 27 January 2022
Accepted for publication 11 July 2022
Published 10 October 2022 Volume 2022:15 Pages 5665—5675
DOI https://doi.org/10.2147/JIR.S360207
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Monika Sharma
Amal Hasan,1 Ebaa Al-Ozairi,2,3 Nosiba YM Hassan,4 Shamsha Ali,5 Rasheed Ahmad,1 Nada Al-Shatti,6 Salem Alshemmari,3,7 Fahd Al-Mulla8
1Department of Immunology and Microbiology, Dasman Diabetes Institute, Dasman, Kuwait City, Kuwait; 2Clinical Research Unit, Dasman Diabetes Institute, Dasman, Kuwait City, Kuwait; 3Department of Medicine, Faculty of Medicine, Kuwait University, Kuwait City, Kuwait; 4Department of Internal Medicine, Jaber Al-Ahmad Hospital, Ministry of Health, Kuwait City, Kuwait; 5Special Services Facility, Dasman Diabetes Institute, Dasman, Kuwait City, Kuwait; 6Immunology & HLA Laboratory, Kuwait Cancer Control Center, Ministry of Health, Kuwait City, Kuwait; 7Department of Hematology, Kuwait Cancer Control Center, Ministry of Health, Kuwait City, Kuwit; 8Department of Genetics and Bioinformatics, Dasman Diabetes Institute, Dasman, Kuwait City, Kuwait
Correspondence: Amal Hasan, Department of Immunology and Microbiology; Dasman Diabetes Institute, Dasman, Kuwait, Tel +965 2224 2999 Ext. 4312, Fax +965 2249 2406, Email [email protected]
Purpose: Severe coronavirus disease 2019 (COVID-19) is linked to insufficient control of viral replication and excessive inflammation driven by an unbalanced immune response. Plasmacytoid dendritic cells (pDCs) are specialized in the rapid production of interferons in response to viral infections, and can also prime and activate T-cells. Conventional DCs (cDCs) are critical for the elimination of viral infections owing to their specialized ability to prime and activate T cells. We assessed the frequency and phenotype of pDCs and cDCs in survivors and non-survivors of COVID-19.
Patients and methods: Patients with COVID-19 were enrolled, and 22 were included in this study. Peripheral whole blood was obtained during the 2nd week of illness, stained with antibodies specific for lineage markers, human leukocyte antigen-DR isotype (HLA-DR), CD11c, and CD123, and analyzed by flow cytometry. Patients were followed-up during hospital admission and grouped into survivors (n=17) and non-survivors (n=5) of COVID-19.
Results: The ratio of pDCs to pre-cDCs was significantly lower (P=0.0005) in non-survivors compared to survivors. The frequency of pDCs was significantly higher than cDC2-like cells (P=0.0002) and pre-cDCs (P< 0.0001) in survivors but not in non-survivors. HLA-DR expression level on pDCs and cDC2-like cells was lower in non-survivors compared to survivors (P=0.02 and P=0.058, respectively), and HLA-DR was inversely correlated with disease severity rating (pDCs: r= – 0.47, P=0.027; cDC2-like cells: r= – 0.45, P=0.037). CD123 expression level on pDCs was significantly lower (P=0.038) in non-survivors compared to survivors, and CD123 was inversely correlated with disease severity rating (r=– 0.5, P=0.016). CD11c expression level on cDC2-like cells was significantly lower (P=0.03) in non-survivors compared to survivors, and CD11c was inversely correlated with disease severity rating (r=– 0.47, P=0.025).
Conclusion: A lower frequency of pDCs compared to other circulating DCs, and lower expression levels of HLA-DR, CD123 or CD11c on DCs is associated with fatal COVID-19.
Keywords: SARS-CoV-2, COVID-19, inflammation, innate immunity, plasmacytoid dendritic cells, conventional dendritic cells
Introduction
Coronavirus disease 2019 (COVID-19), caused by the novel coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), can range in severity from asymptomatic to fatal disease.1,2 In the vast majority of patients, the disease is characterized by flu-like symptoms, which resolves after elimination of the infection (first/only phase). However, some patients progress to a second phase where the disease becomes severe; the clinical sequelae include immune dysfunction, lymphopenia, sustained inflammation, secondary bacterial infection, acute respiratory distress syndrome (ARDS), coagulation activation, thrombosis, myocardial injury, and hepatic and kidney injury.3–13 Progression to severe/critical disease is linked to insufficient control of viral replication, persistent viral load, defective antiviral defense pathways, and excessive inflammation driven by an unbalanced immune response.1,2,7–12 Identification of the underlying cellular and molecular mechanisms is critical to guide the development of effective therapies.
Dendritic cells (DCs) are specialized antigen-presenting cells (APCs) that play a central role in priming and activating naïve T-cells.14 Mature DCs activate CD4+T-cells and CD8+T-cells by presenting antigens in association with major histocompatibility complex class II (MHC-II; also known as human leukocyte antigen-DR isotype, HLA-DR) and MHC-I molecules, respectively. Although antigenic peptides presented by MHC-II molecules are thought to be mainly generated from extracellular sources; intracellular antigens can also be presented by MHC-II molecules through non-classical pathways. In this regard, proteins within APCs can endogenously access the MHC-II pathway and their epitopes presented to CD4+T-cells.15,16
Human peripheral blood contains two main populations of DCs: plasmacytoid DCs (pDCs) and conventional DCs (cDCs).17 pDCs originate in the bone marrow, circulate in the blood, and migrate to secondary lymphoid organs, and sites of inflammation. pDCs are specialized in the production of large amounts of type I interferons (IFN-I) in response to viral infections, which exceeds those produced by any other cell type.18,19 This rapid IFN-I response is critical for antiviral immunity, which is also the case with SARS-CoV-2 infection. Indeed, the inborn errors of IFN-I immunity have been shown to be a major pathway involved in COVID-19 severity.20 Moreover, neutralizing autoantibodies against IFN-I is another major pathway leading to life-threatening COVID-19 pneumonia.21 cDCs, which link the innate and adaptive immune response, are also critical for the elimination of SARS-CoV-2 infection. This is primarily due to their specialized ability to prime and activate naïve T cells.22 In addition, pDCs have been suggested to function as APCs. pDCs can cross-present extracellular antigens coupled with MHC-I molecules to CD8+T-cells,23,24 and can also upregulate MHC-II molecules, acquire a mature dendritic phenotype, and present antigens to CD4+T-cells.15,16,25,26 Although the initial maturation of pDCs is induced through pathogen-derived signals,27,28 later in the immune response, antigen-specific T-cells (among other cell types) expedite the maturation of pDCs in their vicinity through secretion of interleukin (IL)-3. Interaction of IL-3 with its receptor CD123 on the surface of pDCs induces the cells to acquire a mature dendritic phenotype, and thus, an ability to present antigens to T-cells.29
In this study, we analyzed the frequency and phenotype of circulating DCs in survivors and non-survivors of COVID-19. We show previously unrecognized low expression levels of HLA-DR, CD123 or CD11c on circulating DCs, as well as reduced frequency of pDCs compared to other circulating DCs, in patients with fatal COVID-19.
Methods
Study Participants
The study was approved by the Ethical Review Committee of the Ministry of Health, Kuwait (1578/2020), and conducted in accordance with the ethical principles of the Declaration of Helsinki. The inclusion criteria included adults aged ≥21 years, a polymerase chain reaction (PCR)-confirmed diagnosis of COVID-19, and an ability to understand and give consent. The exclusion criteria included pre-existing autoimmune disease (except for type 1 diabetes), organ transplantation, use of steroids/immunosuppressants for longer than 1 month, vaccination against SARS-CoV-2, and any kind of acute infection/recent vaccination. Patients who fulfilled these criteria were enrolled at Jaber Al-Ahmad Hospital, Kuwait, and a total of 22 patients were included for this study. All patients were informed about the purpose of the study and consented to participate. COVID-19 severity was judged according to the Clinical Guidance of the Ministry of Health for the Management of Patients with Confirmed COVID-19. Peripheral blood was obtained during the 2nd week of illness, and patients were given a score based on their disease severity as shown in Table 1. The patients were followed-up during hospital admission and grouped into survivors (n = 17) and non-survivors (n = 5) of COVID-19. Comorbidities, COVID-19-related treatments, and clinical parameters/biomarkers of the study groups are shown in Table 2. Fatal cases were associated with acute respiratory distress syndrome, respiratory failure, and septic shock.
 |
Table 1 Disease Severity Judgement According to Clinical Guideline |
 |
Table 2 Comorbidities, COVID-19-related Treatments, and Clinical Parameters/Biomarkers of the Study Groups |
Clinical and Laboratory Measurements
The participant’s body weight (kg) and height (m) were measured, and the body mass index was calculated as weight/height2 (kg/m2). Peripheral blood samples were obtained, and plasma glucose, serum total cholesterol, triglycerides, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, alanine aminotransferase, alkaline phosphatase, aspartate aminotransferase, lactate dehydrogenase, and magnesium were measured using the AU5800 Clinical Chemistry Analyser (Beckman Coulter, USA). C-reactive protein was measured using IMMAGE. Vitamin D was measured using the Cobas 6000 Analyser (Roche Diagnostics, Switzerland). D-dimer was measured using the Stago Coagulation Analyzer (Sysmex, Japan). Troponin was measured using the DxI platform (Beckman Coulter, USA). Hemoglobin A1c was measured using the D-100 System (Bio-Rad Laboratories, USA). The white blood cell count and differentials were measured using the XN-1000 Haematology Analyser (Sysmex, USA). The erythrocyte sedimentation rate was measured using the VES-MATIC Analyser (Table 2).
Reagents
Mouse anti-human lineage cocktail (containing CD3 [FITC, clone SK7, IgG1], CD14 [FITC, clone MφP9, IgG2b], CD16 [FITC, clone 3G8, IgG1], CD19 [FITC, clone SJ25C1, IgG1], CD20 [FITC, clone L27, IgG1], and CD56 [FITC, clone NCAM16.2, IgG2b]; RUO (GMP); Cat. No. 340546), CD123 (IL-3Rα; PE, clone BALB/c, IgG1; RUO (GMP); Cat. No. 340545), HLA-DR (PerCP, clone L243, IgG2a; CE_IVD; Cat. No. 347402), HLA-DR (APC-H7, clone L243, IgG2a; CE_IVD; Cat. No. 641411), and CD11c (APC, clone SHCL-3, IgG2b; CE_IVD; Cat. No. 333144) were purchased from BD Biosciences. BD FACS™ Lysing Solution (10X; CE_IVD; Cat. No. 349202) and BD CellFIX™ (10X; CE_IVD; Cat. No. 340181) were purchased from BD Biosciences. Dulbecco’s phosphate-buffered saline (DPBS; Cat. No. 14190250) (1X) was purchased from ThermoFisher Scientific.
Immunofluorescence Staining of pDCs in Whole Blood
pDCs were stained in whole blood using clinical-grade fluorochrome-conjugated antibodies, as per manufacturer’s instructions. Briefly, 100μL of whole blood was added to 12x75-mm Falcon™ polystyrene test tubes, and then antibodies specific for lineage markers (CD3, CD14, CD16, CD19, CD20, CD56, FITC), HLA-DR (PerCP or APC-H7), CD11c (APC), and CD123 (PE) were added. Samples were incubated at room temperature in the dark for 15 minutes. After staining, whole blood was lysed in 1X BD FACS lysing solution at room temperature in the dark for 10 minutes, and then centrifuged at 300 X g for 5 minutes. Cells were washed in Dulbecco’s phosphate-buffered saline containing 0.1% sodium azide, fixed in 1X BD CellFix, and analyzed by flow cytometry within 18 hours.
Flow Cytometric Analysis
Data acquisition was performed on a FACSCanto II flow cytometer. A total of 150,000 cellular events were acquired from 0.1 mL of whole blood, and data were analysed using FlowJo software (Version 10.8.0). Gating strategy was as follows: Apoptotic cells and duplicates were excluded based on forward scatter and side scatter characteristics. The first gate was set on the lymphocyte and monocyte area, and the second gate was set on lineage− and HLA-DR+ cells. The pDCs were identified as Lineage−HLA-DR+CD123+CD11c− (Q3); the cDC2-like cells were identified as Lineage−HLA-DR+CD123+CD11c+ (Q2); the pre-cDCs were identified as Lineage−HLA-DR+CD123−CD11c+ (Q1) (Figure 1; and Supplementary Figure 1). Unstained cells and fluorescence minus one (FMO) controls were used to identify and gate positive populations.
Statistical Analysis
Statistical analysis was conducted using the GraphPad Prism software (Version 9.2.0; San Diego, CA, USA). To test whether data come from a “Gaussian Distribution”, the Shapiro–Wilk normality test was performed. Since some of the group data were not normally distributed, and given the small number of patients in each group, the non-parametric Mann–Whitney test or the Wilcoxon matched-pairs signed rank test was applied to compare between medians. The matched-pairs non-parametric Friedman test was applied for 3 group comparisons. Correlation analysis was conducted using the non-parametric Spearman’s r test. A P-value <0.05 was considered statistically significant.
Results
Analysis of Circulating DC Subsets Among Lin−HLA-DR+ DCs of Patients with COVID-19
The frequency and phenotype of circulating DCs was analysed in patients with COVID-19. Three main subsets of DCs were identified, these included CD123+CD11c− pDCs, CD123−CD11c+ pre-cDCs, and CD123+CD11c+ DCs. The latter expressed significantly (P = 0.0005, n = 21) higher levels of surface CD11c compared to CD123−CD11c+ pre-cDCs but similar (although slightly higher, P = 0.055 after removal of 3 outliers, ROUT (Q = 1%)) levels of surface CD123 to that expressed by CD123+CD11c− pDCs (Supplementary Figure 2A and B). Therefore, and since CD123+CD11c+ DCs expressed significantly (P<0.0001) higher levels of CD11c compared to CD123 (Supplementary Figure 2C), these cells were referred to as cDC2-like cells (Supplementary Figure 2C). There was no significant difference in the level of surface HLA-DR expression between CD123+CD11c− pDCs and CD123+CD11c+ cDC2-like cells; however, both cell types expressed significantly (P = 0.0005 and P = 0.0004, respectively, n = 21) higher levels of surface HLA-DR compared to pre-cDCs (Supplementary Figure 2D).
Fatal COVID-19 is Associated with Lower Frequency of pDCs During the 2nd Week of Illness
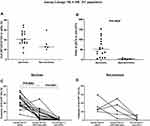
Individuals with COVID-19 were given a score based on disease severity as described in Table 1, and were as follows: asymptomatic disease (1), mild disease (2), moderate disease (3), severe disease (4), and fatal disease (5). The frequency of pDCs (among total Lineage−HLA-DR+ DC population) was lower in non-survivors (n=5) compared to survivors (n=17) (Figure 2A). After the removal of “likely outliers” (n=1 in the non-survivors group; ROUT, Q=5%), this difference reached statistical significance (P=0.0127). The ratio of pDCs to pre-cDCs was significantly (P=0.0005) lower in non-survivors compared to survivors (Figure 2B). In addition, the frequency of pDCs was significantly higher than cDC2-like cells (P=0.0002) and pre-cDCs (P<0.0001) in survivors; however, this was not the case in non-survivors (Figure 2C and D).
Fatal COVID-19 is Associated with Lower Expression Levels of HLA-DR on pDCs and cDC2-Like Cells During the 2nd Week of Illness
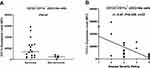
Upregulation of surface HLA-DR (as well as co-stimulatory molecules) expression by DCs is a sign of maturation, which is critical for DC function.15,16,25,26 We assessed the expression level of HLA-DR on circulating DCs in survivors and non-survivors. Our data showed that the expression level of HLA-DR on CD123+CD11c− pDCs was significantly (P=0.02) lower in non-survivors (n=5) compared to survivors (n=17). Similarly, the expression level of HLA-DR on CD123+CD11c+ cDC2-like cells was lower in non-survivors compared to survivors; however, this did not reach statistical significance (P=0.058) (Figure 3A and B). These findings were corroborated by data showing an inverse correlation between the level of HLA-DR expression on pDCs (r=–0.47, P=0.027, n=22), as well as cDC2-like cells (r=–0.45, P=0.037, n=22), and disease severity rating (Figure 3C and D).
Fatal COVID-19 is Associated with Lower Expression Levels of CD123 on pDCs and CD11c on cDC2-Like Cells During the 2nd Week of Illness
CD123 interaction with IL-3 is central to pDC biology.29 We evaluated the expression level of CD123 on pDCs and cDC2-like cells in survivors and non-survivors. Our data showed that the expression level of CD123 on pDCs was significantly (P=0.038) lower in non-survivors compared to survivors. However, this was not the case with cDC2-like cells (Figure 4A and B). These findings were corroborated by data showing an inverse correlation (r=–0.5, P=0.016, n=22) between the level of CD123 expression on pDCs, but not cDC2-like cells, and disease severity rating (Figure 4C and D). Interestingly, the expression level of CD11c on cDC2-like cells was significantly lower (P=0.03) in non-survivors compared to survivors. This was corroborated by data showing an inverse correlation (r=–0.47, P=0.025) between CD11c expression on cDC2-like cells and disease severity rating (Figure 5A and B).
Discussion
pDCs are specialized in the rapid production of large quantities of IFN-I in response to viral infections, which puts them at the forefront of antiviral immunity.18,19 pDCs can also upregulate MHC-II molecules, acquire a mature dendritic phenotype, and present antigens to naïve T-cells leading to their activation and proliferation.15,16,23–26 Although initial pDC maturation is induced through pathogen-derived signals,27,28 at a later stage, IL-3 (the ligand for CD123) is required to expedite the maturation of neighbouring pDCs in the microenvironment.29 Given the importance of pDCs in antiviral immunity, we investigated whether there are any differences in the frequency of circulating pDCs (and cDCs), as well as the expression level of HLA-DR (critical for antigen presentation to T-cells) and CD123 (IL-3 receptor that is central to pDC biology) on pDCs (as well as HLA-DR and CD11c expression on cDCs) among survivors and non-survivors of COVID-19.
Initially, the focus of our analysis was aimed at the two main DC subsets in whole blood, namely CD123+CD11c− pDCs and CD123−CD11c+ pre-cDCs/cDCs. However, we identified a double positive DC subset which expressed both CD123 and CD11c, with the latter molecule being very highly expressed. In fact, the CD123+CD11c+ DC subset expressed higher levels of CD11c and HLA-DR compared to CD123−CD11c+ pre-cDCs. Hence, the CD123+CD11c+ DC subset was referred to as cDC2-like cells thereafter. Our data showed that the frequency of pDCs was highest, followed by cDC2-like cells, and lastly, pre-cDCs in survivors of COVID-19; however, there was no such difference among circulating DCs in non-survivors.
Our data also showed that there was no difference in the frequency of pDCs in non-survivors compared to survivors; however, this was likely due to the low number of patients analyzed. Indeed, pDCs/pre-cDC ratio was lower in non-survivors compared to survivors; of note, this might have been a consequence of severe disease rather than the cause. Although reduced pDC frequency could be attributed to increased pDCs migration to the site of inflammation in the lung, this was unlikely since a recent study reported similar frequencies of pDCs in the circulation and lungs of patients with COVID-19.30
Furthermore, we found reduced expression levels of HLA-DR and CD123 on pDCs, as well as HLA-DR, CD123 (although both non-significant) and CD11c on cDC2-like cells, in non-survivors when compared to survivors. Similarly, correlation analysis showed that the expression levels of HLA-DR and CD123 on pDCs, as well as HLA-DR and CD11c on cDC2-like cells, were inversely correlated with disease severity rating. There could be several explanations for our findings. First, pDCs from non-survivors may inherently express lower levels of HLA-DR and CD123, thereby hindering pDC function. Second, pDCs (and potentially cDC2-like cells) may have failed to upregulate HLA-DR and acquire a mature phenotype, perhaps as a result of reduced IL-3/CD123 interaction. A reduction in CD123 expression would render pDCs less responsive to the biological effects of IL-3, and thus, less able to acquire a mature phenotype (as evidenced by the lower HLA-DR expression). This would ultimately hinder the ability of pDCs to prime and activate T-cells. Similarly, a reduction in IL-3, perhaps as a result of T-cell exhaustion, would have an even greater impact on these already compromised pDCs. In support of our findings, a recent study reported reduced IL-3 levels in patients with fatal COVID-19.30 This reduced IL-3 level could be due to lymphopenia, which is known to be associated with severe COVID-19.13 Moreover, since IL-3 is critical for pDC survival,29 the reduced frequency of pDCs (although non significant) and pDC/pre-cDC ratio in non-survivors could be due to suboptimal IL-3/CD123 interaction leading to increased pDC apoptosis.
Third, lower expression levels of HLA-DR (and possibly CD123) in non-survivors may suggest dampening of the immune response, which could be mediated by IL-10 (already shown to be high in severe COVID-19) and cortisol (stress response). In fact, IL-10 has been reported to downregulate HLA-DR on monocytes during systemic inflammation.31 In other words, downregulation of HLA-DR and CD123 on pDCs may have occurred as part of a compensatory anti-inflammatory response syndrome (CARS). CARS can act as a protective mechanism; however, when it is prolonged/extensive, it can become detrimental. In fact, a decrease in HLA-DR expression by monocytes has been reported to be indicative of CARS.32 Similarly, reduced HLA-DR expression on pDCs from non-survivors may suggest the development of CARS. Indeed, an unrelenting/prolonged inflammatory burst/cytokine storm may eventually result in downregulation of the immune system as a protective mechanism against overwhelming systemic inflammation. As such, it would be imperative to monitor HLA-DR expression on circulating DCs (as well as monocytes), particularly in patients treated with immunosuppressants (such as corticosteroids), which might exacerbate CARS and immunoparalysis. In our study, patients with moderate or severe disease accompanied by pneumonia were treated with glucocorticoid (dexamethasone), which included patients in both the survivors (41%) and non-survivors (60%) groups (1 patient in the non-survivors group was treated with hydrocortisol bringing the total percentage to 80%). Our findings are unlikely to have been due to glucocorticoid exacerbation of CARS; however, since the patients’ number was very small, further studies are required to assess this possibility. Monitoring HLA-DR and CD123 expression may help identify patients most at risk of CARS, and help inform/guide medical management.
Overall, our data show that having a lower pDCs/pre-cDCs ratio, as well as lower levels of HLA-DR and CD123 on pDCs, and HLA-DR and CD11c on cDC2-like cells, is associated with disease severity and outcome. Accordingly, we propose the use of these biomarkers to identify patients most at risk of CARS, and thus the use of corticosteroids with extra caution in such patients.
In further research, we intend to measure the levels of IL-3 and other inflammatory and anti-inflammatory cytokines, as well as explore the expression levels of specific markers of DC maturation (such as CD80, CD86, and CD83) using peripheral blood mononuclear cell (PBMC) samples from the same individuals. Further research may also be aimed at assessing the phenotype of the three DC subsets in more detail using more specific markers, such as AXL receptor tyrosine kinase, CD2, CD5, CD33, CD45RA, BDCA-2, BDCA-4, CC-chemokine receptor 7, and Sialic acid-binding Ig-like lectin 6. Critically, future research is required to elucidate whether immunosuppressant drugs used in the treatment of COVID-19 are associated with reduced HLA-DR, CD123, or CD11c expression on circulating DCs.
Acknowledgments
The project was funded by Kuwait Foundation for the Advancement of Sciences under project code: CORONA PROP 10. We especially thank all individuals who participated in the study. We also thank Dr. Ahmed Sobhy for his assistance with patient recruitment, Mr. Sohan Singh for his support in the laboratory, and the staff from Clinical Laboratory Department at Jaber Al-Ahmad Hospital for their support.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269.
2. Hasan A, Al-Ozairi E, Al-Baqsumi Z, Ahmad R, Al-Mulla F. Cellular and humoral immune responses in COVID-19 and immunotherapeutic approaches. Immunotargets Ther. 2021;10:63–85.
3. Zhang B, Zhou X, Qiu Y, et al. Clinical characteristics of 82 cases of death from COVID-19. PLoS One. 2020;15:e0235458.
4. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513.
5. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506.
6. Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069.
7. Wang F, Hou H, Luo Y, et al. The laboratory tests and host immunity of COVID-19 patients with different severity of illness. JCI Insight. 2020;5:e137799.
8. Pedersen SF, Ho YC. SARS-CoV-2: a storm is raging. J Clin Invest. 2020;130:2202–2205.
9. Yan R, Zhang Y, Li Y, et al. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444–1448.
10. Hadjadj J, Yatim N, Barnabei L, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–724.
11. Olagnier D, Farahani E, Thyrsted J, et al. SARS-CoV2-mediated suppression of NRF2-signaling reveals potent antiviral and anti-inflammatory activity of 4-octyl-itaconate and dimethyl fumarate. Nat Commun. 2020;11:4938.
12. Galani IE, Rovina N, Lampropoulou V, et al. Untuned antiviral immunity in COVID-19 revealed by temporal type I/III interferon patterns and flu comparison. Nat Immunol. 2021;22:32–40.
13. Huang I, Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. J Intensive Care. 2020;8:36.
14. Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811.
15. Bikoff E, Birshtein BK. T cell clones specific for IgG2a of the a allotype: direct evidence for presentation of endogenous antigen. J Immunol. 1986;137:28–34.
16. Eisenlohr LC, Hackett CJ. Class II major histocompatibility complex-restricted T cells specific for a virion structural protein that do not recognize exogenous influenza virus. Evidence that presentation of labile T cell determinants is favored by endogenous antigen synthesis. J Exp Med. 1989;169:921–931.
17. Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol. 2007;7:19–30.
18. Colonna M. Trinchieri G and Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–1226.
19. Reizis B, Colonna M, Trinchieri G. Barrat F and Gilliet M. Plasmacytoid dendritic cells: one-trick ponies or workhorses of the immune system? Nat Rev Immunol. 2011;11:558–565.
20. Zhang Q, Bastard P, Liu Z, et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370:eabd4570.
21. Bastard P, Rosen LB, Zhang Q, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370:eabd4585.
22. Wack A. Monocyte and dendritic cell defects in COVID-19. Nat Cell Biol. 2021;23:445–447.
23. Salio M, Palmowski MJ, Atzberger A. Hermans IF and Cerundolo V. CpG-matured murine plasmacytoid dendritic cells are capable of in vivo priming of functional CD8 T cell responses to endogenous but not exogenous antigens. J Exp Med. 2004;199:567–579.
24. Hoeffel G, Ripoche AC, Matheoud D, et al. Antigen cross presentation by human plasmacytoid dendritic cells. Immunity. 2007;27:481–492.
25. Sapoznikov A, Fischer JA, Zaft T, et al. Organ-dependent in vivo priming of naive CD4+, but not CD8+, T cells by plasmacytoid dendritic cells. J Exp Med. 2007;204:1923–1933.
26. Young LJ, Wilson NS, Schnorrer P, et al. Differential MHC class II synthesis and ubiquitination confers distinct antigen-presenting properties on conventional and plasmacytoid dendritic cells. Nat Immunol. 2008;9:1244–1252.
27. Colonna M. Krug A and Cella M. Interferon-producing cells: on the front line in immune responses against pathogens. Curr Opin Immunol. 2002;14:373–379.
28. Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145.
29. Janke M, Witsch EJ, Mages HW. Hutloff A and Kroczek RA. Eminent role of ICOS costimulation for T cells interacting with plasmacytoid dendritic cells. Immunology. 2006;118:353–360.
30. Benard A, Jacobsen A, Brunner M, et al. Interleukin-3 is a predictive marker for severity and outcome during SARS-CoV-2 infections. Nat Commun. 2021;12:1112.
31. Kim OY, Monsel A, Bertrand M, Coriat P, Cavaillon JM, Adib-Conquy M. Differential down-regulation of HLA-DR on monocyte subpopulations during systemic inflammation. Crit Care. 2010;14:R61.
32. Frazier WJ, Hall MW. Immunoparalysis and adverse outcomes from critical illness. Pediatr Clin North Am. 2008;55:647–68, xi.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.