Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 17
Factors Associated with the Development of Chronic Kidney Disease in Patients with Arterial Hypertension
Authors Goicochea-Rios EDS, Chian-García AM, Yupari-Azabache IL , Gómez Goicochea NI
Received 9 November 2023
Accepted for publication 24 February 2024
Published 29 March 2024 Volume 2024:17 Pages 113—123
DOI https://doi.org/10.2147/IJNRD.S448986
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pravin Singhal
Evelyn Del Socorro Goicochea-Rios, Ana María Chian-García, Irma Luz Yupari-Azabache, Néstor Iván Gómez Goicochea
School of Medicine, Universidad César Vallejo, Trujillo, Peru
Correspondence: Irma Luz Yupari-Azabache, Email [email protected]
Introduction: Chronic kidney disease affects a large part of the population with hypertension, diabetes mellitus as well as those over 50 years of age. Research reported that male sex and other comorbidities such as obesity and anemia are more frequent in Chronic kidney disease, as well as uncontrolled diabetes mellitus or hypertension.
Objective: To determine the risk factors associated with the development of chronic kidney disease in adults with arterial hypertension.
Material and Methods: Retrospective cohort study of 455 patients with hypertension treated in a primary health care hospital. Medical records and laboratory information were reviewed for the diagnosis of chronic kidney disease and its staging. Patients aged 40 years and older, of both sexes and evaluated between the years 2015 − 2017 were included. Logistic regression analysis allowed the identification of risk factors associated with the development of chronic kidney disease.
Results: 63.7% were female and 36.3% male. The average age for 2015 was 69.79 ± 9.03, more than half of participants had diabetes mellitus and controlled hypertension and the predominant nephroprotection was with Losartan (53%) that year. Male sex (OR 1.68, CI 1.03– 2,76), age: 60 years or older (OR 6.38, CI 2.65– 15,37) and anemia (OR 1.71, CI 1.03– 2,85), were risk factors for the development of chronic kidney disease (p < 0.05), whereas nephroprotection (OR 0.39, CI 0.18– 0,88) and controlled diabetes mellitus (OR: 0.18, CI 0.07– 0,47) were shown to be protective factors (p < 0.05). The prevalence of chronic kidney disease between 2015 and 2017 was 19% and 45%, respectively, with predominance of category G2. The comparison group is the same cohort analyzed in each year under study.
Conclusion: Male sex, age over 60 years, and anemia are risk factors for chronic kidney disease. Nephroprotection, controlled diabetes mellitus, and patient follow-up are factors that prevent its development.
Keywords: chronic kidney disease, classification renal disease, glomerular filtration rate, hypertension, primary health care
Introduction
Chronic kidney disease (CKD) is a disease of public health interest due to its impact on the quality of life, cardiovascular comorbidity and mortality.1 In patients with arterial hypertension (AHT) and diabetes mellitus (DM) treated in primary healthcare, its prevalence can reach 40%.2
The prevalence of CKD worldwide varies between 11.7% and 15.1%, and it is estimated that about 6’000 000 patients reach stage 5.3 These figures vary from one country to another, highlighting advanced cases in the United States and Spain.2,4 In Cuba, the estimated prevalence of CKD is 2.5–3.5 patients/1000 inhabitants.5 In Peru, the adjusted prevalence of CKD was 1.5 per 1000 patients for 2017,6 and the number of nephrologists and hemodialysis services are insufficient at the national level, so early detection of CKD in primary health care facilities is urgently needed.7
Arterial hypertension (AHT) is the persistent elevation of systemic arterial pressure, with values ≥140 mm Hg/90 mm Hg and is one of the first causes of CKD and It is described that 7% of patients with a recent diagnosis of AHT already have albuminuria.8 The pathogenesis of AHT in patients with CKD begins with hypertrophy and sclerosis in the wall of the glomerular afferent arteriole, conditioning the ischemia of certain glomeruli and post-glomerular structures.9
There is lesion of the vascular or glomerular compartment by renal progression that gives rise to inflammation with progressive reduction of the glomerular filtration surface and loss of nephrons. Likewise, interstitial lesion is presented that begins with inflammation, increase of angiotensin II production and sclerosis of renal vessels with loss of capillaries in the cortical interstitium and tubular pericapillary ischemia, ending with fibrosis and tubular.10 In summary, the mechanisms by which AHT leads to CKD are multiple and interrelated: including hydrosaline retention, stimulation of the renin-angiotensin-aldosterone system (RAAS) and hyperactivity of the sympathetic nervous system.11
AHT is involved in the natural history of CKD as an initiating factor of renal damage, one of the factors that increase the risk of developing CKD, one of the factors of progression of CKD, and a factor that increases morbidity and mortality in CKD.11 Other initiating factors of renal damage have also been described, such as autoimmune diseases, systemic and urinary tract infections, urolithiasis, nephrotoxic substances; progression factors such as persistent proteinuria, uncontrolled DM, smoking, dyslipidemia, anemia, obesity, and factors that increase morbidity and mortality in CKD such as, hypoalbuminemia and anemia.12 Atherosclerosis in the elderly and fibromuscular dysplasia among the young are another cause of CKD associated with decreased renal arterial flow.4
In Peru, the etiology of CKD has changed in recent years. In 2015, nephropathy due to DM accounted for 44.1% of cases, followed by chronic glomerulonephritis with 23.2%, obstructive nephropathy with 15.3%, nephropathy due to HTN with 12.4% and other causes with 5.11%; whereas by the year 2021, 54.28% of G5 CKD cases had hypertensive nephropathy as etiology.6 Based on these reports, CKD requires priority attention because it influences overall morbidity and mortality.13
It is important to bear in mind that individuality and various risk factors such as age,5,14,15 male sex,(5,15,) race and DM,14 anemia,16,17 obesidad2,18,19 and others can determine the development of kidney disease14 and that in the follow-up of patients with AHT, early detection of CKD or delaying its development can have an impact in order to prevent the patient from reaching kidney failure.
CKD is defined as abnormalities of kidney structure or function, present for >3 months, with implications for health.20 Presence of an alteration in the urinary sediment (elevated proteinuria) or other alterations of tubular origin, alteration in the renal structure evidenced by imaging or biopsy with a duration of more than 3 months, with or without impairment of renal function;3 or a glomerular filtration rate (GFR) <60 mL/min/1.73 m2 without other signs of renal disease.1–3
A single criterion of alterations in renal structure or function is sufficient to diagnose CKD, but if the GFR is >60 mL/min/1.73 m2, the presence of markers of renal injury is required to diagnose CKD2. Proteinuria is a determinant for the diagnosis of CKD and several studies show that albuminuria levels are directly related to renal health prognosis. In addition, it is considered a marker of systemic damage as it is related to endothelial dysfunction, arterial remodeling and high cardiovascular risk.12 CKD is classified based on Cause, GFR category (G1-G5), and Albuminuria category (A1-A3).20 For G1 and G2, the existence of proteinuria or other signs of renal damage is required, whereas for G3 to G5, the finding of a GFR decrease below 60 mL/min/1.73 m2 is sufficient.8,20
The development of CKD in patients with AHT has been documented by a mean decrease in GFR of 5.8 mL/min/year and a statistically significant change from one stage to another of 3.2 years. Likewise, CKD is maintained in initial stages in patients recently diagnosed with AHT and then evolves rapidly21. CKD progression is defined as a decrease in glomerular filtration rate (GFR) >5 mL/min/year or >10 mL/min in five years together with the appearance of local or systemic complications, however, progression is multifactorial and may be associated with AHT, DM, black skin, sedentary lifestyle and age over 60 years.21 Also, having relatives with CKD, renal hypoplasia and low birth weight.22
In a Colombian retrospective cohort study of 5872 patients in a nephroprotection program, 49% showed progression of CKD, with a predominance of G3 to G5 in people aged 60 or older with AHT, non-adherent to treatment, and male sex,2 and in a Cuban cross-sectional study of 227 hypertensive patients it is found mostly in people aged 60–69 years with obesity and DM and G2 CKD.5
In Peru, the CKD affects 10% of the population over 20 years of age, of which 0.1% would have terminal CKD.6 The number of patients treated for this damage in outpatient clinics shows an increasing trend, as do the number of cases requiring dialysis. ESSALUD level III and IV hospitals treat about 70% of all patients with G4 and G5 CKD nationwide, which causes a high economic cost to the institution and a high social cost for patients and their families,7 so it is important to strengthen early detection of the CKD.
Therefore, this study carried out in a primary healthcare (level I hospital) allows us to identify the early stages of CKD and the factors associated with CKD in the adult population with arterial hypertension, both risk factors and protective factors, results that can be extrapolated to similar realities within ESSALUD and that contribute to reducing the development of the CKD.
The quality of life of patients with CKD may be affected by anxiety and depression as mentioned in a systematic review, where a high prevalence of anxiety symptoms and anxiety disorders (43% and 19% respectively) was found in all continents studied, especially in pre-dialysis and dialysis patients.23 Depression affects approximately a quarter of adults with CKD, being higher among patients with CKD stage 5D (22.8%), and for CKD stages 1–5 (21.4%).24
At the local level, there are no studies on the risk factors and clinical characteristics of patients with CKD treated in Essalud hospitals at the first level of care, so we believe that this research is indicated to systematize and analyze the information collected in outpatient clinics and has the following objectives: to identify the biological and clinical factors associated with the development of CKD, to establish the development of CKD during the study period, and to identify the prevalence of CKD and its stages in patients with AHT.
Materials and Methods
Study Design, Population and Sample
An observational retrospective cohort study was developed with a target population of 2100 patients with AHT assisted in a chronic disease control program, between the years 2015 to 2017 at the Hospital Albrecht - ESSALUD of the Province and District of Trujillo, Peru.
From this cohort, the medical records of 455 patients were selected who met the following inclusion criteria: male or female, 40 years of age or older, with a diagnosis of AHT, proteinuria results, proteinuria/creatinuria ratio, determination of glomerular filtration rate using the MDRD 4 formula25, and a minimum of 4 controls per year, 2 medical and 2 nursing controls.
Medical records of patients with sequelae of cardiovascular disease, patients with glomerulonephritis, renal hypoplasia or monorenal disease, patients with metabolic diseases other than DM or obesity, and patients on dialysis were excluded because the control and treatment of these pathologies is provided at another level of care to which they are referred. Those who stopped attending check-ups during the study period were also excluded.
Data Collection
The documentary analysis technique was used to obtain information from the medical records and the ESSALUD follow-up book.26 The instrument designed by the authors made it possible to collect information on biological factors: age and sex, and clinical factors: blood pressure, body mass index and comorbidities recorded in the medical records. Also, serum creatinine values, microalbuminuria/creatinuria ratio and glomerular filtration rate. This information made it possible to identify the staging and development of CKD in the population studied.
Likewise, the information from the medical and nursing controls was transcribed in terms of blood pressure, treatment, laboratory findings of each patient, and participation in “renal health” workshops conducted by the nurses.
Regarding AHT, it was considered controlled if the values were lower than 140/90 mm Hg in all the evaluations performed on each patient, controlled DM if the HbA1c <6.9%, and obesity if the body mass index was 30 or more. In the case of anemia if Hb was <12 g/dL for the female sex and <13 g/dL for the male sex. CKD was considered if the albuminuria/creatinuria ratio was >30 for females and >20 for males, and the MDRD 4 formula was used for staging.25
If the patient was treated with angiotensin-converting enzyme inhibitors (ACE inhibitors) or angiotensin II receptor blockers (ARBs) was considered nefroprotection.27 The drugs used for this purpose in Essalud are as follows: enalapril 10 and 20 mg, and captopril 25 mg (ACE inhibitors), losartan 50 mg, valsartan 80 mg and irbesartan 150 mg (ARBs).
Based on the selection criteria, this information was organized by patient and by year. All the variables recorded by the responsible professional were considered. Subsequently, the information was analyzed and the scientific article was written.
The results have been presented considering the STROBE checklist.
Statistical Analysis
Descriptive statistics were used, organizing the data in tables and graphs to analyze the behavior of the study variables. For the inferential analysis, the measures of association were processed in JAMOVI, an open-access statistical software designed for data analysis and statistical tests.28 Likewise, a logistic regression model was developed to predict the factors associated with the development of CKD. In this model we can find the OR, with its confidence intervals at 95%, which indicates whether the associated factor is a risk factor for the development of CKD.
Ethical Considerations
This research has the favorable opinion 080-CEI-EPM-UCV-2022 of the Ethics Committee of the School of Medicine - UCV and the permission of the Hospital I Albrecht by means of a certificate from the Training Committee (NIT 2032–2022-251). This committee has the functions of an institutional review board for research projects. The manuscript complies with the Declaration of Helsinki for retrospective reviews. We declare that the privacy of the participants has been preserved and that the data were anonymized. The confidentiality and veracity of the data collected during the study were respected and faithfully presented. Authorship contributions and transparency in conflicts of interest were reported.29
Results
Table 1 shows the characteristics of the patients with follow-up in the Hypertension Control Program in the years 2015–2017. In biological factors, the average age in the three years studied is between 69.79 ± 9.03 to 71.51 ± 9.18 years, the majority are female, average BMI is from 27.77 ± 4.14 to 27.33. ±4.39. In clinical factors, hemoglobin in women reflects an average of 12.37 ± 1.10 to 12.85 ± 0.24, in men, 13.61 ± 1.21 to 13.43 ± 1.25, 22% to 25% with obesity, creatinine average of 0.77 ± 0.20 to 0.88 ± 0.29, around 50% with controlled diabetes and the majority of patients have nephroprotection with enalapril and losartan.
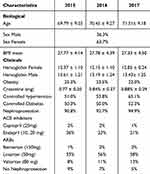 |
Table 1 Characteristics of Patients with Follow-Up in the Hypertension Control Program in the Years 2015–2017 |
Table 2 shows that 28.8% of the people who developed CKD were female and 40.9% were aged 60 years or older.
 |
Table 2 Biological Factors According to the Development of Chronic Kidney Disease in Hypertensive Patients. Period 2015–2017 |
Table 3 shows that anemia and DM remained with similar percentages in the years under study whereas obesity decreased. The percentages of nephroprotection and controlled AHT increased with a significant difference between the years 2015 and 2017 (p < 0.05).
 |
Table 3 Clinical Factors According to Development of Chronic Kidney Disease in Hypertensive Patients. Period 2015–2017 |
Table 4 shows the analysis of a logistic regression model for each year of the study. In 2015 the variables, sex (OR 1.68, CI 1.03–2.76) and anemia (OR 1.71, CI 1.03–2.85) enter as risk factors for CKD (p < 0.05). However, controlled DM (OR 0.18, CI 0.07–0.47) and nephroprotection (OR 0.39, CI 0.18–0.88) were protective factors (p < 0.05).
 |
Table 4 Logistic Regression Model for the Development of Chronic Kidney Disease in Hypertensive Patients According to Factors and Year. Period 2015–2017 |
In Figure 1, it is observed that from 2015 to 2017 there was an increase in the prevalence of CKD in the population studied.
 |
Figure 1 Prevalence of chronic kidney disease in patients with arterial hypertension. |
Figure 2 shows the development of CKD as the years progress, being so that from 2015 to 2017, stage 1 increased by 55.2%, in stage 2 and 3A the increase was more than double with respect to 2015, with stage 2 predominating and in stages 3B and 4 new cases appeared.
 |
Figure 2 Classification of chronic kidney disease according to stage in patients with arterial hypertension. |
Discussion
The medical literature reviewed indicates that kidney disease increases with age and is more prevalent in people aged 55 years and older.2,14,15,30 This is also evident in the present study, where 40.9% of patients with CKD are aged 60 years or older, with average age of 69.79,70.45 and 71.5 years between 2015 and 2017. In the studies consulted, the variable age is related to the progression of CKD, for participants aged 60 years and older14,15,30. It is important to point out that the higher the age, the higher the frequency of CKD, mainly due to a decrease in renal function due to aging15 secondary to glomerular sclerosis and a decrease in the glomerular filtration rate of 1mL/min/1.73 m2 after the age of 40 years.2
The prevalence of CKD was more frequent in the female sex, with almost one-third of the women with this diagnosis. This could be conditioned to the predominance of female patients in the chronic disease control program (63.7% female and 36.3% male), as well as the greater attendance to controls and evaluations in health facilities than men,2 since more women than men request attention for chronic health problems.31 It has also been described that in the Peruvian population pyramid, women predominate after the age of 60 years32 and that survival is greater in this group.4
In the present study, both age 60 years and older and male sex are risk factors for the development of CKD, as reported in other studies14,30 that find that CKD is up to three times higher in men than in women, with a statistically significant relationship between CKD progression and male sex.2,4
Regarding the evolution of the clinical factors studied, it can be seen that there is no difference in the percentages of anemia between 2015 and 2017. The cases of anemia found probably have percentages similar to those of the general population since anemia, especially due to iron deficiency, is common in the Peruvian population, where one in four older adults has anemia.16 Most of the patients in this study are in the early stages of CKD, stages in which the effect of endogenous erythropoietin deficiency, chronic inflammation, decreased iron available for erythropoiesis, vitamin B12, and folic acid deficiency, and other changes characteristic of the late stages of CKD have not yet been observed.17 However, it cannot be ruled out that the presence of anemia in early stages of CKD is of renal cause since it can coexist with ferropenia (iron deficiency)3,17 and in the present study, anemia is a risk factor for the development of CKD.
Regarding obesity, it is noted that there was a reduction from 25.3% to 22% between 2015 and 2017, which could be related to the health education on nutrition and physical activity provided to these patients by family physicians and nurses; as well as the nutritional control by nutrition graduates who are part of the program’s integral health care team. Several authors recognize obesity as a risk factor for the progression of CKD because it generates glomerular hyperfiltration,2,18,19 increased functional demand related to the increase in body mass index with the same number of nephrons, glomerulosclerosis, and renal damage that could occur with leptin hypersecretion.10,33 In the context of this study, the decrease in obesity contributed to the fact that renal disease remained predominant in the early stages.
AHT is one of the causes of the progression of CKD associated with obesity, and significant renovascular disease can develop more easily.18,19 Non-controlled blood pressure has been described as a risk factor for CKD, whereas controlled blood pressure has a protective behavior.2 The results of the present study indicate that controlled AHT increased over the years, with a significant difference between 2015 and 2017 (p < 0.05). Therefore, participating in the program to control older adults with chronic disease improves the follow-up and control of AHT over time, as well as DM. In this study, 50% or more of the population in each year of follow-up had controlled blood pressure and DM. It has also been documented that patients with obesity and uncontrolled AHT and DM have a higher risk of progression of renal disease,18 which is why control of both diseases is recommended as a key objective to reduce renal deterioration.2
The overall prevalence of CKD increased over the study years from 19% in 2015 to 45% in 2017. 2015 was the baseline year, in which the care of patients with hypertension and those over 55 years of age was systematized for the early detection of CKD, through the evaluation of the microalbuminuria/creatinuria and serum creatinine ratio. The staging remained mostly between G1 and G3A, coinciding with that described in the literature, although it will be necessary to evaluate the cohort studied after the fifth year of follow-up, where the change in staging to 3B, IV or more may be much more rapid.2,4,12,14 It is important to mention that in the first level of care of Essalud, the confirmation of stage G3B or G4 is a criterion for referral to nephrology services located in level III hospitals. Likewise, the follow-up of only three years has probably not allowed the detection of more G3B or G4 cases.
The nephroprotection received by more than 90% of the population studied, with ACE inhibitors or ARBs (predominantly enalapril 10 and 20 mg and losartan 50 mg, respectively), was linked to less development of kidney disease in the study group, ie, it acted as a protective factor, according to the results described for the 2015 model, delaying the development of CKD. Nephroprotection is a combined, multidisciplinary therapeutic approach to delay or prevent tubulointerstitial fibrosis and atrophy and modifiable risk factors for CKD progression such as proteinuria, intraglomerular hypertension, and metabolic control in diabetes mellitus.10 Treatment with ACE inhibitors or ARBs has been shown to reduce albuminuria excretion and slow the progression of CKD. On the other hand, RAAS inhibitors are recommended as antihypertensives of choice in patients with CKD and albuminuria with or without diabetes and have been shown to have cardiovascular benefits beyond blood pressure control.27 Likewise, Irbesartan has been shown to decrease the rate of albuminuria in people with diabetes mellitus, independently of AHT.34
Sodium-glucose cotransporter 2 (SGLT2) inhibitors, in addition to their glucose-lowering effects, prevent renal damage, CKD and cardiovascular events;35 however, they are not included in the Essalud pharmacological formulary.
Likewise, in the results it was only possible to establish logistic regression models for CKD risk factors in 2015 and 2016. In 2017, there was no significant model manifesting the behavior of this disease. This could be due to the influence of the control program for adults with chronic disease2 and nephroprotection,27 as it has been shown to improve the monitoring and control of the described risk factors. The possible differences that may arise between the population studied and the current population could be related to the increase in morbimortality (especially cardiovascular morbidity and mortality associated with renal damage) and the greater longevity of the world population.1
We believe that the results regarding anemia and obesity warrant further investigation in the short term, as well as comparing groups with follow-up and nephroprotection with groups without these criteria. The key areas that warrant research to prevent the progression of CKD in patients with hypertension are early screening, periodic monitoring, use of nephroprotective drugs, education on CKD risk factors, diet, physical activity, and treatment adherence.
Scientific Contribution
This article reports the importance of detecting risk factors and protective factors of chronic kidney disease in a program for the control of AHT and DM, which should not only evaluate the controlled condition but also prevent the deleterious effects of CKD on the target organs.
Limitations of the Study
Specific to the retrospective observational design used, the migration to a computerized system in 2018 made it difficult to obtain follow-up information on the study population (between 2015 and 2017, it was collected in manual records within the HTA control program). In addition, the COVID-19 pandemic delayed the collection of information and made it difficult to access the medical records archive due to the lack of responsible personnel.
Conclusions
Biological factors, age 60 years or older and male sex are risk factors for the development of CKD.
Clinical factors such as non-controlled diabetes mellitus and anemia are risk factors for the development of CKD.
Nephroprotection by treatment with ACE inhibitors or ARBs is a protective factor for CKD.
The prevalence of CKD increased throughout the years of the study with a predominance of stage II.
Acknowledgment
We are grateful to Universidad César Vallejo for their support in financing this study.
Author Contributions
All authors made a significant contribution to the manuscript in the conception, study design, execution, data acquisition, analysis and interpretation. They participated in the drafting, critical revision of the article, gave final approval to the version to be published, agreed on the journal to which the article was submitted, as well as accountability for all aspects of the work.
Funding
This study was financed by the Universidad César Vallejo.
Disclosure
The authors declare that they have no conflicts of interest in conducting this research.
References
1. Sellarés VL, Rodríguez DL. Enfermedad Renal Crónica. Nefrologiaaldia.org. Sociedad Española de Nefrología; 2023 Available from: http://www.nefrologiaaldia.org/es-articulo-enfermedad-renal-cronica-136.
2. Bastidas BLB, Quirós Gómez OI. Factores demográficos y clínicos que explican la progresión de la Enfermedad Renal Crónica en un programa de nefroprotección del departamento de Nariño, Colombia 2016–2018. Rev médica Risaralda. 2020;26(2). doi:10.22517/25395203.24533
3. García-Maset R, Bover J, Segura de la morena J, et al. Documento de información y consenso para la detección y manejo de la enfermedad renal crónica. Nefrologia. 2022;42(3):233–264. doi:10.1016/j.nefro.2021.07.010
4. Gorostidi M, Sánchez-Martínez M, Ruilope LM, et al. Prevalencia de enfermedad renal crónica en España: impacto de la acumulación de factores de riesgo cardiovascular. Nefrologia. 2018;38(6):606–615. doi:10.1016/j.nefro.2018.04.004
5. MBl C, Gómez EAO, Hernández AO, García LRF, Barrera MC. Desarrollo de la enfermedad renal crónica en pacientes con hipertensión arterial y/o diabetes mellitus. Universidad Méd Pinareña. 2019;15(1):13–20.
6. Loza-Munarriz CA, Ramos-Muñoz WC Análisis de la situación de la Enfermedad Renal Crónica en el Perú, 2015. Available from: https://www.spn.pe/archivos/ANALISIS%20DE%20LA%20SITUACION%20DE%20LA%20ENFERMEDAD%20RENAL%20CRONICA%20EN%20%20EL%20PERU%201.pdf.
7. Herrera-Añazco P, Atamari-Anahui N, Flores-Benites V. Número de nefrólogos, servicios de hemodiálisis y tendencia de la prevalencia de enfermedad renal crónica en el Ministerio de Salud de Perú. Rev Peru Med Exp Salud Publica. 2019;36(1):62–67. doi:10.17843/rpmesp.2019.361.4253
8. Escalona-González SO, González-Milán ZC, Alarcón-González R. Determinación de enfermedad renal crónica mediante estimación de albuminuria en pacientes con hipertensión arterial de la Atención Primaria de Salud. EsTuSalud. 2020;2(1):1.
9. López RO, Andreu ML, Montemayor VEG, Olmo RS. Hipertensión arterial en la enfermedad renal crónica. Medicine. 2023;13(83):4891–4897. doi:10.1016/j.med.2023.06.019
10. Górriz JL, Górriz-Zambrano C, Pallarés-Carratalá V. Fisiopatología renal y mecanismos farmacológicos de nefroprotección. Semergen. 2023;49(102021):102021. doi:10.1016/j.semerg.2023.102021
11. Arroyo D, Quiroga B, de la Fuente G, de A. Hipertensión arterial en la enfermedad renal crónica. Medicine. 2019;12(81):4772–4778. doi:10.1016/j.med.2019.06.003
12. Figueroa-García J, Granados-García V, Hernández-Rivera JCH, Lagunes-Cisneros M, Alvarado-Gutiérrez T, Paniagua-Sierra JR. Evolution of the stage of chronic kidney disease from the diagnosis of hypertension in primary care. Aten Primaria. 2022;54(7):102364. doi:10.1016/j.aprim.2022.102364
13. Centro Centro Nacional de Epidemiología, Prevención y Control de Enfermedades. Boletín epidemiológico del Perú Volumen 27, SE 52; 2024. Available fom: https://www.dge.gob.pe/portal/docs/vigilancia/boletines/2018/52.pdf.
14. Abascal REC. Aproximación al riesgo de progresión de la enfermedad renal crónica. Exper en Botsuana Acta Médica del Centro. 2018;12(3):293–301.
15. Nieto-Ríos JF, Zuluaga Quintero M, Ariza-Parra EJ, Bello-Márquez DC, Gómez-Castro LT. Es hora de adaptar la definición de la enfermedad renal crónica de acuerdo con la edad. Acta Med Colomb. 2021;46(4):43–45. doi:10.36104/amc.2021.2080
16. Tarqui-Mamani C, Sanchez-Abanto J, Alvarez-Dongo D, Espinoza-Oriundo P, Jordan-Lechuga T. Prevalencia de anemia y factores asociados en adultos mayores peruanos. Rev Peru Med Exp Salud Publica. 2015;32(4):687. doi:10.17843/rpmesp.2015.324.1759
17. Cases A, Egocheaga MI, Tranche S, et al. Anemia en la enfermedad renal crónica: protocolo de estudio, manejo y derivación a Nefrología. Nefrologia. 2018;38(1):8–12. doi:10.1016/j.nefro.2017.09.004
18. Obesidad y Progresión de la Enfermedad Renal. Nefrologiaaldia.org. [citado el 28 de abril de 2023]; 2023. Available from: http://www.nefrologiaaldia.org/es-articulo-obesidad-progresion-enfermedad-renal-21.
19. Mendoza-Niño C, Martínez-Robles JD, Gallardo-García I. Relación entre sobrepeso y obesidad con la progresión de la enfermedad renal crónica en pacientes del Centro Médico Naval en México. Enferm Nefrol. 2023;26(1):60–66. doi:10.37551/s225428842023007
20. Cheung AK, Chang TI, Cushman WC, et al. KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int. 2021;99(3):S1–87. doi:10.1016/j.kint.2020.11.00
21. Araújo DL, Betancourt DB, Gabriela D, et al.La Hipertensión Arterial es factor de riesgo para el desarrollo y progresión de la Enfermedad Renal Crónica; 2023. Available from: http://www.scielo.edu.uy/pdf/rumi/v1n3/v01n03a01.pdf.
22. Tocora DGG, Del Valle KMP, Hernández Sevillano B, de la Fuente G de A. Nefropatías vasculares: hipertensión vasculorrenal y nefropatía isquémica. Medicine. 2019;12(81):4779–4785. doi:10.1016/j.med.2019.06.004
23. Huang CW, Wee PH, Low LL, et al. Prevalence and risk factors for elevated anxiety symptoms and anxiety disorders in chronic kidney disease: a systematic review and meta-analysis. Gen Hosp Psych. 2021;69:27–40. doi:10.1016/j.genhosppsych.2020.12.003
24. Palmer S, Vecchio M, Craig JC, et al. Prevalence of depression in chronic kidney disease: systematic review and meta-analysis of observational studies. Kidney Int. 2013;84(1):179–191. doi:10.1038/ki.2013.77
25. Filtración Glomerular Primaria (MDRD-4) Ecuación: mediCalculato: scyMed; 2023. Available from: http://www.scymed.com/es/smnxps/psdgp182.htm.
26. Resolución No 1185-GG-ESSALUD-2020 de Seguro Social de Salud del Perú (ESSALUD), 13–10-2020. vLex. [citado el 3 de noviembre de 2022].; 2024. Available from: https://vlex.com.pe/vid/resolucion-n-1185-gg-875321031.
27. Egocheaga MI, Drak Y, Otero V. Nefroprotección clásica: inhibidores del sistema renina angiotensina aldosterona. Semergen. 2023;49(102018):102018. doi:10.1016/j.semerg.2023.102018
28. Jamovi - open statistical software for the desktop and cloud. Jamovi.org; 2024. Available from: https://www.jamovi.org/.
29. Declaración de Helsinki de la AMM – Principios éticos para las investigaciones médicas en seres humanos. Wma.net. [citado el 30 de setiembre de 2022].; 2024. Available from: https://www.wma.net/es/policies-post/declaracion-de-helsinki-de-la-amm-principios-eticos-para-las-investigaciones-medicas-en-seres-humanos/.
30. De la Fuente G, Del Valle KMP A, Gaitán Tocora DG, Puyol DR. Hipertensión arterial y riñón. Medicine. 2019;12(81):4759–4764.
31. Instituto Nacional de Estadística e Informática. Estadística con enfoque de género. Informe técnico N;.2024.
32. Pirámides de población del mundo desde 1950 a 2100. PopulationPyramid.net. 2024. Available from: https://www.populationpyramid.net/es/mundo/2023/.
33. Gustavo N, Leopoldo A. Obesidad y enfermedad renal crónica: una peligrosa asociación. Rev méd Chile. 2015;143(1):77–84. doi:10.4067/S0034-98872015000100010
34. Sasso FC, Carbonara O, Persico M, et al. Irbesartan reduces the albumin excretion rate in microalbuminuric type 2 diabetic patients independently of hypertension. Diabetes Care. 2002;25(11):1909–1913. doi:10.2337/diacare.25.11.1909
35. Salvatore T, Galiero R, Caturano A, et al. An overview of the cardiorenal protective mechanisms of SGLT2 inhibitors. Int J Mol Sci. 2022;23(7):3651. doi:10.3390/ijms23073651
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
