Back to Journals » Neuropsychiatric Disease and Treatment » Volume 14
Factors related to improvement of symptoms, function, and caregiver burden in Chinese patients with schizophrenia after switching to paliperidone palmitate once-monthly from oral antipsychotics
Authors Li N , Feng Y, Lu HF, Cai SL, Zhuo JM, Si TM, Zhang LL
Received 28 November 2017
Accepted for publication 9 February 2018
Published 22 March 2018 Volume 2018:14 Pages 825—837
DOI https://doi.org/10.2147/NDT.S158353
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Wai Kwong Tang
Nan Li,1 Yu Feng,2 Huafei Lu,3 Shang Li Cai,3 Jianmin Zhuo,4 Tianmei Si,5,6 Lili Zhang3
1Research Center of Clinical Epidemiology, Peking University Third Hospital, Beijing, People’s Republic of China; 2Regional Medical Affairs, Janssen Pharmaceutical Companies of Johnson and Johnson, Singapore; 3Medical Affairs, Xian Janssen Pharmaceutical Ltd, Beijing, People’s Republic of China; 4Department of Statistics, Janssen China Research and Development, Shanghai, People’s Republic of China; 5National Clinical Research Center for Mental Disorders and The Key Laboratory of Mental Health, Ministry of Health (Peking University), Beijing, People’s Republic of China; 6Peking University Institute of Mental Health (The Sixth Hospital), Beijing, People’s Republic of China
Background: Paliperidone palmitate once-monthly (PP1M) demonstrated symptomatic and functional remission in patients with schizophrenia. This post hoc analysis aimed to identify factors associated with improved clinical outcomes in patients switching to PP1M (75–150 mg eq.).
Methods: The improved patient outcomes were observed as Positive and Negative Symptom Scale (PANSS, symptoms) score <70:66.7% (407/610), Personal and Social Performance (PSP, function) score >70:34.3% (199/581), and Involvement Evaluation Questionnaire (IEQ, caregiver burden) reduction ≥6:50.2% (270/538). Independent variables including demographics, disease duration, employment status, and clinical scores were screened individually using a univariate analysis and subsequently, variables (cutoff p<0.15) were analyzed using a multivariate regression analysis for association with better clinical outcomes at week 13.
Results: The factors significantly associated with favorable clinical outcomes were reduction in PANSS at week 5 (odds ratio [OR]=1.14, 95% CI=1.11–1.17) with symptom reduction; baseline PSP total score (OR=1.07, 95% CI=1.05–1.10), PSP change at week 5 (OR=1.07, 95% CI=1.05–1.10), PANSS reduction at week 5 (OR=1.06, 95% CI=1.03–1.08) with functional improvement, reduction in PANSS at week 5 (OR=1.02, 95% CI=1.01–1.03), and total IEQ score at baseline (OR=1.09, 95% CI=1.07–1.11) with caregiver burden reduction.
Conclusion: Thus, symptom and functional improvements with caregiver burden reduction were observed in patients, and PANSS reduction at week 5 was commonly associated with favorable outcomes.
Keywords: caregiver burden, clinical outcomes, post hoc analyses, psychosocial function, remission
Introduction
Management of schizophrenia, a chronic debilitating disorder, includes clinically meaningful improvement in symptoms along with improved social functioning.1 Impairments in interpersonal relations and daily living skills, and poor interactions in occupational, social, and community settings that reduce the patients’ quality of life are common features of schizophrenia.2,3 Although most antipsychotics improve the acute symptoms of schizophrenia within a couple of weeks, complete functional improvement requires long-term treatment.4–6 Several factors could contribute as predictors of treatment outcomes in a chronic multidimensional disorder such as schizophrenia. These factors include severity of disease at baseline, employment and financial status, disease duration, hospitalizations, medication dose, and disease- and medication-associated scores (adherence, satisfaction, and preference), and have been analyzed for their potential association with treatment outcomes.7–9
Management of psychotic symptoms along with meeting basic living needs can be an overwhelming burden for the patient and, hence, the need for caregiver assistance arises.10 Routine disturbances, emotional stress, as well as social and financial pressure escalate with severity of the patients’ symptoms and also contribute to reduction in the caregivers’ quality of life.11 This increased caregiver burden may impact treatment adherence as well as long-term outcomes in patients with chronic disease. Previous studies on caregiver burden were descriptive; however, some recent studies have attempted to measure caregiver burden objectively using validated instruments such as an Involvement Evaluation Questionnaire (IEQ), which evaluated factors affecting reduction of caregiver burden.12,13
Long-acting injectables (LAIs) were developed for schizophrenia treatment with the aim to increase adherence among patients by avoiding daily treatment with oral antipsychotics and having the advantage of reduced frequency of doses and administration with physician’s monitoring.14 Relapses within the first 5 years of onset of schizophrenia are a common observation.15 A recent literature review summarizes evidence of LAIs lowering the relapse rates when used in the treatment of first episode psychosis or recent-onset schizophrenia.16 LAIs are also recommended for relapse patients with a history of self-harm, self-neglect, or violence.17 A neuroprotective effect of promoting intracortical myelination, essential for delaying chronic disease progression, has been observed within a year of LAI therapy as compared with oral antipsychotics in patients with recent-onset schizophrenia.18
It is thus important for clinicians to not only evaluate whether LAIs could potentially be utilized as first-line therapy in patients with acute schizophrenia but also while switching from oral antipsychotics because of unsatisfactory response. Identification of factors influencing improvement in clinical outcomes might further aid the choice of therapy and facilitate informed decision-making for switching therapy (in the case of ineffective therapy).19
Paliperidone palmitate once-monthly (PP1M) LAI is approved for use globally in many countries and has demonstrated efficacy and safety in acute and long-term, randomized controlled studies.20–25 Additionally, patients switching to PP1M (due to poor adherence to previous oral antipsychotics) remained adherent as reported by follow-up studies carried out in naturalistic settings.26,27 In a primary study in patients with schizophrenia from People’s Republic of China, switching to PP1M from previously unsatisfactory oral antipsychotics demonstrated a reduction in schizophrenia symptoms and improved patient functioning, with a safety comparable to other global short-term studies.22,23,28 In the current post hoc analyses of the primary study, we aimed to explore the factors associated with improvement in clinical outcomes with PP1M therapy.
Methods
The current post hoc analyses are part of a multicenter, single-arm, open-label, prospective Phase IV study conducted in patients with schizophrenia. The methodology for this study has been described previously and is reviewed here briefly.28,29 The protocol of the current study was reviewed and approved by the Independent Ethics Committee of Peking University Sixth Hospital.
Patients
Adult patients from People’s Republic of China (18–65 years, inclusive), meeting Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) criteria for schizophrenia; with Positive and Negative Symptom Scale (PANSS) score 70–120 (inclusive) at baseline and screening; with stable disease and unsatisfactory response to previous oral antipsychotics, were enrolled. The major exclusion criteria were DSM-IV-TR Axis I diagnosis; severely suicidal or violent behavior (12 months before screening); history of paliperidone or risperidone allergy or resistance, and presence of any serious or unstable systemic disease.
The study protocol was approved by the local Institutional Review Board and the study was conducted in accordance with the ethical principles that originated in the Declaration of Helsinki, the International Conference on Harmonization and Good Clinical Practice guidelines, and applicable regulatory requirements. All participants provided written informed consent to participate in the study.
Study drug
The PP1M doses are expressed as milligram equivalent (mg eq.) wherein pharmacologically active paliperidone of 75, 100, and 150 mg eq. correspond to 117, 156, and 234 mg of paliperidone palmitate, respectively. PP1M was supplied as a suspension in a prefilled syringe.
Study design
This open-label study was conducted at 22 sites in the People’s Republic of China from October, 2012 to November, 2013. It consisted of three phases: screening phase (up to 7 days), acute treatment phase (13 weeks), and follow-up phase (1 year). Patients received the following treatment with PP1M: day 1, 150 mg eq.; day 8, 100 mg eq.; and later once-monthly flexible dosing of 75–150 mg eq.20
Study outcomes
The current post hoc analyses evaluated clinical improvement by PANSS total scores, psychosocial functioning by Personal and Social Performance (PSP) score, and caregiver burden by IEQ reduction. Better clinical outcomes were defined as PANSS total score <70, PSP total score >70, and IEQ reduction ≥6 after 13 weeks of treatment. The PANSS total score ≤60 (mildly ill condition) and PANSS ≤75 (moderately ill condition) were considered as a “gold-standard” in a study assessing symptomatic remission.30,31 The current study utilizes PANSS <70 (a score midway between the previously used scores) as representative of symptomatic reduction (mild-to-moderate severity). The rationale for utilizing PSP total score >70 was based on a previous study wherein this cutoff was indicative of good overall functioning corresponding to functional remission.7 IEQ reduction in total score of ≥6 was considered a reasonable estimate of lowering caregiver burden. The following factors influencing these outcomes were analyzed:
- Demographics and other factors – sex, age, employment status (full, temporary, or unemployed), monthly incomes, disease duration (classified as ≤3 or >3 years, >5 or ≤5 years),32,33 and dose of third injection (75, 100, or 150 mg eq.).
- Clinical scores – Medication Adherence Rating Scale (MARS) total score at baseline; Medication Preferences Questionnaire (MPQ) status at baseline with the first question analyzed – tablet or injection favored; Medication Satisfaction Questionnaire (MSQ) score at baseline (MSQ score of both patients and caregivers); Clinical Global Impression (CGI) score at baseline; IEQ total score at baseline; PSP total score at baseline, PSP change at week 5 and 13 as compared with baseline; PANSS total score at baseline and PANSS change at week 1, 5, and 13 from baseline.
Statistical analysis
The post hoc analyses were conducted on the full analysis set, comprising all patients who had at least one dose of PP1M and had at least one post-baseline efficacy assessment. As the last dose of PP1M was administered at week 13, the assessments conducted at this time were included. The outcomes were analyzed descriptively. For all the clinical scores, mean (SD) or median (range) values and for categorical data, number, percentage, or ratios were summarized. The hypothesis was two-sided with p<0.05 considered to be significant. Odds ratio (OR) and p-values were calculated to determine the association of different factors with clinical outcomes.
Symptoms (PANSS), functionality (PSP), and caregiver burden (IEQ) scores
The PANSS score was analyzed at baseline, weeks 1, 5, 9, and 13; PSP was analyzed at baseline, weeks 5 and 13; and IEQ was analyzed at baseline and week 13. The data were summarized based on better clinical outcomes using the last observation carried forward approach.
Better clinical outcomes
The factors were summarized descriptively. Comparison among groups (PANSS <70 vs ≥70, PSP >70 vs ≤70 and IEQ reduction ≥6 vs <6) for demographics and other factors was done using chi-square test or Fisher’s exact probability test. For clinical scores, comparisons were done using analysis of covariance or by Wilcoxon rank sum test.
Factors associated with better clinical outcomes
The factors were considered as single independent variables and were evaluated for better clinical outcomes as response variables. Initially, a univariate analysis screen was applied and independent variables with p<0.15 as candidate variables were tested in the multivariate logistic regression analyses model (Tables S1–S3 and Box S1). A multivariate logistic regression analysis was performed using the backward elimination method to determine the variables associated with the response. The 95% CIs along with the p-values were also calculated.
Results
Patient disposition and characteristics
Detailed efficacy and safety analysis of PP1M in this study has been published previously.28,29 Of the 616 patients from People’s Republic of China enrolled in the study, 610 were part of the full analysis set. The proportion of men (55.1%) was higher than women (44.9%). The mean (SD) age was 31.5 (10.85) years and body mass index was 23.22 (3.77) kg/m2.
Better clinical outcomes
During the course of treatment, PANSS scores on average displayed improvement with a gradual decrease toward <70 from baseline (mean [SD], 91.83 [12.54]) to week 13 (60.88 [19.74]). At week 13, 407 of the 610 patients presented with PANSS score <70 (66.7%) and PANSS reduction rate of ≥30% (73.4%). The PSP scores improved from baseline (44.92 [13.65]) to week 13 (64.11 [13.63]) with PSP total score >70 in 34.3% of the patients (199/581) and PSP change of ≥10% in 69.7% patients. The IEQ scores improved from baseline (44.92 [13.65]) to week 13 (23.72 [12.75]). In total, 50.2% patients (270/538) had a reduction in the IEQ score (≥6) (Table 1).
Factors influencing improvements in symptoms, function, and caregiver burden
Factors affecting clinical symptoms
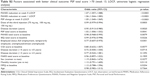
At week 13, there was a significant difference between the patient group with PANSS <70 vs PANSS ≥70 with regard to factors such as disease duration (p=0.0123), disease duration ≤3 years vs >3 years (p=0.0228), dose of third injection (p=0.0008), MSQ score of patients (p=0.0261), CGI score (p=0.0008), PSP total score of patients (p=0.0080), PSP change at week 5 (p<0.0001), PANSS total score (p<0.0001), and PANSS change at weeks 1 and 5 (both p<0.0001) (Table 2). Though many factors were significantly different between the two groups, further multiple regression analysis revealed that only PANSS reduction at week 5 associated with PANSS score <70 (OR=1.14, 95% CI=1.11–1.17, p<0.0001). PANSS total score at baseline and disease duration (>3 years vs ≤3 years) associated with less probability of PANSS score <70 (Table 3).
  | Table 3 Factors associated with better clinical outcomes: PANSS <70, PSP >70, and IEQ reduction ≥6 (week 13, LOCF, multivariate logistic regression analysis*) |
Factors affecting psychosocial function
Disease duration (p=0.0129), dose of third injection (p=0.0011), CGI score (p=0.0334), IEQ total score (p=0.0438), PSP total score (p=0.0049), PSP change at week 5 (p<0.0001), PANSS total score (p=0.0051), and its change at weeks 1 and 5 (both p<0.0001) were significantly different between the PSP >70 and PSP ≤70 groups at week 13 (Table 4). Multiple regression analyses of factors influencing PSP >70 demonstrate that PSP total score at baseline (OR=1.07, 95% CI=1.05–1.10, p<0.0001), its change at week 5 (OR=1.07, 95% CI=1.05–1.10, p<0.0001), and PANSS reduction at week 5 (OR=1.06, 95% CI=1.03–1.08, p<0.0001) associated with PSP >70. PANSS total score at baseline had no effect on the PSP outcome (Table 3).
Factors affecting caregiver burden
Factors such as disease duration (p=0.0460), disease duration >3 years vs ≤3 years (p=0.0366), CGI score (p=0.0099), IEQ total score (p<0.0001), PSP change at week 13 (p=0.0001), and PANSS change at week 1 (p=0.0061) and week 5 (p=0.0027) were significantly different between the groups with IEQ reduction (five-classification data) ≥6 and <6 (Table 5). Factors significantly associated with reduction of IEQ ≥6 based on multiple regression analysis include the total IEQ score at baseline (OR=1.09, 95% CI=1.07–1.11, p<0.0001) and PANSS reduction at week 5 (OR=1.02, 95% CI=1.01–1.03, p=0.0056) (Table 3).
Discussion
The current post hoc analyses were aimed at determining the factors associated with better clinical outcomes of symptoms (PANSS <70), psychosocial function (PSP >70), and caregiver burden (IEQ reduction ≥6) in patients with schizophrenia from People’s Republic of China switching from oral antipsychotics to PP1M. Overall, the analyses demonstrated an improvement in all of these outcomes at week 13. Factors such as disease duration, CGI score, and PANSS change at weeks 1 and 5 differed significantly between the patient groups (PANSS <70 vs ≥70, PSP >70 vs ≤70, IEQ reduction ≥6 vs <6; p<0.05 for all). There was a significant difference between PANSS <70 vs ≥70 and PSP >70 vs ≤70 patient groups with respect to the factor of dose of the third injection (PP1M monthly maintenance dose, p<0.05 for all) probably as the lower dose of the third injection was administered in patients with less severity of disease. A significant difference between PANSS <70 vs ≥70 and IEQ reduction ≥6 vs <6 patient groups was also observed with respect to the factor of disease duration >3 years vs ≤3 years (p<0.05 for all). Multivariate regression analyses indicate that factors such as shorter disease duration (≤3 years), PSP total score at baseline, PSP change at week 5, IEQ total score at baseline, and PANSS reduction at week 5 are all associated with good clinical outcomes. Specifically, PANSS reduction at week 5 was the only common factor associated with favorable response in clinical outcomes of symptoms, function, and caregiver burden.
Previous pharmacokinetic studies demonstrated that PP1M achieves therapeutic, steady-state plasma levels rapidly on initiation without the necessity of oral supplementation.34 Early symptomatic improvement (within a few days) observed with PP1M therapy may be attributed to its unique pharmacokinetics, though the complete therapeutic effect requires several weeks (eg, longer time to relapse with PP1M compared to oral antipsychotics).24,35–38 These results are consistent with the association of PANSS reduction at week 5 on better symptomatic outcomes observed in the current study. An analysis of 12 studies also identified early symptomatic improvement as one of the predictors of symptomatic remission, thus corroborating the findings of the current study.39 The current analyses also demonstrated that PANSS reduction at week 5 was associated with an improvement in the mean PSP score >70. The correlation between clinical and functional improvement was consistent with findings from other studies, wherein PANSS total score was established as the best predictor of improved functioning7 and PANSS subscores were a contributing factor in PSP total score improvements in patients switching from oral antipsychotics to risperidone LAI.40
The role of the caregiver is important in chronic diseases; however, of the many studies in patients with schizophrenia, relatively few focus on the influence of caregiver burden on therapy.12 The analyses in this study demonstrated that caregiver burden is reduced at week 13 and is associated with reduction in PANSS score at week 5. Consistent results were observed in a study from People’s Republic of China, which reported that patient functioning and PANSS score were good predictors of caregiver burden, in patients with schizophrenia.41 Thus, PANSS reduction at week 5 has a significant association with symptoms, function, and caregiver burden in a short period of 13 weeks with PP1M therapy. Hence, it becomes increasingly important that short-term efficacy of all LAIs be investigated for their consideration as first-line therapy in patients with schizophrenia.
Studies have revealed that the initial few years, post–schizophrenia diagnosis, are crucial for therapeutic intervention to achieve desirable long-term outcomes, as beyond this period, schizophrenia symptoms are refractory.42,43 Therefore, previous studies applied a 5-year cutoff to distinguish between recent and chronic schizophrenia. However, recently, studies have utilized a 3-year cutoff period to analyze efficacy of LAIs.32 This cutoff was based on a 15-year study following the natural course of schizophrenia in patients, which revealed that disease chronicity increases gradually up to four episodes (after which the disease is established as chronic)44 and the characterization of these patients revealed an average illness duration of 3 years.33 The current study utilized both these cutoff periods (3 years and 5 years) for the analysis to identify the time period influencing better clinical outcomes on PP1M therapy. Multivariate regression analysis revealed that the duration of disease ≤3 years has a significant association with symptomatic remission, thus corroborating the data from the other studies.45,46
Clinical outcome reporting routinely focuses on improvement rates from a specific baseline to the end of treatment.23,47,48 However, in the real-world setting, variability in the baseline of individual patients limits the evaluation of achievement of these clinical goals based on improvement rate. Instead, threshold values of scales depicting current patient status as used in this study are considered more appropriate. However, the short duration of this study also restricts its predictive value for long-term therapy to assess symptoms, functional, and caregiver burden improvement. Schizophrenia management can be complex because various factors such as age, employment, disease duration, disease severity at baseline, medication adherence, and medication preference may affect disease prognosis. Based on the limited evidence available in published literature, clinical outcomes were selected for the current analysis;7,30,31 however, there is a lack of consensus on clinical scores most appropriate for the identification of better clinical outcomes.49,50 The cutoff for better clinical outcomes identified in the current study requires further statistical validation in other studies. Additionally, the current study is restricted only to PP1M therapy and, hence, the findings cannot be extrapolated for comparison with other antipsychotics.
Recent evidence and guidelines suggest that LAIs must also be considered earlier in therapy.51,52 The current study adds to this body of growing evidence for the consideration of PP1M therapy in the acute phase, as early improvement in symptomatic and functional outcomes were demonstrated. To the best of our knowledge, there are no previous reports wherein factors associated with clinical outcomes and caregiver burden were analyzed in patients switching over to PP1M from oral antipsychotics.
Conclusions
The findings of these analyses reveal that significant improvements in symptoms, functionality, and caregiver burden were observed with PP1M treatment in patients with schizophrenia from People’s Republic of China switching from oral antipsychotics. Demographic factors, dose of third injection, and MARS, MSQ, and MPQ scores were not significantly associated with the better clinical outcomes discussed here. The PANSS reduction at week 5 was commonly associated with all favorable outcomes in these patients.
Acknowledgments
Dr Sonia Philipose (SIRO Clinpharm Pvt. Ltd.) provided writing assistance and Dr Ellen Baum (Janssen Research and Development, LLC) provided additional editorial support for this manuscript. The authors also thank the study participants, without whom this study would never have been accomplished, and the investigators for their participation in this study. This study was supported by funding from Janssen Research and Development, People’s Republic of China.
Author contributions
YF and TMS were involved in study design and NL, TMS, and YF were the lead scientists for the study, contributing to analysis of samples and data interpretation. YF, TMS, HFL, LLZ, and SLC were clinical leads for the study, and were also involved in data interpretation. JMZ and NL were the project statisticians. All authors had access to the study data, contributed to the data interpretation for the results, provided direction and comments on the manuscript, made the final decision about where to publish these data, and approved submission to the journal.
Disclosure
Nan Li has been a consultant and/or advisor to Janssen Research and Development (Beijing), Lundbeck, Astellas, Sanofi, Merck Serono, and Hisun Pfizer. He received honoraria and/or grant support from Janssen Research and Development (Beijing), Lundbeck, Astellas, Sanofi, Merck Serono, and Hisun Pfizer. Tianmei Si has been a consultant and/or advisor to Janssen Research and Development (Beijing); Pfizer, Lundbeck, and Otsuka. She received honoraria and/or grant support from Janssen Research and Development (Beijing), Lundbeck, Pfizer, and Otsuka. Jianmin Zhuo is from Janssen Research and Development, Shanghai, People’s Republic of China. Feng Yu, Zhang Lili, and Huafei Lu are from Janssen Research and Development, Beijing, People’s Republic of China. Shang Li Cai was an employee of Janssen Research and Development, Beijing, People’s Republic of China at the time of this analysis. The authors report no other conflicts of interest in this work.
References
Lysaker PH, Roe D, Buck KD. Recovery and wellness amidst schizophrenia: definitions, evidence, and the implications for clinical practice. J Am Psychiatr Nurses Assoc. 2010;16(1):36–42. | ||
Best MW, Gupta M, Bowie CR, Harvey PD. A longitudinal examination of the moderating effects of symptoms on the relationship between functional competence and real world functional performance in schizophrenia. Schizophr Res Cogn. 2014;1(2):90–95. | ||
Valencia M, Fresán A, Barak Y, Juárez F, Escamilla R, Saracco R. Predicting functional remission in patients with schizophrenia: a cross-sectional study of symptomatic remission, psychosocial remission, functioning, and clinical outcome. Neuropsychiatr Dis Treat. 2015;11:2339–2348. | ||
Ventura J, Subotnik KL, Guzik LH, et al. Remission and recovery during the first outpatient year of the early course of schizophrenia. Schizophr Res. 2011;132(1):18–23. | ||
Juckel G, Morosini PL. The new approach: psychosocial functioning as a necessary outcome criterion for therapeutic success in schizophrenia. Curr Opin Psychiatry. 2008;21(6):630–639. | ||
Nasrallah HA, Targum SD, Tandon R, McCombs JS, Ross R. Defining and measuring clinical effectiveness in the treatment of schizophrenia. Psychiatr Serv. 2005;56(3):273–282. | ||
Pinna F, Tusconi M, Bosia M, Cavallaro R, Carpiniello B; Cagliari Recovery Group Study. Criteria for symptom remission revisited: a study of patients affected by schizophrenia and schizoaffective disorders. BMC Psychiatry. 2013;13(1):235. | ||
Suttajit S, Arunpongpaisal S, Srisurapanont M, et al. Psychosocial functioning in schizophrenia: are some symptoms or demographic characteristics predictors across the functioning domains? Neuropsychiatr Dis Treat. 2014;11:2471–2477. | ||
Jelastopulu E, Giourou E, Merekoulias G, Mestousi A, Moratis E, Alexopoulos EC. Correlation between the Personal and Social Performance scale (PSP) and the Positive and Negative Syndrome Scale (PANSS) in a Greek sample of patients with schizophrenia. BMC Psychiatry. 2014;14(1):197. | ||
Awad AG, Voruganti LN. The burden of schizophrenia on caregivers. Pharmacoeconomics. 2008;26(2):149–162. | ||
Caqueo-Urízar A, Gutiérrez-Maldonado J, Miranda-Castillo C. Quality of life in caregivers of patients with schizophrenia: a literature review. Health Qual Life Outcomes. 2009;7(1):84. | ||
Schene AH, van Wijngaarden B, Koeter MW. Family caregiving in schizophrenia: domains and distress. Schizophr Bull. 1998;24(4):609–618. | ||
Gopal S, Xu H, McQuarrie K, et al. Caregiver burden in schizophrenia: pooled analysis of the involvement evaluation questionnaire data for paliperidone palmitate 3-month formulation. Value Health. 2016;19(3):A191. | ||
Simpson GM. A brief history of depot neuroleptics. J Clin Psychiatry. 1984;45(5 Pt 2):3–4. | ||
Robinson D, Woerner MG, Alvir JM, et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 1999;56(3):241–247. | ||
Stevens GL, Dawson G, Zummo J. Clinical benefits and impact of early use of long-acting injectable antipsychotics for schizophrenia. Early Interv Psychiatry. 2016;10(5):365–377. | ||
Correll CU, Citrome L, Haddad PM, et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry. 2016;77(Suppl 3):1–24. | ||
Bartzokis G, Lu PH, Amar CP, et al. Long acting injection versus oral risperidone in first-episode schizophrenia: differential impact on white matter myelination trajectory. Schizophr Res. 2011;132(1):35–41. | ||
Kirson NY, Weiden PJ, Yermakov S, et al. Efficacy and effectiveness of depot versus oral antipsychotics in schizophrenia: synthesizing results across different research designs. 2013;74(6):568–575. | ||
Janssen Pharmaceuticals. INVEGA SUSTENNA® (paliperidone palmitate) extended release injectable suspension [prescribing information]. 2017; Available from: https://www.invegasustenna.com/important-product-information. Accessed August 22, 2017. | ||
Kramer M, Litman R, Hough D, et al. Paliperidone palmitate, a potential long-acting treatment for patients with schizophrenia. Results of a randomized, double-blind, placebo-controlled efficacy and safety study. Int J Neuropsychopharmacol. 2010;13(5):635–647. | ||
Gopal S, Hough DW, Xu H, et al. Efficacy and safety of paliperidone palmitate in adult patients with acutely symptomatic schizophrenia: a randomized, double-blind, placebo-controlled, dose-response study. Int Clin Psychopharmacol. 2010;25(5):247–256. | ||
Nasrallah HA, Gopal S, Gassmann-Mayer C, et al. A controlled, evidence-based trial of paliperidone palmitate, a long-acting injectable antipsychotic, in schizophrenia. Neuropsychopharmacology. 2010;35(10):2072–2082. | ||
Gopal S, Vijapurkar U, Lim P, Morozova M, Eerdekens M, Hough D. A 52-week open-label study of the safety and tolerability of paliperidone palmitate in patients with schizophrenia. J Psychopharmacol. 2011;25(5):685–697. | ||
Hough D, Gopal S, Vijapurkar U, Lim P, Morozova M, Eerdekens M. Paliperidone palmitate maintenance treatment in delaying the time-to-relapse in patients with schizophrenia: a randomized, double-blind, placebo-controlled study. Schizophr Res. 2010;116(2–3):107–117. | ||
Attard A, Olofinjana O, Cornelius V, Curtis V, Taylor D. Paliperidone palmitate long-acting injection-prospective year-long follow-up of use in clinical practice. Acta Psychiatr Scand. 2014;130(1):46–51. | ||
Taylor DM, Sparshatt A, O’Hagan M, Dzahini O. Paliperidone palmitate: factors predicting continuation with treatment at 2 years. Eur Neuropsychopharmacol. 2016;26(12):2011–2017. | ||
Si T, Zhang K, Tang J, et al. Efficacy and safety of flexibly dosed paliperidone palmitate in Chinese patients with acute schizophrenia: an open-label, single-arm, prospective, interventional study. Neuropsychiatr Dis Treat. 2015;11:1483–1492. | ||
Si T, Fan J, Wang X, et al. A subgroup analysis of Chinese patients switched to paliperidone palmitate one-month injectable by prior oral antipsychotic treatment. Pharmacopsychiatry. 2016;49(1):32–41. | ||
Opler MG, Yang LH, Caleo S, Alberti P. Statistical validation of the criteria for symptom remission in schizophrenia: preliminary findings. BMC Psychiatry. 2007;7(1):35. | ||
Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean? Schizophr Res. 2005;79(2–3):231–238. | ||
Rosen K, Garety P. Predicting recovery from schizophrenia: a retrospective comparison of characteristics at onset of people with single and multiple episodes. Schizophr Bull. 2005;31(3):735–750. | ||
Dubois V, Peuskens J, Geerts P, Detraux J. Clinical outcomes of long-acting risperidone in recent versus long-term diagnosed Belgian schizophrenic patients: results from electronic Schizophrenia Treatment Adherence Registry (e-STAR) and Trial for the Initiation and Maintenance Of REmission in Schizophrenia with risperidone (TIMORES). Early Interv Psychiatry. 2014;8(1):39–49. | ||
Si T, Su Y, Liu Y, et al. Pharmacokinetics and tolerability of paliperidone palmitate injection in Chinese subjects. Hum Psychopharmacol. 2014;29(2):203–210. | ||
Bishara D. Once-monthly paliperidone injection for the treatment of schizophrenia. Neuropsychiatr Dis Treat. 2010;6:561–572. | ||
Schreiner A, Aadamsoo K, Altamura AC, et al. Paliperidone palmitate versus oral antipsychotics in recently diagnosed schizophrenia. Schizophr Res. 2015;169(1–3):393–399. | ||
Alphs L, Bossie CA, Sliwa JK, Ma YW, Turner N. Onset of efficacy with acute long-acting injectable paliperidone palmitate treatment in markedly to severely ill patients with schizophrenia: post hoc analysis of a randomized, double-blind clinical trial. Ann Gen Psychiatry. 2011;10(1):12. | ||
Bossie CA, Sliwa JK, Ma YW, Fu DJ, Alphs L. Onset of efficacy and tolerability following the initiation dosing of long-acting paliperidone palmitate: post-hoc analyses of a randomized, double-blind clinical trial. BMC Psychiatry. 2011;11(1):79. | ||
Lambert M, Karow A, Leucht S, Schimmelmann BG, Naber D. Remission in schizophrenia: validity, frequency, predictors, and patients’ perspective 5 years later. Dialogues Clin Neurosci. 2010;12(3):393–407. | ||
Gharabawi G, Bossie C, Turkoz I, Kujawa M, Mahmoud R, Simpson G. The impact of insight on functioning in patients with schizophrenia or schizoaffective disorder receiving risperidone long-acting injectable. J Nerv Ment Dis. 2007;195(12):976–982. | ||
Tang VW, Leung SK, Lam LC. Clinical correlates of the caregiving experience for Chinese caregivers of patients with schizophrenia. Soc Psychiatry Psychiatr Epidemiol. 2008;43(9):720–726. | ||
Lieberman JA, Perkins D, Belger A, et al. The early stages of schizophrenia: speculations on pathogenesis, pathophysiology, and therapeutic approaches. Biol Psychiatry. 2001;50(11):884–897. | ||
McGorry PD, Killackey E, Yung A. Early intervention in psychosis: concepts, evidence and future directions. World Psychiatry. 2008;7(3):148–156. | ||
Wiersma D, Nienhuis FJ, Slooff CJ, Giel R. Natural course of schizophrenic disorders: a 15-year followup of a Dutch incidence cohort. Schizophr Bull. 1998;24(1):75–85. | ||
Canuso CM, Bossie CA, Amatniek J, Turkoz I, Pandina G, Cornblatt B. Paliperidone extended-release tablets in patients with recently diagnosed schizophrenia. Early Interv Psychiatry. 2010;4(1):64–78. | ||
Macfadden W, Bossie CA, Turkoz I, Haskins JT. Risperidone long-acting therapy in stable patients with recently diagnosed schizophrenia. Int Clin Psychopharmacol. 2010;25(2):75–82. | ||
Pandina G, Lane R, Gopal S, et al. A double-blind study of paliperidone palmitate and risperidone long-acting injectable in adults with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(1):218–226. | ||
Patrick DL, Burns T, Morosini P, Gagnon DD, Rothman M, Adriaenssen I. Measuring social functioning with the personal and social performance scale in patients with acute symptoms of schizophrenia: interpretation of results of a pooled analysis of three Phase III trials of paliperidone extended-release tablets. Clin Ther. 2010;32(2):275–292. | ||
Suzuki T. Which rating scales are regarded as ‘the standard’ in clinical trials for schizophrenia? A critical review. Psychopharmacol Bull. 2011;44(1):18–31. | ||
Suzuki T, Uchida H, Watanabe K, Kashima H. Treatment target in schizophrenia: a critical review and a clinical suggestion. Psychopharmacol Bull. 2008;41(4):80–102. | ||
Jeong HG, Lee MS. Long-acting injectable antipsychotics in first-episode schizophrenia. Clin Psychopharmacol Neurosci. 2013;11(1):1–6. | ||
Llorca PM, Abbar M, Courtet P, Guillaume S, Lancrenon S, Samalin L. Guidelines for the use and management of long-acting injectable antipsychotics in serious mental illness. BMC Psychiatry. 2013;13(1):340. |
Supplementary materials
  | Box S1 Supplementary information for Table 3 |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.