Back to Journals » Clinical Ophthalmology » Volume 17
Factors Affecting Visual Recovery in Patients with Ethambutol-Induced Optic Neuropathy
Authors Srithawatpong S, Chaitanuwong P , Yospaiboon Y
Received 30 December 2022
Accepted for publication 27 January 2023
Published 9 February 2023 Volume 2023:17 Pages 545—554
DOI https://doi.org/10.2147/OPTH.S401916
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Supaporn Srithawatpong,1,2 Pareena Chaitanuwong,1,2 Yosanan Yospaiboon3
1Department of Ophthalmology, Rajavithi Hospital, Ministry of Public Health, Bangkok, Thailand; 2Department of Ophthalmology, College of Medicine, Rangsit University, Bangkok, Thailand; 3Department of Ophthalmology, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand
Correspondence: Yosanan Yospaiboon, Department of Ophthalmology, Faculty of Medicine, Khon Kaen University, 123 Mitraparb Highway, Khon Kaen, 40002, Thailand, Tel +66-4336-3010, Fax +66-4334-8383, Email [email protected]
Purpose: To study visual recovery and identify the factors that may affect it in patients with ethambutol-induced optic neuropathy (EON).
Patients and Methods: Medical charts of patients who developed optic neuropathy after ethambutol (EMB) treatment for tuberculosis infection were retrospectively reviewed. Demographic details and clinical data were examined to assess visual recovery after discontinuation of ethambutol treatment. The univariate and multivariate relationships between various factors and visual recovery were evaluated using regression analysis.
Results: Of 5394 patients diagnosed with tuberculosis infection and treated with EMB, 23 patients (0.43%) were diagnosed with EON. Logistic regression analysis found that female sex was the categorical factor significantly associated with good visual recovery with an odds ratio of 12.0 (95% confidence interval 1.56, 92.29; p = 0.02), while linear regression analysis identified good initial visual acuity as the numerical factor significantly related with it (p < 0.001). After adjustment with multivariate analysis, initial visual acuity was found to be the only significant factor associated with visual recovery. All patients with initial visual acuity of better than 20/200 at first visit achieved good visual recovery.
Conclusion: The incidence of EON in patients treated with EMB was 0.43% in this hospital-based study. Good visual recovery was noted in 39.13% of these patients, and initial visual acuity was the factor that affected visual recovery. It is recommended that patients on EMB have regular screening by an ophthalmologist for early detection of the disease, and if it is discovered, that the use of the drug be immediately discontinued in order to prevent potentially devastating visual loss.
Keywords: ethambutol, optic neuropathy, visual recovery
Introduction
Tuberculosis is a communicable disease that is a major cause of ill health and one of the causes of death worldwide.1 In the treatment of tuberculosis, isoniazid, rifampin, ethambutol (EMB), and pyrazinamide are considered first-line drugs and form the core of standard treatment regimens. Although EMB is effective in treating mycobacterial infections when combined with other anti-tuberculous drugs, it can cause mitochondrial optic neuropathy.2 Ethambutol-induced optic neuropathy (EON) is an uncommon but well-recognized adverse event in patients receiving EMB treatment for tuberculosis. Its reported incidence has varied widely across studies, ranging from 0.5% to 2.25% of patients taking the drug at the WHO recommended doses.3–8 Some previous studies have reported that EON is dose-related, and that it is reversible after immediate EMB discontinuation, but others have revealed irreparable damage.9–12 Due to EON’s potentially catastrophic visual impact, numerous studies have emphasized the importance of early detection of the disease and signs of its risk factors.8,13–16 Mandal et al conducted a prospective study to evaluate indicators for early detection of EON and found that, in 46% of eyes, subclinical damage was signaled by an increase in visual-evoked potential (VEP) latency, a decrease in retinal nerve fiber layer thickness and a distinct ganglion cells layer loss detected by optical coherence tomography (OCT).8 Menon et al also reported that pattern VEP and visual field examinations were sensitive tests for detection of early EMB toxicity,13 and Choi et al emphasized the importance of color vision test in identification of early subclinical EON.14 Age, dose and duration of EMB treatment, hypertension, and renal diseases have also been reported to be risk factors for toxicity;15,16 however, data relating to visual recovery and its associated factors in patients with EON are as yet limited and inconclusive.17 This study, conducted in a large tertiary eye center, aimed to study visual recovery and determine the factors that may affect it in patients with EON.
Patients and Methods
This retrospective observational study was performed in accordance with the principles of the Declaration of Helsinki, the International Conference on Harmonization, and the Good Clinical Practice Guidelines, and it was approved by the institutional ethics committee of Rajavithi Hospital. From January 2012 to December 2021, we recruited patients who complained of decreased vision after EMB treatment for tuberculosis infection. Although patient consent to review their medical records was not required by the ethics committee, the data in case report forms nevertheless had no linkage to the patient identities and the patient data confidentiality was protected. EON was diagnosed by ophthalmologists when there was decreased visual acuity, abnormal color vision identified by Ishihara isochromatic test, and central or paracentral scotoma on the Goldmann or Humphrey perimeter. Abnormal visual field defects, other than central or paracentral scotomas, optic disc pallor, and abnormal OCT findings, were also used for diagnosis. Patients were excluded if they had other retina or macular diseases, cataract, glaucoma, or other optic neuropathies.
The collected data included: age; gender; body weight; underlying diseases, such as diabetes mellitus, hypertension, dyslipidemia, and HIV infection; and history of smoking. Site of tuberculosis infection, daily dose and duration of EMB treatment, visual acuity, visual field, and color vision test results were recorded. Fundus photography (Kowa VX-20, Kowa, Japan) and Optical Coherence Tomography (Spectralis, Heidelberg Engineering, Germany) images were also obtained if available.
The primary outcome was visual recovery of EON patients measured by the difference between initial visual acuity at first visit when the patient complained of decreased vision and best final visual acuity after discontinuation of EMB treatment. Patients were defined as having good visual recovery when they had visual improvement and final best-corrected visual acuity (BCVA) of 20/50 or better. Those who had no visual improvement or some improvement but final BCVA worse than 20/50 were defined as having poor visual recovery. Visual acuity was recorded in Snellen notation and converted to logMAR using the formula: logMAR = -log(Snellen decimal).
Counting fingers (CF) was recorded as 20/1000, hand movement (HM) as 20/2000 and light perception (LP) as 20/4000 Snellen visual acuity, in accordance with the criteria employed by Steinberg et al.18 Mean initial and final visual acuity for analytical purposes were taken as the mean logMAR acuity of both eyes in all patients.
Statistical Analysis
All analyses were performed with SPSS Statistics Version 22.0 (IBM Corp., Chicago, IL, USA). Data were presented as mean and standard deviation (SD) unless otherwise noted. Either Pearson’s Chi-square test or Fisher’s Exact test was used for comparison between two categorical variables, and Student’s t-test was employed to compare numerical variables between two groups. The univariate and multivariate relationships between various factors and visual recovery were analyzed by logistic and linear regression. A p-value of less than 0.05 was considered statistically significant.
Results
During the 10-year period (2012–2021), 5394 patients were diagnosed with tuberculosis infection and treated with multiple drugs, including EMB. Of these, 23 (0.43%) who complained of blurred vision after EMB treatment were diagnosed as having EON: 14 (60.87%) had primary pulmonary tuberculosis; five (21.74%) had tuberculosis of the spine; one had tuberculosis of the knee; one had pleural tuberculosis; one had tuberculous liver abscess; and one had tuberculous meningitis.
The mean age of the patients at the time of starting medication was 58.2 ± 11.4 (95% CI 53.3, 63.1) years, range 27 to 74 years. Of these 23 patients, 15 were male and 8 were female, and their mean body weight was 56.7±8.5 kg. Five (21.74%) had diabetes mellitus, 9 (39.13%) had hypertension, 4 (17.39%) had dyslipidemia, 4 (17.39%) had a history of cigarette smoking, and 1 (4.3%) had a history of alcoholism. There were no HIV cases in this study. The mean daily dose of EMB was 18.8±3.5 (95% CI 17.6, 19.9) mg/kg/day, and the mean duration of EMB intake before the onset of symptoms was 7.9±4.1 (95% CI 6.7, 9.1; range 1–20) months. The average glomerular filtration rate (GFR) was 85.9±28.4 (95% CI 70.9, 99.1) mL/min/1.73 m2, and renal impairment was found in only one patient (4.35%), as shown in Table 1. The mean follow-up time was 24.1±25.4 (95% CI 11.5, 36.8) months (range, 2–83 months).
 |
Table 1 Demographic and Clinical Data of Patients in the Study |
All 23 patients had impaired vision in both eyes. One patient had normal visual acuity (20/20) in both eyes but abnormal visual field test. Mean initial visual acuity was 1.4±0.6 logMAR and mean final best vision after EMB discontinuation was 0.9±0.7 logMAR. Good visual recovery was achieved in 9 patients (39.13%) and poor visual recovery was noted in 14 (60.87%). Color vision test was abnormal in 9 subjects (39.13%), and 1 (4.35%) reported normal color vision and good visual acuity but had a visual field defect; the remaining (13) patients were unable to take the color vision test because of their poor vision. Ten patients had abnormal visual field: 2 had central scotoma; 1 had paracentral scotoma; 2 had bitemporal field defect; and 5 had generalized reductions, while the remaining 13 patients could not have the reliability test due to their poor vision. Two patients with bitemporal field defect were investigated with MRI to rule out other causes and had negative results. Pale disc on fundus examination and abnormal OCT findings were investigated to assist in the diagnosis of EON, with optic disc pallor noted in 13 patients (56.52%) and normal optic disc observed in the other 10 (43.48%). OCT was available in 14 patients, and all had abnormal findings which included a decrease in the peripapillary retinal nerve fiber layer thickness and a marked ganglion cell layer loss.
The factors that may have affected visual recovery after discontinuation of EMB treatment were analyzed, and the results are displayed in Table 1.
Gender
Female sex was significantly associated with EON, with six females (66.7%) in the good visual recovery group and only two (14.3%) in the poor outcomes group (p = 0.02).
Age
The mean age of patients in the good visual recovery group (53.7±15.2 years, 95% CI 42, 65.3 years) was lower than that of those in the other group (61.1±7.4 years, 95% CI 56.8, 65.4 years), but the difference was not statistically significant (p = 0.20).
Body Weight
The mean body weight of patients in the good visual recovery group (53.5±6.4 kg, 95% CI 42, 65.3 kg) was lower than that observed in the other group (59.1±9.3 kg, 95% CI 56.8, 65.4 kg); however, this difference was without statistical significance (p = 0.12).
Daily Dose of Ethambutol
The mean daily EMB dose of patients in the good visual recovery group (19.3±3.2 mg/kg/day) was slightly higher than that received by their counterparts in the other group (18.3±3.7 mg/kg/day), but there was no statistically significant difference between the two groups (p = 0.55).
Duration of Ethambutol Treatment
The mean duration of EMB treatment of patients in the good recovery group was 8.1±5.6 months compared with 7.9±2.9 months in the other group, but this difference was not statistically significant (p = 0.92).
Accumulative Dose of Ethambutol
The mean accumulative dose of EMB of patients in the good visual recovery group (4736.6±3407.9 mg) was not significantly different from that of those in the other group (4332.0±1406.1 mg; p = 0.75).
Glomerular Filtration Rate
The mean GFR of patients in the good recovery group, 99.5±20.9 (95% CI 81.9, 117) mL/min/1.73 m2, was higher than that of their counterparts in the other group, 76.1±29.1 (95% CI 52.6, 94.2) mL/min/1.73 m2; however, this difference did not reach a level of significance (p = 0.06).
Initial BCVA at First Visit
The mean initial BCVA in the good visual recovery group was 0.9±0.6 logMAR compared to 1.7±0.2 logMAR in the other group, and this difference was statistically significant (p < 0.001).
Time to Recovery
The mean time to recovery of the good visual recovery group (18.5±18.2 months) was shorter than that of the poor visual recovery group (26.7±28.7 months), but this difference lacked statistical significance (p = 0.46).
Univariate analysis of categorical variables was performed with logistic regression and identified gender as the main factor associated with good visual recovery. As shown in Table 2, females had a better chance of good visual recovery than their male counterparts, with an odds ratio of 12 (95% CI 1.6, 92.3; p = 0.02). Univariate analysis of the numerical variables was performed with linear regression and showed that initial BCVA at first visit was significantly associated with good visual recovery (Table 3), while further multivariate analysis demonstrated that initial BCVA was the only factor affecting visual recovery in these EON patients (Table 4). Further analysis of initial BCVA of the 46 eyes at the first visit when patients complained of decreased vision revealed that 9 eyes (19.57%) had initial vision better than 20/200 and 37 eyes (80.43%) had vision of 20/200 or worse. All eyes with initial BCVA better than 20/200 had good visual recovery compared with 24.32% of eyes with initial BCVA of 20/200 or worse. Eyes with BCVA of 20/200 had a 28.57% chance of good visual recovery, while those with CF had only a 16.67% chance, and those with HM or PL were found to have no chance at all (Table 5).
 |
Table 2 Univariate Analysis of the Categorical Factors by Logistic Regression |
 |
Table 3 Univariate Analysis of the Numerical Factors by Linear Regression |
 |
Table 4 Multivariate Analysis of the Factors That Were Significant in Univariate Analysis |
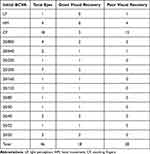 |
Table 5 Initial BCVA and Good Visual Recovery |
Discussion
Previous researchers have reported EON incidence rates varying widely from 0.5% to 2.25%.3–8 Our hospital-based study found 23 EON patients (0.43%) among the 5394 patients who were diagnosed with tuberculosis infection and treated with EMB. This incidence is quite low compared to those reported in the literature, and there could be a number of reasons for this. First, the data relating to EON patients were retrieved from hospital medical charts via ICD-10 codes, and some EON patients may have been omitted due to coding errors. Second, our institute is a tertiary eye center to which affiliated hospitals send more complicated cases for treatment while more straightforward ones are usually treated in the primary hospitals; thus; our results may not be representative of the entire population. Finally, some patients may not have been examined ophthalmologically, and this could have resulted in an underestimation of the actual incidence of EON patients in our study.
A previous study reported that age over 65 years was a significant risk factor for EON;16 however, our study examined only 6 patients (28.1%) in this age range and found no significant association between this factor and EON (as shown in Table 2).
EON has mainly been reported in patients who have been receiving EMB for at least 6–7 months, and it rarely occurs earlier than 2 months after commencement of this treatment.17 In our study, the mean duration of EMB treatment before the onset of EON was 7.9±4.1 months (range, 1–20 months), which was in agreement with the findings of a previous study.17 It was noted that one patient developed blurred vision after one month of EMB treatment, and Lee et al also reported that some patients developed visual symptoms as early as one month after starting EMB treatment.6
Visual field defect in EON usually involves a central or paracentral scotoma; however, the most common visual field defect in our study was generalized reduction (50%), followed by central or paracentral scotoma (30%) and bitemporal field defect (20%) which was due to toxic damage to the nasal crossing fibers in the optic chiasm.19
Optic atrophy on presentation has been reported to be a poor prognostic indicator,6 and Lee et al revealed that patients with pale disc did not achieve visual improvement.6 In our study, optic disc pallor was also more common in the poor recovery group (10 of 14 patients; 71.4%) than in patients with good recovery (3 of 9 patients; 33.3%), but this difference was not statistically significant.
There is no known effective treatment for EON, but it is generally recommended that EMB treatment be stopped immediately in all cases. Vision may gradually recover in some cases, but the damage can also be irreversible. If we took the definition of visual recovery as ≥2-line visual improvement on the Snellen chart, 30 of the 46 eyes (65.22%) in our study would have been considered to have achieved it at the final follow-up, and this is comparable to the rate of 42.2–62.9% reported in the literature.3,9,17 In our study, however, we did not consider that a mere ≥2-line visual improvement represented good visual recovery; instead, we defined it as visual improvement and best final BCVA ≥20/50. Using this definition, only 18 eyes (39.13%) were classified as having achieved good visual recovery in our study.
Woung et al found that EON visual recovery time after discontinuation of EMB treatment was 3–4 months (range from 1–12 months)10 while Tsai et al reported 50% visual recovery after 1–3 years of follow-up.11 In our study, time to best visual recovery after stopping EMB use was 23.54±25.19 months, which was consistent with the range found in previous studies.10,11
We found that initial VA at first visit was the only factor that appeared to affect visual recovery in EON patients: all eyes with initial BCVA of better than 20/200 had good visual recovery, whereas eyes with 20/200 and those with counting fingers had just a 28.57% and 16.67% chance respectively. In our study, EON damage was not always reversible, and eyes with initial vision of hand movement and light perception were found to have no chance of visual recovery. Optic nerve head structural changes may not correlate with considerably improved visual function, and visual improvement has been reported to occur despite progressive structural changes.20
To the best of our knowledge, this study is the first aimed at identifying the factors that may affect visual recovery in EON patients; however, it had some limitations. Firstly, the disease is quite rare, and our small sample size may have affected the low levels of statistical significance found in the analysis. Secondly, owing to the study’s long-term retrospective design, some clinical data may have been missing, and certain special investigative techniques, such as VEP and OCT, may have been unavailable at the time when patients were examined. Thirdly, our hospital is a tertiary eye center to which affiliated hospitals refer more difficult cases, and this means that simpler cases are normally treated at the primary hospitals, so that our results may not be generalizable to the entire Thai population. Finally, some patients may not have been examined ophthalmologically and may therefore not have been recruited into the study: this may have resulted in underestimation of the actual EON incidence, and it may also explain why the incidence was lower in our study than found in previous research. Although our sample size was small, the results of the study identified some factors that may affect visual recovery in EON patients.
Conclusion
In conclusion, the incidence of EON in patients treated with EMB was 0.43% in our hospital-based study. Female sex and better initial visual acuity at first visit were factors identified by univariate analysis as affecting the visual recovery of EON patients. Multivariate analysis, however, revealed that initial visual acuity on first visit was the only factor that affected visual recovery, with patients who had initial visual acuity of better than 20/200 having the best chance of good visual outcomes. All patients on EMB should undergo regular screening by an ophthalmologist to enable early detection of EON, and if it is found, immediate discontinuation of the drug should be made in order to prevent devastating visual loss.
Acknowledgments
The authors would like to thank Dr. Phalin Kamolwat, the Director of the Division of Tuberculosis, Department of Disease Control, Ministry of Public Health, Thailand, for data and valuable suggestions; Associate Professor Dr. Dusit Sujirarat, Department of Research and Technology Assessment, Rajavithi Hospital, for assistance with the statistical analysis; Dr. Niracha Arjkongharn, Department of Ophthalmology, Rajavithi Hospital, for assistance with data collection; and Mr. John Flanagan for assistance with the English-language presentation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. Global Tuberculosis Report. World Health Organization; 2022.
2. Wang MY, Sadun AA. Drug-related mitochondrial optic neuropathies. J Neuroophthalmol. 2013;33:172–178. doi:10.1097/WNO.0b013e3182901969
3. Chen SC, Lin MC, Sheu SJ. Incidence and prognostic factor of ethambutol-related optic neuropathy: 10-year experience in southern Taiwan. Kaohsiung J Med Sci. 2015;31(7):358–362. doi:10.1016/j.kjms.2015.05.004
4. Chamberlain PD, Sadaka A, Berry S, Lee AG. Ethambutol optic neuropathy. Curr Opin Ophthalmol. 2017;28(6):545–551. doi:10.1097/ICU.0000000000000416
5. Yang HK, Park MJ, Lee JH, Lee CT, Park JS, Hwang JM. Incidence of toxic optic neuropathy with low-dose ethambutol. Int J Tuberc Lung Dis. 2016;20(2):261–264. doi:10.5588/ijtld.15.0275
6. Lee EJ, Kim SJ, Choung HK, et al. Incidence and clinical features of ethambutol-induced optic neuropathy in Korea. J Neuroophthalmol. 2008;28:269–277. doi:10.1097/WNO.0b013e31818e3c6b
7. Ezer N, Benedetti A, Darvish-Zargar M, Menzies D. Incidence of ethambutol related visual impairment during treatment of active tuberculosis. Int J Tuberc Lung Dis. 2013;17:447–455. doi:10.5588/ijtld.11.0766
8. Mandal S, Saxena R, Dhiman R, et al. Prospective study to evaluate incidence and indicators for early detection of ethambutol toxicity. Br J Ophthalmol. 2021;105(7):1024–1028. doi:10.1136/bjophthalmol-2020-316897
9. Kumar A, Sandramouli S, Verma L, et al. Ocular ethambutol toxicity: is it reversible? J Clin Neuroophthalmol. 1993;13(1):15–17.
10. Woung LC, Jou JR, Liaw SL. Visual function in recovered ethambutol optic neuropathy. J Ocul Pharmacol Ther. 1995;11:411–419. doi:10.1089/jop.1995.11.411
11. Tsai RK, Lee YH. Reversibility of ethambutol optic neuropathy. J Ocul Pharmacol Ther. 1997;13:473–477. doi:10.1089/jop.1997.13.473
12. Melamud A, Kosmorsky GS, Lee MS. Ocular ethambutol toxicity. Mayo Clin Proc. 2003;78:1409–1411. doi:10.4065/78.11.1409
13. Menon V, Jain D, Saxena R, et al. Prospective evaluation of visual function for early detection of ethambutol toxicity. Br J Ophthalmol. 2009;93:1251–1254. doi:10.1136/bjo.2008.148502
14. Choi SY, Hwang JM. Optic neuropathy associated with ethambutol in Koreans. Korean J Ophthalmol. 1997;11:106–110. doi:10.3341/kjo.1997.11.2.106
15. Talbert Estlin KA, Sadun AA. Risk factors for ethambutol optic toxicity. Int Ophthalmol. 2010;30:63–72. doi:10.1007/s10792-009-9293-z
16. Chen HY, Lai SW, Muo CH, Chen PC, Wang IJ. Ethambutol-induced optic neuropathy: a nationwide population-based study from Taiwan. Br J Ophthalmol. 2012;96:1368–1371. doi:10.1136/bjophthalmol-2012-301870
17. Ambika S, Lakshmi KP, Gopal M, Noronha OV. Visual outcomes of toxic optic neuropathy secondary to Ethambutol: a retrospective observational study from India, an endemic country. Indian J Ophthalmol. 2022;70:3388–3392. doi:10.4103/ijo.IJO_2996_21
18. Steinberg EP, Tielsch JM, Schein OD, et al. The VF-14. An index of functional impairment in patients with cataract. Arch Ophthalmol. 1994;112:630–638. doi:10.1001/archopht.1994.01090170074026
19. Lim SA. Ethambutol-associated optic neuropathy. Ann Acad Med Singapore. 2006;35:274–278.
20. Masvidal D, Parrish RK, Lam BL. Structural-functional dissociation in presumed ethambutol optic neuropathy. J Neuroophthalmol. 2010;30(4):305–310. doi:10.1097/WNO.0b013e3181e08ecb
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
