Back to Journals » Journal of Asthma and Allergy » Volume 13
Factors Associated with Failure of Intermittent Nebulization with Short-Acting Beta-Agonists in Children with Severe Asthma Exacerbation
Authors Kulalert P, Phinyo P , Patumanond J , Smathakanee C , Chuenjit W, Nanthapisal S
Received 16 April 2020
Accepted for publication 11 August 2020
Published 25 August 2020 Volume 2020:13 Pages 275—283
DOI https://doi.org/10.2147/JAA.S258549
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Amrita Dosanjh
Prapasri Kulalert,1 Phichayut Phinyo,2,3 Jayanton Patumanond,3 Chutima Smathakanee,4 Wantida Chuenjit,4 Sira Nanthapisal5
1Department of Clinical Epidemiology, Faculty of Medicine, Thammasat University, Pathum Thani, Thailand; 2Department of Family Medicine, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand; 3Center for Clinical Epidemiology and Clinical Statistics, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand; 4Department of Pediatrics, Hat Yai Hospital, Songkhla, Thailand; 5Department of Pediatrics, Faculty of Medicine, Thammasat University, Pathum Thani, Thailand
Correspondence: Phichayut Phinyo
Department of Family Medicine, Faculty of Medicine, Chiang Mai University, 110/510 Intawaroros Road, Suthep, Mueang Chiang Mai District, Chiang Mai, Chiang Mai Province 50200, Thailand
Tel +66 89 850 1987
Email [email protected]
Purpose: Intermittent nebulization of short-acting beta-agonists (SABA) is the initial treatment of choice for children with asthma exacerbation. However, children with severe asthma exacerbation (SAE) may not show an adequate response and need aggressive stepwise therapy. We aimed to explore factors associated with a poor response to intermittent nebulized SABA in children with SAE.
Methods: A retrospective cohort study of children with SAE diagnosed according to the definition of the British Guidelines on the Management of Asthma, who were admitted at Hat Yai Hospital from January 1, 2015, to December 31, 2017. All children were treated with intermittent SABA nebulization. Treatment failure was defined as children needing escalated therapy. Logistic regression with confounding score adjustment was used to explore the predictors of treatment failure.
Results: One hundred thirty-three children were included in the analysis, 59 were in the failure group and 74 were in the success group. After adjusting for potential confounders, they were significantly associated with a previous history of intubation (adjusted OR 6.46, 95% CI 1.13 to 36.79, p=0.036), receiving < 3 doses of nebulized salbutamol in the emergency room (ER, aOR 3.21, 95% CI 1.15 to 9.02, p=0.027), ER measured oxygen saturation (SpO2) < 92% (adjusted OR 3.02, 95% CI 1.18 to 7.75, p=0.022), and exacerbation triggered by pneumonia (adjusted OR 2.67, 95% CI 1.19 to 6.00, p=0.017).
Conclusion: We identified four prognostic factors of treatment failure in children with SAE: a previous history of intubation; receiving < 3 doses of nebulized salbutamol in the ER, SpO2 at ER < 92%; and exacerbation triggered by pneumonia. Further prospective studies are required to confirm our findings before clinical implementation.
Keywords: status asthmaticus, hospitalization, nebulization, treatment failure, prognosis
Introduction
The incidence of children with severe asthma exacerbation (SAE) who require intensive care treatment and monitoring is increasing.1,2 Children with inadequate responses at the emergency room (ER) were admitted for continuation of treatment until their symptoms subsided. The first-line therapy for these children generally includes intermittent nebulized short-acting beta-agonists (SABA) every one to four hours, administration of systematic corticosteroids (eg, oral prednisolone 1–2 mg/kg/day), and oxygen supplementation to achieve an oxygen saturation (SpO2) >95%.3–5 Clinical guidelines and experts recommend nebulization of ipratropium bromide plus fenoterol (Berodual) and subcutaneous (sc) injection of terbutaline as adjunctive therapies.4–7 However, approximately 20% of children with SAE respond poorly to nebulized SABA and subsequently required a more aggressive stepwise approach (ie, intravenous (IV) magnesium sulfate, IV terbutaline, admission to a pediatric intensive care unit (PICU), endotracheal intubation, and mechanical ventilation).8–10
In children with SAE, failure of the initial regimen is the leading cause of asthma complications, increased costs, and prolonged hospital stays.11,12 Several studies have proposed factors associated with fatal or near-fatal events (ie, PICU admission, endotracheal intubation and mechanical ventilation. These include 1) a previous history of endotracheal intubation and mechanical ventilation due to asthma exacerbation; 2) ≥2 asthma admissions within the previous 12 months; 3) ≥3 ER visits for asthma within the previous 12 months; 4) not currently using asthma controller medication; 5) obesity; 6) SpO2 at first presentation <92%; or 7) exacerbation triggered by pneumonia.5,6,13–17
One meta-analysis confirmed the benefit of the timely administration of systemic corticosteroids within 60 minutes of presentation to an ER in reducing the need for hospitalization.18 In our setting, we observe that children who had inadequate nebulization (<3 doses of nebulized SABA) in the ER were more likely to be hospitalized. Thus, inadequate management in the ER may be an important factor influencing the initial response and prognosis.
There are currently no studies that have evaluated factors associated with the failure of intermittent nebulized SABA. Recognizing these predictors could guide physicians in identifying high-risk children and lead to better first-line care.
In this study, we aimed to explore potential prognostic factors associated with a failure response to intermittent nebulized SABA in children with SAE.
Materials and Methods
Study Design
Prognostic factor research with a retrospective cohort design was conducted. We retrieved admission records of children with asthma exacerbation who were admitted to Hat Yai Hospital, a regional referral hospital in southern Thailand, from January 1, 2015, to December 31, 2017.
The Institutional Review Board of the Hat Yai Hospital (Protocol number 33/2561) and the Faculty of Medicine, Thammasat University (MTU-EC-ES-0-061/61), approved the study protocol. Informed consent was waived as the data collection was retrospective and without any patient identifiers (ie, all data were anonymized). Asthma diagnosis was based on the International Classification of Diseases, Tenth Revision, or ICD-10 discharge diagnosis codes J45 and J46.
We screened the medical records and included the following children for analysis: 1) age 1–15 years, 2) prior documented evidence of physician-diagnosed asthma or symptoms suggestive of asthma according to GINA guidelines, 3) diagnosis of SAE according to the criteria of the British Guideline on the Management of Asthma of the British Thoracic Society (BTS) (Table 1),19 4) documented treatment with nebulized SABA (salbutamol 2.5 mg/dose) delivered via face mask with an oxygen flow rate of 6–8 L/min every 1–4 hours, and 5) received the recommended dose of systemic corticosteroid and adequate supplemental oxygen therapy.3,4 We did not include children aged <12 months in this study, as an accurate diagnosis of asthma in this age group is difficult.
 |
Table 1 Severity Classification of Asthma Exacerbation According to the British Guideline on the Management of Asthma for Hospitalized Children |
Children who were initially prescribed with treatment regimens other than intermittent nebulized SABA (eg, nebulized adrenaline or nebulized 3% NaCl), with chronic pulmonary comorbidity, who were referred to or from other hospitals before their resolution of asthma symptoms, and those with incomplete data (pre-specified prognostic factors and confounders) were excluded from our study.
In the ER, all children with asthma exacerbation were managed according to standardized local practice guidelines by delivering up to three dosages of 2.5 mg salbutamol, nebulized every 15–20 minutes with the optional use of sc terbutaline. Children with an inadequate clinical response in the ER would be admitted to the pediatric ward or PICU. According to our inclusion criteria, only children admitted to the hospital for intermittent nebulization were included. After the completion of intermittent nebulization, children failing nebulized salbutamol were defined as any one of the followings: 1) the need for continuous SABA nebulization, 2) prescription of IV terbutaline, 3) requiring airway support: continuous positive airway pressure (CPAP), bi-level positive airway pressure, or endotracheal intubation and mechanical ventilation. The success group was defined as children whose asthma exacerbation completely resolved after intermittent nebulization with SABA and were discharged from the hospital.
Candidate Prognostic Factors
We explored ten candidate factors for their association with treatment failure. All factors, including their cut-off values, were chosen according to previously published studies: 1) ≥1 previous history of endotracheal intubation and mechanical ventilation due to exacerbation of asthma,3,5,14 2) ≥2 admissions for asthma within the previous 12 months,5 3) ≥3 ER visits for asthma within the past 12 months,5,16 4) not using controller medications,3,6 5) obesity,15 6) SpO2 at initial presentation in the ER <92%,16 7) exacerbation triggered by pneumonia,13,17 8) inadequate salbutamol nebulization in the ER (<3 doses),3,4,19 9) delayed administration of systemic corticosteroids (>60 mins after ER arrival),3 and 10) received terbutaline injections given in the ER.13 The 90th percentile for weight-for-age defined obesity.20
Other Pre-Specified Confounders
Other than pre-selected candidate factors, relevant confounders may arise during exploratory analysis. Age and gender were not well-established prognostic factors of asthma,3 and results across studies are inconsistent.13,16,17,21,22 Nevertheless, we included age and sex in our logistic regression model. We also included the administration of any concomitant medication into the model for statistical adjustments, such as sc terbutaline injection and Berodual nebulizer that may have been prescribed at the discretion of the attending pediatrician.5–7
Data Collection
Data were collected on gender, age, weight, diagnosis of asthma, use of controller medications, number of visits to the ER within the past 12 months, number of hospitalizations within the past 12 months, and history of intubation due to asthma exacerbation, number of salbutamol nebulization in the ER and in the hospital, prescription of concomitant drugs, and the trigger for the current exacerbation episode.
Statistical Analysis
All statistical analyses were performed using Stata version 16 (StataCorp, Lakeway, Texas, USA). Frequencies/percentages described the categorical variables and mean and standard deviation or median and interquartile ranges (IQR) were used to describe continuous variables, as appropriate. Categorical data were analyzed by chi-squared or Fisher’s exact test, as appropriate. An independent t-test was used for continuous data with a normal distribution and the Wilcoxon rank sum test was used for skewed data. A two-sided p-value <0.05 was considered statistically significant.
Derivation of Confounding Score and Multivariable Modeling
For the derivation of a confounding score (disease risk score or balancing score),23,24 logistic regression was used for statistical modeling of pretreatment confounders and confounders by concomitant medications to estimate the probability of failing first-line therapy. For the age variable and other continuous variables, second-level fractional polynomial was used to include the variable within the model. Finally, two confounding scores, one for pretreatment and another for concomitant medications, were derived separately from two logistic models.
For the exploratory analysis, multivariable logistic regression with confounding score adjustment was used to model the ten candidate prognostic factors. This model was reduced by manual backward elimination of non-significant predictors, yielding the final reduced model. The results are reported as the adjusted odds ratios (aOR) with their 95% confidence intervals (CI).
Results
A total of 568 medical charts were screened. Three-hundred and thirty-six records did not fulfill the inclusion criteria: 154 were moderate asthma exacerbation, 22 were life-threatening asthma exacerbation, 64 were not managed according to our study protocol, 41 were not diagnosed with asthma and did not have symptoms suggestive of asthma according to GINA guidelines, 34 were aged <12 months, and 21 did not have asthma (eg, bronchiolitis or anaphylaxis) (Figure 1). Thus, we identified 232 children with SAE. Of these, 99 were excluded from the analysis: 15 did not receive intermittent nebulization of SABA as their first-line therapy, 21 had chronic cardiopulmonary comorbidity, 12 were referred to other hospitals before complete resolution of their exacerbation, 3 were referred from other hospitals, and 48 had incomplete data on their treatment in the ER.
 |
Figure 1 Study flow diagram of patient cohort. |
The final dataset consisted of 133 children with SAE were included. Treatment failures and successes numbered 59 (44.4%) and 74 (55.6%), respectively. The details on escalation therapy in the failure group were as follows: 47 children were converted to continuous SABA nebulization, eight were injected with intravenous terbutaline, and four were intubated and mechanically ventilated.
There were no significant differences between groups in terms of clinical characteristics, pretreatment prognostic factors, and concomitant medications between groups (Table 2).
 |
Table 2 Clinical Characteristics, Pre-Specified Prognostic Factors, and Confounders of the Study Patients |
In the failure group, there were significantly higher proportions of children with history of endotracheal intubation (17.0% vs 2.7%, p=0.006), exacerbation triggered by pneumonia (54.2% vs 27.0%, p=0.002), SpO2 in the ER <92% (33.9% vs 14.9%, p=0.013), nebulized salbutamol <3 doses in the ER (30.5% vs 10.8%, p=0.007), and those receiving injection of terbutaline (17.0% vs 5.4%, p=0.045).
Factors Associated with Failure of Intermittent SABA Therapy
The logistic modeling results to derive the confounding scores are presented in Table 3. The generated confounding scores were subsequently included in the multivariable logistic regression with all ten potential predictors of failing first-line intermittent nebulization with SABA (Table 4).
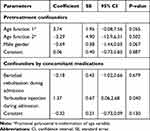 |
Table 3 Multivariable Logistic Regression Model to Derive the Confounding Scores |
 |
Table 4 Multivariable Logistic Regression for Predictors of Intermittent SABA Failure with Prognostic Score Adjustment |
In the full model, four variables were significantly associated with treatment failure: a previous history of endotracheal intubation, exacerbation triggered by pneumonia, a SpO2 <92% in the ER, and receiving <3 doses of nebulized salbutamol in the ER. After the backward elimination of non-contributing factors, these four factors remained significant explanatory factors. History of endotracheal intubation (aOR 6.46, 95% CI 1.13 to 36.79, p=0.036) was identified as the strongest prognostic factor, followed by <3 doses of nebulized salbutamol (aOR 3.21, 95% CI 1.15 to 9.02, p=0.027), SpO2 <92% (aOR 3.02, 95% CI 1.18 to 7.75, p=0.022), and exacerbation triggered by pneumonia (aOR 2.67, 95% CI 1.19 to 6.00, p=0.017).
Discussion
In this study, four factors were associated with failure of intermittent nebulization with SABA in children who were diagnosed with SAE; a history of endotracheal intubation due to asthma exacerbation, inadequate of initial nebulization treatment with salbutamol at ER, oxygen saturation less than 92% upon presentation, and exacerbation triggered by pneumonia.
Of all four factors, a prior history of intubation for a previous asthma exacerbation was the most potent predictor of treatment failure. This is in concordance with a systematic review and meta-analysis, which reported that patients with a history of intubation had an increased risk of a fatal or near-fatal asthma exacerbation.14 The second strongest factor was an inadequate nebulization of SABA, mostly salbutamol in our setting, in the ER. Standard asthma treatment guidelines in our hospital recommend an initial nebulization with three doses of SABA every 15–20 minutes, together with adequate supplemental oxygen, and systemic corticosteroids within the first hour of the patient’s arrival in the ER.3,4,19 Despite these recommendations, almost 20% of children with severe asthma exacerbation were not properly nebulized in the ER before their admission to hospital, and these children had a 3-fold increased risk of treatment failure. Thus, it is mandatory to encourage emergency physicians to follow local guidelines and complete the three doses of nebulization in children with SAE.
A low SpO2 <92% at initial presentation was another independent, ominous factor for treatment failure. This cut-off has also been identified in earlier studies as a predictive factor for hospitalization,25 admission to an intensive care unit, and a requirement for IV terbutaline or intubation.16 Clinical guidelines recommend oxygen saturation as an objective and useful measure to assess and classify the severity of asthma exacerbations.26 As oxygen saturation measuring devices like pulse oximeters are cheap, practical and readily available in hospitals, we suggest it should always be used in asthma patients as an indicator for predicting failure to intermittent nebulization with SABA.
Children whose SAE was triggered by pneumonia were also at an increased risk of failing the first-line intermittent nebulization therapy. Pneumonia, as a trigger of SAE, has been reported by others as a risk factor for admission to an intensive care unit.13,17 Pneumonia is a lower respiratory tract infection that involves alveolar inflammation, which does not improve after treatment with bronchodilators alone. Therefore, such children should be treated with antibiotics or antivirals if their pneumonia is of bacterial or viral origin, respectively.27
Some clinically relevant factors have been reported to be associated with poor asthma outcomes but did not show statistical significance in our study. Children who were prescribed with sc terbutaline in the ER had a higher risk of failing continuous nebulization with SABA.13 In our study, the magnitude of the effect of sc terbutaline on the risk of treatment failure was large but was not statistically significant; this may have been due to our limited sample size and the small number (n=8) of children prescribed with sc terbutaline in the ER. Previous studies report that children with frequent, ≥3 ER visits for asthma exacerbation within the past 12 months, had an increased risk of admission to intensive care.16 Our study did not reconfirm this due to its small size.
Corticosteroids are widely accepted as beneficial in acute asthma and are recommended by many treatment guidelines. Their use decreases inflammatory cytokines, reduces airway swelling, and up-regulates the β-receptors in the bronchial smooth muscle with enhanced efficacy of β-agonists in reducing bronchoconstriction.28 Prompt steroid administration within 60 minutes of presentation reduces hospital admission.18 In our study, only 52 (39%) children were prescribed corticosteroids within 60 minutes, but we were unable to demonstrate statistical significance, due to our small sample size. Our result emphasizes the need for guidelines to be followed, and ER physicians to manage asthma patients rapidly.
Our study provides evidence of prognostic factors linked to the failure of intermittent nebulization of SABA in children with SAE. These factors are easy to evaluate through a brief history taking, a focused clinical examination, and, if practical, a chest X-ray. The knowledge of these factors can alert ER physicians and pediatricians to prioritize such children, treat them promptly following recommended guidelines, and prevent severe adverse outcomes, such as more frequent monitoring for clinical deterioration, early escalation of therapy, or admission to an intensive care unit.
The application of confounding score adjustment incorporated pretreatment confounders and confounders of concomitant medications is a useful technique for controlling of confounder in an observational study and added strength to our study. The statistical power was preserved because the total number of covariates was reduced. After model reduction through backward elimination, it was evident that the precisions of all estimates were improved. There were some limitations to our study. First, the data were retrospectively reviewed and collected from an electronic medical database of Hat Yai hospital. Thus, several patients with incomplete data were excluded from the analysis, which may give rise to selection bias. However, our study was a cohort, where all included children were followed since their admissions to the hospital until their dispositions. Therefore, the effect of reported prognostic factors on the outcome may be validly drawn. Second, the study size was small and might not be adequately powered to identify statistically significant of some predictors in the full model. Before clinical implementation of our findings, more supporting evidence from larger, prospective studies or clinical trials is required. Third, according to our inclusion and exclusion criteria, our study results were not generalizable to children aged less than one year, children with an unclear diagnosis of asthma, and children with moderate or life-threatening asthma exacerbation. Finally, even after confounding score adjustment, residual confounding may still be present. However, as the direction and magnitude of the association between the predictors in the final model and the outcome were sensible, the effect of residual confounding is likely minimal.
Conclusion
In conclusion, children with SAE are at higher risk of failing intermittent nebulization with SABA if they have a previous history of intubation, a low SpO2 <92%, are inadequately treated with nebulized salbutamol in the ER, and who have pneumonia. Such children need rapid treatment and close observation. However, due to important limitations in study design, further prospective clinical studies are needed to confirm our findings before clinical implementation.
Acknowledgments
The authors would like to thank Ms Debra Kim Liwiski, writer/international instructor, Clinical Research Center, and Dr. Walter Robert John Taylor, an international instructor at Research Administration Section, Faculty of Medicine, for Scientific English editing.
Disclosure
The authors have no potential conflicts of interest to declare with respect to the authorship and publication of this article or this work.
References
1. Al-Eyadhy AA, Temsah MH, Alhaboob AA, et al. Asthma changes at a pediatric intensive care unit after 10 years: observational study. Ann Thorac Med. 2015;10(4):243–248. doi:10.4103/1817-1737.165302
2. Boeschoten SA, Buysse CMP, Merkus P, et al. Children with severe acute asthma admitted to Dutch PICUs: a changing landscape. Pediatr Pulmonol. 2018;53(7):857–865. doi:10.1002/ppul.24009
3. Global Initiative for Asthma. Global strategy for asthma management and prevention; 2019. Available from: https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf.
4. Pardue Jones B, Fleming GM, Otillio JK, Asokan I, Arnold DH. Pediatric acute asthma exacerbations: evaluation and management from emergency department to intensive care unit. J Asthma. 2016;53(6):607–617. doi:10.3109/02770903.2015.1067323
5. Camargo CA
6. Fergeson JE, Patel SS, Lockey RF. Acute asthma, prognosis, and treatment. J Allergy Clin Immunol. 2017;139(2):438–447. doi:10.1016/j.jaci.2016.06.054
7. Nievas IF, Anand KJ. Severe acute asthma exacerbation in children: a stepwise approach for escalating therapy in a pediatric intensive care unit. J Pediatr Pharmacol Ther. 2013;18(2):88–104. doi:10.5863/1551-6776-18.2.88
8. Bratton SL, Odetola FO, McCollegan J, Cabana MD, Levy FH, Keenan HT. Regional variation in ICU care for pediatric patients with asthma. J Pediatr. 2005;147(3):355–361. doi:10.1016/j.jpeds.2005.05.008
9. Hartman ME, Linde-Zwirble WT, Angus DC, Watson RS. Trends in admissions for pediatric status asthmaticus in New Jersey over a 15-year period. Pediatrics. 2010;126(4):e904–11. doi:10.1542/peds.2009-3239
10. Newth CJ, Meert KL, Clark AE, et al. Fatal and near-fatal asthma in children: the critical care perspective. J Pediatr. 2012;161(2):214–21.e3. doi:10.1016/j.jpeds.2012.02.041
11. Carroll CL, Zucker AR. The increased cost of complications in children with status asthmaticus. Pediatr Pulmonol. 2007;42(10):914–919. doi:10.1002/ppul.20682
12. Chiang BL, Hsieh CT, Wang LC, et al. Clinical course and outcome of children with status asthmaticus treated in a pediatric intensive care unit: a 15-year review. J Microbiol Immunol Infect Wei Mian Yu Gan Ran Za Zhi. 2009;42(6):488–493.
13. Kenyon CC, Fieldston ES, Luan X, Keren R, Zorc JJ. Safety and effectiveness of continuous aerosolized albuterol in the non-intensive care setting. Pediatrics. 2014;134(4):e976–82. doi:10.1542/peds.2014-0907
14. Alvarez GG, Schulzer M, Jung D, Fitzgerald JM. A systematic review of risk factors associated with near-fatal and fatal asthma. Can Respir J. 2005;12(5):265–270. doi:10.1155/2005/837645
15. Okubo Y, Nochioka K, Hataya H, Sakakibara H, Terakawa T, Testa M. Burden of obesity on pediatric inpatients with acute asthma exacerbation in the United States. J Allergy Clin Immunol Pract. 2016;4(6):1227–1231. doi:10.1016/j.jaip.2016.06.004
16. Belessis Y, Dixon S, Thomsen A, et al. Risk factors for an intensive care unit admission in children with asthma. Pediatr Pulmonol. 2004;37(3):201–209. doi:10.1002/ppul.10443
17. Grunwell JR, Travers C, Fitzpatrick AM. Inflammatory and comorbid features of children admitted to a PICU for status asthmaticus. Pediatr Crit Care Med. 2018;19(11):e585–e594. doi:10.1097/pcc.0000000000001695
18. Rowe BH, Spooner C, Ducharme FM, Bretzlaff JA, Bota GW. Early emergency department treatment of acute asthma with systemic corticosteroids. Cochrane Database Syst Rev. 2001;1:Cd002178. doi:10.1002/14651858.Cd002178
19. British Thoracic Society, Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma. Thorax. 2014;69(Suppl 1):1–192.
20. Gamliel A, Ziv-Baran T, Siegel RM, Fogelman Y, Dubnov-Raz G. Using weight-for-age percentiles to screen for overweight and obese children and adolescents. Prev Med. 2015;81:174–179. doi:10.1016/j.ypmed.2015.08.017
21. Lyell PJ, Villanueva E, Burton D, Freezer NJ, Bardin PG. Risk factors for intensive care in children with acute asthma. Respirology. 2005;10(4):436–441. doi:10.1111/j.1440-1843.2005.00726.x
22. Cundiff KM, Gerard JM, Flood RG. Critical care interventions for asthmatic patients admitted from the emergency department to the pediatric intensive care unit. Pediatr Emerg Care. 2018;34(6):385–389. doi:10.1097/pec.0000000000001163
23. Tadrous M, Gagne JJ, Sturmer T, Cadarette SM. Disease risk score as a confounder summary method: systematic review and recommendations. Pharmacoepidemiol Drug Saf. 2013;22(2):122–129. doi:10.1002/pds.3377
24. Arbogast PG, Ray WA. Performance of disease risk scores, propensity scores, and traditional multivariable outcome regression in the presence of multiple confounders. Am J Epidemiol. 2011;174(5):613–620. doi:10.1093/aje/kwr143
25. Keahey L, Bulloch B, Becker AB, Pollack CV
26. Carroll CL, Sala KA. Pediatric status asthmaticus. Crit Care Clin. 2013;29(2):153–166. doi:10.1016/j.ccc.2012.12.001
27. Sandora TJ, Harper MB. Pneumonia in hospitalized children. Pediatr Clin North Am. 2005;52(4):1059–1081, viii. doi:10.1016/j.pcl.2005.03.004
28. Rehder KJ. Adjunct therapies for refractory status asthmaticus in children. Respir Care. 2017;62(6):849–865. doi:10.4187/respcare.05174
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
