Back to Journals » Risk Management and Healthcare Policy » Volume 16
Factors Influencing Functional Coronary Artery Ischemia: A Gender-Specific Predictive Model
Received 16 August 2023
Accepted for publication 17 November 2023
Published 30 November 2023 Volume 2023:16 Pages 2649—2660
DOI https://doi.org/10.2147/RMHP.S435766
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jongwha Chang
Wen-Jing Li, Hong-Wei Xu
Department of Medical Imaging, Fifth Affiliated Hospital of Zhengzhou University, Zhengzhou, 450052, People’s Republic of China
Correspondence: Hong-Wei Xu, Department of Medical Imaging, Fifth Affiliated Hospital of Zhengzhou University, No. 3, Rehabilitation Street, Erqi District, Zhengzhou, 450052, People’s Republic of China, Tel +86 13598861696, Email [email protected]
Objective: The objective of this study was to explore factors that impact functional coronary artery ischemia (FCAI) and develop a gender-specific prognostic model that could serve as a benchmark for predicting FCAI in clinical practice.
Methods: A cumulative total of 330 patients were enrolled comprising 634 main and branch coronary, consisting of 179 men (359 coronary arteries) and 151 women (275 coronary arteries). Based on the computed tomography-fractional flow reserve (CT-FFR), the coronary arteries of male and female patients were classified into the non-ischemic group (CT-FFR ≥ 0.80) and the ischemic group (CT-FFR < 0.80). We screened for factors related to the CT-FFR values of the coronary arteries in male and female patients and developed corresponding gender-specific models.
Results: In male patients, the correlation between FCAI and several indicators, including white blood cell (WBC) count, left anterior descending artery (LAD) lesions, pericoronary fat attenuation index (FAI), and the degree of coronary artery stenosis, was found to be statistically significant. A predictive model was developed using these factors, yielding an area under the curve (AUC) value of 0.812, with a P value of < 0.001 and a 95% confidence interval (CI) ranging from 0.767 to 0.857. This model demonstrated superior predictive capability compared to any individual indicators mentioned above. Significant correlations with FCAI were observed in female patients for hemoglobin (Hb), systolic blood pressure (SBP), FAI, and the degree of coronary artery stenosis. The predictive model, derived from these factors, exhibited robust performance with an area under the curve (AUC) value of 0.818, a P value of < 0.001, and a 95% confidence interval (CI) ranging from 0.764 to 0.872.
Conclusion: Gender disparities exist in the factors affecting FCAI, underscoring the need for a gender-specific predictive model to enhance the precision of FCAI prediction.
Keywords: computed tomography-fractional flow reserve, CT-FFR, coronary artery disease, CAD, degree of coronary artery stenosis, fat attenuation index, FAI, gender differences
Introduction
Coronary artery disease (CAD) is universally acknowledged as the leading cause of mortality among individuals with cardiovascular disorders worldwide. The incidence and mortality rates of CAD have exhibited a consistent upward trend annually, presenting a significant public health challenge. Epidemiological research has highlighted substantial gender-based disparities in various aspects of CAD, encompassing risk factors, clinical presentations, and treatment modalities, emphasizing the distinct differences between male and female patients.1–3 Coronary computed tomography angiography (CCTA) is the most preferred non-invasive imaging modality for CAD diagnosis in contemporary clinical practice. Concurrently, invasive coronary angiography (ICA) is the most common invasive imaging modality. However, both these approaches offer insights primarily into the degree of stenosis from an anatomical perspective and have limitations for analyzing the influence of stenosis on myocardial blood supply from a functional perspective.4
Fractional flow reserve (FFR) is an important indicator to assess FCAI from a physiological perspective.5 It is useful in assessing the degree of coronary artery stenosis, instructing revascularization, and significantly reducing the incidence of cardiac adverse events.6 However, its use in clinical practice is restricted due to its invasiveness, expensiveness, and potential risks during the procedure. The CT-FFR technology is based on CCTA. It provides an evaluation of coronary stenosis from both the anatomical and functional perspectives, and the CT-FFR measurement can be repeated in several blood vessels,7 making it a better choice for the assessment of coronary artery lesions.
Several prospective and multicenter clinical studies conducted in China and other countries8–10 have demonstrated remarkable concordance between non-invasive CT-FFR and invasive FFR in test outcomes. Furthermore, CT-FFR exhibits robust diagnostic capability, accurately evaluating specific ischemic conditions arising from coronary artery stenosis. In this study, our objective was to investigate the gender-specific variations in factors impacting coronary artery CT-FFR values. By delving into these influences, we aimed to formulate more precise predictive models. We anticipate that our study findings will offer valuable clinical insights, serving as a crucial reference point for the prevention and treatment of CAD.
Materials and Methods
Study Participants
Through retrospective screening, we meticulously chose a cohort of 330 patients, collectively encompassing 634 coronary arteries. The selection criteria required that each patient had at least one main artery displaying anatomical stenosis, as identified through CCTA conducted at The Fifth Affiliated Hospital of Zhengzhou University, China, spanning the period from August 2022 to April 2023. Among the 330 patients studied, 179 were male (accounting for a total of 359 coronary arteries) and 151 were female (comprising 275 coronary arteries). The classification of coronary arteries with lesions was based on their CT-FFR values, dividing them into two distinct groups: the non-FCAI group (consisting of arteries with CT-FFR ≥ 0.8) and the FCAI group (comprising arteries with CT-FFR < 0.8). A visual representation of this classification process is depicted in Figure 1.
 |
Figure 1 The research flow chart. |
We analyzed the clinical and imaging data of the patients. Clinical data included age, systolic blood pressure (SBP), diastolic blood pressure (DBP), body mass index (BMI), high blood pressure, diabetes mellitus, hyperlipidemia, history of smoking, history of alcohol use, left ventricular ejection fraction (LVEF), white blood cell (WBC) count, red blood cell (RBC) count, hemoglobin (Hb), red cell distribution width (RDW), platelet distribution width (PDW), creatinine, uric acid (UA), total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDLC), and low-density lipoprotein cholesterol (LDLC).
Inclusion criteria: (1) The diameter of the stenotic coronary artery was ≥ 2 mm. (2) The CCTA image was of sufficient quality for to evaluate CT-FFR using the relevant software. (3) Patients with complete clinical and imaging data.
Exclusion criteria: (1) Patients with a history of cardiac surgery. (2) The image quality of CCTA was poor. (3) Patients with a myocardial bridge that traveled into the stenotic coronary artery.
CCTA Scanning
In this study, all patients underwent CCTA that was performed using a 256-slice spiral CT scanner. To mitigate motion artifacts, patients received training in breath-holding before the scanning procedure. Patients with an elevated heart rate (HR) were administered metoprolol to reduce their HR below 65 bpm. Furthermore, two to five minutes before the scanning commenced, patients were administered nitroglycerin sublingually to induce coronary artery dilation. Following the definition of the scanning range in the CT scout view position, enhanced scanning was conducted utilizing a dual-barrel high-pressure injector. The contrast agent, iohexol, was injected through the elbow vein at a volume of 60–80 mL and a flow rate of 4–5 mL/s, followed by the injection of 40 mL of normal saline at the same flow rate. An automatic threshold tracking and triggering technique was employed, with the bifurcation of the ascending aorta and trachea serving as the triggering point and a threshold of 150 Hounsfield Units (Hu) for triggering the scanning process. The scanning commenced 4.2 seconds after the trigger. Reconstruction was performed utilizing iterative reconstruction technology. The collimator width was set at 128×0.625 mm, the scan layer thickness was 0.90 mm, and the scan interval was 0.450 mm. For reconstruction, the layer thickness was maintained at 0.90 mm, and the interval between reconstructions was 0.450 mm.
Image Analysis
The initial CCTA images were subjected to processing, and those exhibiting superior vascular visualization were meticulously chosen. These selected images were then imported into the artificial intelligence software, uAI Discover-Coronary CTA, to determine the degree of coronary artery stenosis, the FAI value, and the CT-FFR value. The degree of stenosis was categorized into three grades: mild stenosis (1–49%), moderate stenosis (50–69%), and severe stenosis (≥70%).
The measurement for CT-FFR was conducted at a distance of 20 mm from the distal end of the lesion, with a CT-FFR value of < 0.8 signifying functional ischemia and a CT-FFR value of ≥ 0.8 indicating the absence of functional ischemia. For the FAI values of the left anterior descending artery (LAD) and left circumflex artery (LCX), the measurement range extended from the initial point of the blood vessel to 40 mm. The measurement range for the FAI value of the right coronary artery was from 10 mm to 50 mm from the starting point. Fat was measured at the horizontal level of the blood vessel, at a distance equal to one blood vessel diameter from the blood vessel’s outer wall. The CT value for adipose tissue was defined as –190 to –30 HU (Figure 2).
Two qualified professionals experienced in post-procedure processing independently conducted all the procedures. Only the results that demonstrated consistency between the assessments of these two experts were considered for inclusion in this study.
Statistical Analysis
Measurement data are expressed as mean ± standard deviation, while enumeration data are presented as percentages. The Kolmogorov–Smirnov method was used to validate whether the data were normally distributed. For data in normal distribution, continuous variables are expressed as mean ± standard deviation, and the t-test was used for pairwise comparisons. Data in a non-normal distribution are expressed as the median and interquartile range (IQR), and the Mann–Whitney U-test was used for pairwise comparisons. For enumeration data, the chi-squared test was used for pairwise comparisons. Univariate and multivariate logistic regression were used to analyze the correlation between FCAI and other variables. A multivariate logistic regression equation was established using the stepwise method. The predictive power of the model was assessed using the receiver operating characteristic (ROC) curve and area under the curve (AUC). A P value of < 0.05 was considered to indicate a statistically significant difference. SPSS 26.0 statistical software (IBM Inc., Armonk, NY, USA) was used for the data analysis.
Results
Comparison of Clinical Data
As shown in Table 1, there were statistically significant differences in age, WBC count, PDW, TC, and LDLC between the coronary arteries in the male FCAI group and non-FCAI group (P < 0.05), while the two groups had no statistically significant differences in SBP, DBP, LVEF, BMI, high blood pressure, hyperlipidemia, diabetes mellitus, the proportion of patients having a history of smoking or alcohol use, RBC, Hb, RDW, PDW, creatinine, UA, TG, and HDLC (P > 0.05).
 |
Table 1 Comparison of Clinical and Imaging Data of Coronary Arteries Between the Two Groups of Male Patients |
The coronary arteries in the two female groups had statistically significant differences in SBP, Hb, and RDW (P < 0.05), while there were no statistically significant differences in age, DBP, LVEF, BMI, high blood pressure, hyperlipidemia, diabetes mellitus, the proportion of patients having a history of smoking or alcohol use, WBC, RBC, PDW, creatinine, UA, TC, TG, HDLC, and LDLC (P > 0.05), as seen in Table 2.
 |
Table 2 Comparison of Clinical and Imaging Data of Coronary Arteries Between the Two Groups of Female Patients |
Comparison of Imaging Data
There were no statistically significant differences in the LCA lesion in the diseased coronary arteries in the two groups of male patients (P > 0.05), however, there were statistically significant differences in the LAD lesion, right coronary artery (RCA) lesion, FAI, and degree of stenosis (P < 0.05), as displayed in Table 1. The coronary arteries of the two groups of female patients did not differ significantly with respect to LAD, LCX, and RCA lesions (P > 0.05), but had a statistically significant difference in FAI and degree of stenosis (P < 0.05), as shown in Table 2.
Univariate and Multivariate Analysis of FCAI
Logistic Regression Analysis of FCAI in Male Patients
The dependent variable was FCAI, and the independent variables were significantly distinctive indicators, including age, WBC, PDW, TC, LDLC, FAI, degree of stenosis, LAD lesion, and RCA lesion (Table 3). Univariate logistic regression analysis revealed that FCAI was not statistically related to PDW (P > 0.05), but was significantly related to age, WBC, TC, LDLC, FAI, degree of stenosis, LAD lesion, and RCA lesion (P < 0.05).
 |
Table 3 Univariate and Multivariate Analysis of FCAI in Male Patients |
We conducted a multivariate regression analysis, employing an inclusion criterion of 0.05 and an exclusion criterion of 0.10, on the following eight variables: age, WBC, TC, LDLC, FAI, degree of stenosis, LAD lesion, and RCA lesion. We found that the dependent risk factors for FCAI in male patients were WBC count, LAD lesion, FAI, and degree of stenosis (P < 0.05), and that FCAI in men was not significantly related to age, TC, LDLC, or RCA lesion (P > 0.05). We eventually obtained the following multivariate logistic regression equation: (P was defined as the probability of FCAI occurrence) LogitP = 3.455 + 1.222 × WBC + 3.103 × LAD lesion (Yes = 1, No = 0) + 3.421 × degree of stenosis (mild = 1, moderate = 2, severe = 3) + 1.091 × FAI. When the model was validated using the Hosmer-Lemeshow test, the corresponding P value was > 0.05, indicating that the regression model was a good fit for the data.
Logistic Regression Analysis of FCAI in Female Patients
The dependent variable was FCAI, and the independent variables were significantly distinctive indicators, namely, SBP, Hb, RDW, and degree of stenosis (Table 4). According to the results of univariate logistic regression analysis, FCAI was significantly related to all five variables (P < 0.05). We conducted a multivariate regression analysis, with an inclusion criterion of 0.05 and an exclusion criterion of 0.10, considering five variables. The analysis revealed that SBP and degree of stenosis were identified as independent risk factors for FCAI in female patients, while Hb served as a protective factor. The eventual multivariate logistic regression equation obtained was as follows: (P was defined as the probability of FCAI occurrence) LogitP = 0.964 × Hb + 1.023 × SBP + 6.891 × degree of stenosis (mild = 1, moderate = 2, severe = 3) + 1.059 × FAI. The model was validated using the Hosmer-Lemeshow test; the corresponding P value was > 0.05, indicating that the regression model was a good fit for the data.
 |
Table 4 Univariate and Multivariate Analysis of FCAI in Female Patients |
Diagnostic Significance of the Predictive Models
Diagnostic Significance of the Predictive Models for Male Patients
We constructed the regression equation by combining the four indicators, namely, WBC, LAD lesion, FAI, and degree of stenosis. The ROC curve was used to analyze the diagnostic significance of the multivariate regression model. As shown in Figure 3, the AUC, sensitivity, and specificity for the composite indicator model were 0.812 (95% CI ranging between 0.767–0.857, P < 0.001), 78.7%, and 72.3%, respectively. In contrast, when using WBC, LAD lesion, FAI, and degree of stenosis individually to predict FCAI, the corresponding AUC values were 0.582, 0.624, 0.659, and 0.731; the corresponding P values were 0.007, 0.000, 0.000, and 0.000; and the 95% CI were 0.523–0.641, 0.565–0.682, 0.603–0.715, and 0.677–0.784, respectively.
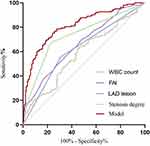 |
Figure 3 The ROC curve of predicting FCAI using the indicators for male patients. |
Upon comparing multiple ROC curves, our analysis demonstrated that the composite model, integrating multiple indicators, outperformed any individual indicator in predicting FCAI with statistically significant differences (P < 0.05). We identified the optimal cut-off value for the composite model as 0.339, utilizing the maximum value of the Youden’s index (0.510).
Diagnostic Significance of the Predictive Models for Female Patients
We constructed the regression equation by combining the four indicators, namely, SBP, Hb, FAI, and degree of stenosis. The diagnostic significance of the regression model was analyzed using the ROC curve. As shown in Figure 4, the AUC, sensitivity, and specificity for the multivariable regression model were 0.818 (95% CI ranging between 0.764–0.872, P < 0.001), 65.6%, and 92.0%, respectively. In contrast, when using SBP, Hb, FAI, and degree of stenosis individually to predict FCAI, the corresponding AUC values were 0.602, 0.412, 0.685, and 0.750; the corresponding P values were 0.004, 0.035, 0.000, and 0.000; and the 95% CI were 0.534–0.669, 0.343–0.482, 0.621–0.748, and 0.690–0.811, respectively.
 |
Figure 4 The ROC curve of predicting FCAI using the indicators for female patients. |
The results of comparison between multiple ROC curves indicated that the composite indicator model was superior to any single indicator in predicting FCAI, and the differences were statistically significant (P < 0.05). The optimal cut-off value for the composite model was 0.527 when using the maximum value of the Youden’s index (0.576).
Discussion
The guidelines jointly issued by the European Society of Cardiology and the European Association for Cardio-Thoracic Surgery advocate for the performance of physiological function assessments in patients with myocardial ischemia before they undergo invasive examinations and treatments.11 The current interpretation of CT-FFR results aligns with that of FFR: revascularization is recommended for patients with lesions displaying CT-FFR values < 0.8, while patients with lesions exhibiting FFR values ≥ 0.8 are initially recommended pharmaceutical treatment.
Earlier studies12 have substantiated that male patients, even with comparable degrees of stenosis, face a higher risk of developing FCAI compared to their female counterparts. Moreover, male patients exhibit distinct risk factors for CAD when compared to female patients. Various explanations have been proposed to account for these observed phenomena. Certain researchers13,14 suggest that estrogens play a regulatory role in lipids, inflammatory markers, and blood coagulation factors, offering some degree of protection to the cardiovascular system. Conversely, other studies15,16 have associated the Y chromosome with men’s heightened susceptibility to CAD. The accumulation of inflammation within the coronary artery wall can result in the development of severe cardiovascular diseases.
In this study, we conducted distinct analyses for diseased coronary arteries in male and female patients, constructing gender-specific predictive models tailored to each group. Our results indicated that in male patients, CT-FFR values of coronary arteries exhibited moderate correlations with WBC count, FAI, degree of stenosis, and lesions in the LAD. Conversely, in female patients, CT-FFR values of coronary arteries were moderately correlated with SBP, Hb, FAI, and degree of stenosis.
A robust correlation between the degree of coronary artery stenosis and the CT-FFR value was observed in both male and female patients (P < 0.001). Nevertheless, as established by numerous studies, it is well-documented that the relationship between the degree of coronary artery stenosis and myocardial ischemia is intricate and multifaceted.17,18 Results of a study trial by Tonino et al19 showed that 20% of the coronary arteries with over 70% stenosis had no functional ischemia. Park et al4 showed that more than half of the coronary arteries with over 50% stenosis had an FFR value above 0.80. A possible explanation is that there is a mismatch between the degree of coronary artery stenosis and myocardial ischemia due to the compensatory ability of collateral circulation in some segments of the diseased coronary artery. These studies demonstrate that assessing coronary artery ischemia solely based on the degree of stenosis is insufficient.
Accurately diagnosing FCAI requires further research into associated factors and the combination of multiple factors. Vasculitis constitutes an early hallmark of coronary atherosclerosis initiation and plaque rupture. Inflammatory blood vessels emit inflammatory signals to adjacent adipose tissue in a paracrine fashion. This process can elevate microvascular permeability, fostering edema around the blood vessels and culminating in augmented fat density surrounding the coronary arteries.20
FAI is a non-invasive imaging technique used to assess coronary artery inflammation that can measure the average density of adipose tissue within the affected area. Hoshino et al21 showed that FAI is an independent predictor of myocardial ischemia. In their study on pericoronary FAI and coronary artery FFR in patients with suspected coronary artery diseases, Nomura et al22 found that there was a correlation between FAI and myocardial ischemia. The results of our study also showed a strong correlation between the FAI value and FCAI, both in male and female patients (P < 0.001).
In their research, Shiono et al23 found that LAD lesions are more likely to cause myocardial ischemia, and the narrowing of the proximal end of the LAD has a significant impact on patient prognosis and the efficacy of revascularization. This indicates that myocardial ischemia is not only related to the degree of coronary artery stenosis but also to the quantity or area of myocardial tissue supplied by the narrowed coronary artery.
In our study, we discovered that lesions in the LAD pose a risk factor for FCAI in male patients. This observation might be attributed to the prevalent left-dominant coronary artery pattern in most men, where the LAD artery supplies the largest myocardial area.24 Hence, in men, lesions in the LAD are more prone to causing myocardial ischemia. Among women, the prevalence of a left-dominant coronary artery pattern is slightly lower, while the incidence of a right-dominant coronary artery pattern is higher. This discrepancy could explain the lack of a significant correlation between LAD lesions and CT-FFR values of coronary arteries in women.
Traditional risk factors for patients with CAD typically include high blood pressure, hyperlipidemia, and diabetes mellitus. However, in this study, we did not find a correlation between these three indicators and FCAI. A probable explanation is that with improved health awareness, many patients are taking antihypertensive, lipid-lowering, and antidiabetic medications. This widespread medication usage could significantly influence the research outcomes and potentially explain the absence of a direct association between these traditional risk factors and FCAI in our study.
In clinical practice, WBC count serves as a frequently utilized and crucial inflammatory marker. The substantial release of inflammatory factors and free radicals by WBCs can lead to damage to the coronary artery endothelium. Lee et al25 found that an increase in WBC count directly affects the incidence of CAD and increases the risk of developing CAD. In this study, we found that WBC was a gender-specific factor that was significantly correlated with men. By contrast, there was no statistically significant difference in WBC count between the two groups of female patients. The reason for this may be that women are more prone to gynecological diseases and are more likely than men to develop immune system disorders.26 Both gynecological diseases and immune system disorders can lead to a decrease in WBC count in female patients, which may have confounded the results of this study. Therefore, it is necessary to establish different models for male and female patients.
High blood pressure is intricately linked to the onset of cardiovascular disease and the subsequent fatalities attributed to it. Prolonged hypertension can result in endothelial cell damage and the accumulation of inflammatory factors, fostering the development of coronary plaques.27 Ultimately, this process leads to the narrowing of coronary arteries and subsequent myocardial ischemia. We included two relevant factors, SBP and DBP, in our study. The results showed that SBP was a significant factor associated with FCAI in female patients, with an odds ratio (OR) of 1.022 (P < 0.05). A possible explanation for this is that in male patients, myocardial ischemia is primarily related to coronary artery stenosis and plaque properties, while in female patients, it is often closely related to coronary artery spasm. Coronary artery spasm can further increase SBP, resulting in a stronger correlation between myocardial ischemia and SBP.
In addition, in this study, we also found that Hb was a protective factor against FCAI in women, with an OR value of 0.965 (P < 0.05). The Hb level was lower in the female FCAI group than in the female non-ischemia group (P < 0.05). Chonchol et al28 also found that the onset of CAD is related to Hb levels. From both pathological and physiological standpoints, a low Hb concentration in females could significantly diminish arterial blood oxygen levels. This phenomenon was not observed in males. Consequently, due to inadequate blood supply, coronary arteries in females are more susceptible to spasms, ultimately leading to myocardial ischemia.29
This study has several limitations: (1) Its retrospective nature confined us to patient data from a specific period, leading to a limited sample size. Moreover, we did not conduct age-based stratification analysis. In future research, we plan to enhance the sample size and implement age-based stratification to explore the relationships between variables within distinct age groups. (2) FCAI is influenced by a multitude of factors, including race, family genetic history, homocysteine levels, and the characteristics and parameters of various plaques. In our future research endeavors, we aim to incorporate a broader range of these variables and investigate their associations with CAD. (3) The factors associated with coronary artery lesions in female patients may differ before and after menopause, potentially leading to distinct predictive models. In this study, we employed random sampling without accounting for menopausal status among women. In future research, we plan to stratify female patients based on their menopausal status to create more precise and tailored predictive models, recognizing the importance of this factor in understanding and predicting coronary artery disease in women.
Conclusion
In conclusion, gender disparities exist in the factors affecting FCAI, emphasizing the need for a gender-specific predictive model to enhance the accuracy of FCAI prediction. In situations where advanced AI software technology for rapid CT-FFR measurement is unavailable, physicians can rely on this model, constructed based on variables such as stenosis degree, WBC count, and SBP, to predict the likelihood of FCAI in patients of different genders. The predictive model developed in this study offers a more comprehensive and accurate insight into a patient’s condition, providing valuable guidance and assistance for disease prevention and subsequent treatment decisions.
Data Sharing Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Ethics Approval and Consent to Participate
I confirm that I have read the Editorial Policy pages. This study was conducted with approval from the Ethics Committee of Fifth Affiliated Hospital of Zhengzhou University (No. KY2023072). This study was conducted in accordance with the declaration of Helsinki. Written informed consent was obtained from all participants.
Acknowledgments
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study.
Funding
No external funding received to conduct this study.
Disclosure
The authors declare that they have no competing interests.
References
1. Jneid H, Fonarow GC, Cannon CP, et al. Sex differences in medical care and early death after acute myocardial infarction. Circulation. 2008;118(25):2803–2810. doi:10.1161/CIRCULATIONAHA.108.789800
2. López L, Wilper AP, Cervantes MC, Betancourt JR, Green AR. Racial and sex differences in emergency department triage assessment and test ordering for chest pain, 1997–2006. Acad Emerg Med. 2010;17(8):801–808. doi:10.1111/j.1553-2712.2010.00823.x
3. Martínez-Sellés M, García Robles JA, Prieto L, et al. Systolic dysfunction is a predictor of long term mortality in men but not in women with heart failure. Eur Heart J. 2003;24(22):2046–2053. doi:10.1016/j.ehj.2003.07.007
4. Park SJ, Kang SJ, Ahn JM, et al. Visual-functional mismatch between coronary angiography and fractional flow reserve. JACC: Cardiovasc Interv. 2012;5(10):1029–1036. doi:10.1016/j.jcin.2012.07.007
5. Toth G G, Johnson NP, Wijns W, et al. Revascularization decisions in patients with chronic coronary syndromes: results of the second International Survey on Interventional Strategy (ISIS-2). Int J Cardiol. 2021;336:38–44. doi:10.1016/j.ijcard.2021.05.005
6. Tonino PA, De Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360(3):213–224. doi:10.1056/NEJMoa0807611
7. Koo BK. The present and future of fractional flow reserve. Circ J. 2014;78(5):1048–1054. doi:10.1253/circj.cj-14-0249
8. Wang ZQ, Zhou YJ, Zhao YX, et al. Diagnostic accuracy of a deep learning approach to calculate FFR from coronary CT angiography. J Geriatr Cardiol. 2019;16(1):42–48. doi:10.11909/j.issn.1671-5411.2019.01.010
9. Nørgaard BL, Leipsic J, Gaur S, et al. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: next Steps). J Am Coll Cardiol. 2014;63(12):1145–1155. doi:10.1016/j.jacc.2013.11.043
10. Xia YM, Gao H, Wang QS, Feng X, Wang YQ, Xu ZX. Characteristics of traditional Chinese medicine syndrome in patients with coronary heart disease at different disease stages. World J Tradit Chin Med. 2022;8:218–224. doi:10.4103/wjtcm.wjtcm_65_21
11. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. Linee guida ESC/EACTS 2018 sulla rivascolarizzazione miocardica. Task Force sulla Rivascolarizzazione Miocardica della Società Europea di Cardiologia (ESC) e dell’Associazione Europea di Chirurgia Cardiotoracica (EACTS) [2018 ESC/EACTS Guidelines on myocardial revascularization. The Task Force on myocardial revascularization of the European Society of Cardiology (ESC) and European Association for Cardio-Thoracic Surgery (EACTS)]. G Ital Cardiol. 2019;20(7–8 Suppl 1):1S–61S. Italian. doi:10.1714/3203.31801
12. Lakhter V, Alkhouli M, Zack CJ, et al. Sex differences in fractional flow reserve-guided revascularization: a nationwide analysis. J Womens Health. 2017;26(2):109–115. doi:10.1089/jwh.2016.5806
13. Martínez-Sellés M, Díez-Villanueva P, Álvarez Garcia J, et al. Influence of sex and pregnancy on survival in patients admitted with heart failure: data from a prospective multicenter registry. Clin Cardiol. 2018;41(7):924–930. doi:10.1002/clc.22979
14. Martínez-Sellés H, Martínez-Sellés D, Martínez-Sellés M. Sex, lies, and coronary artery disease. J Clin Med. 2021;10(14):3114. doi:10.3390/jcm10143114
15. Eales JM, Maan AA, Xu X, et al. Human Y chromosome exerts pleiotropic effects on susceptibility to atherosclerosis. Arterioscler Thromb Vasc Biol. 2019;39(11):2386–2401. doi:10.1161/ATVBAHA.119.312405
16. Haitjema S, Kofink D, van Setten J, et al. Loss of Y chromosome in blood is associated with major cardiovascular events during follow-up in men after carotid endarterectomy. Circ Cardiovasc Genet. 2017;10(4):e001544. doi:10.1161/CIRCGENETICS.116.001544
17. Ahmadi A, Kini A, Narula J. Discordance between ischemia and stenosis, or PINSS and NIPSS: are we ready for new vocabulary? JACC Cardiovasc Imaging. 2015;8(1):111–114. doi:10.1016/j.jcmg.2014.11.010
18. Adjedj J, Stoyanov N, Muller O. Comparison of coronary angiography and intracoronary imaging with fractional flow reserve for coronary artery disease evaluation: an anatomical-functional mismatch. Anatol J Cardiol. 2018;20(3):182–189. doi:10.14744/AnatolJCardiol.2018.42949
19. Tonino PA, Fearon WF, De Bruyne B, et al. Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography in multivessel evaluation. J Am Coll Cardiol. 2010;55(25):2816–2821. doi:10.1016/j.jacc.2009.11.096
20. Margaritis M, Antonopoulos AS, Digby J, et al. Interactions between vascular wall and perivascular adipose tissue reveal novel roles for adiponectin in the regulation of endothelial nitric oxide synthase function in human vessels. Circulation. 2013;127(22):2209–2221. doi:10.1161/CIRCULATIONAHA.112.001133
21. Hoshino M, Yang S, Sugiyama T, et al. Peri-coronary inflammation is associated with findings on coronary computed tomography angiography and fractional flow reserve. J Cardiovasc Comput Tomogr. 2020;14(6):483–489. doi:10.1016/j.jcct.2020.02.002
22. Nomura CH, Assuncao-Jr AN, Guimarães PO, et al. Association between perivascular inflammation and downstream myocardial perfusion in patients with suspected coronary artery disease. Eur Heart J Cardiovasc Imaging. 2020;21(6):599–605. doi:10.1093/ehjci/jeaa023
23. Shiono Y, Kubo T, Tanaka A, et al. Impact of myocardial supply area on the transstenotic hemodynamics as determined by fractional flow reserve. Catheter Cardiovasc Interv. 2014;84(3):406–413. doi:10.1002/ccd.25300
24. Leone AM, De Caterina AR, Basile E, et al. Influence of the amount of myocardium subtended by a stenosis on fractional flow reserve. Circ Cardiovasc Interv. 2013;6(1):29–36. doi:10.1161/CIRCINTERVENTIONS.112.971101
25. Lee CD, Folsom AR, Nieto FJ, Chambless LE, Shahar E, Wolfe DA. White blood cell count and incidence of coronary heart disease and ischemic stroke and mortality from cardiovascular disease in African-American and White men and women: atherosclerosis risk in communities study. Am J Epidemiol. 2001;154(8):758–764. doi:10.1093/aje/154.8.758
26. Zhu Y, Zhang Y, Choi HK. The serum urate-lowering impact of weight loss among men with a high cardiovascular risk profile: the Multiple Risk Factor Intervention Trial. Rheumatology. 2010;49(12):2391–2399. doi:10.1093/rheumatology/keq256
27. Fuchs FD, Whelton PK. High Blood Pressure and Cardiovascular Disease. Hypertension. 2020;75(2):285–292. doi:10.1161/HYPERTENSIONAHA.119.14240
28. Chonchol M, Nielson C. Hemoglobin levels and coronary artery disease. Am Heart J. 2008;155(3):494–498. doi:10.1016/j.ahj.2007.10.031
29. Most AS, Ruocco NA, Gewirtz H. Effect of a reduction in blood viscosity on maximal myocardial oxygen delivery distal to a moderate coronary stenosis. Circulation. 1986;74(5):1085–1092. doi:10.1161/01.cir.74.5.1085
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

