Back to Journals » Therapeutics and Clinical Risk Management » Volume 19
Factors Affecting Radial Artery Occlusion After Right Transradial Artery Catheterization for Coronary Intervention and Procedures
Authors Xu D , Liu Y, Xu C , Liu X , Chen Y , Feng C, Lyu N
Received 17 March 2023
Accepted for publication 18 June 2023
Published 24 June 2023 Volume 2023:19 Pages 525—533
DOI https://doi.org/10.2147/TCRM.S403410
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Garry Walsh
Dujuan Xu,1,* Ying Liu,1,* Chao Xu,2,* Xuekui Liu,3 Ye Chen,1 Chunguang Feng,4 Nan Lyu1
1Department of Ultrasound, Xuzhou Central Hospital, Xuzhou, People’s Republic of China; 2Department of Radiology, Xuzhou Children’s Hospital, Xuzhou, People’s Republic of China; 3Department of Central Laboratory, Xuzhou Central Hospital, Xuzhou, People’s Republic of China; 4Department of Cardiology, Xuzhou Central Hospital, Xuzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Nan Lyu; Chunguang Feng, Email [email protected]; [email protected]
Objective: To determine the factors influencing proximal radial artery occlusion (PRAO) right radial artery after coronary intervention.
Methods: This is a single-center prospective observational study. A total of 460 patients were selected to undergo coronary angiography (CAG) or percutaneous coronary intervention (PCI) via the proximal transradial approach (PTRA) or distal transradial approach (DTRA). The 6F sheath tube were received by all patients. Radial artery ultrasound was performed 1 day before procedure and 1– 4 days after procedure. Patients were divided into the PRAO group (42 cases) and the non-PRAO group (418 cases). General clinical data and preoperative radial artery ultrasound indexes of the two groups were compared to analyze related factors leading to PRAO.
Results: The total incidence of PRAO was 9.1%, including 3.8% for DTAR and 12.7% for PTRA. The PRAO rate of DTRA was significantly lower than that of PTRA (p < 0.05). Female, low body weight, low body mass index (BMI) and CAG patients were more likely to develop PRAO after procedure (p < 0.05). The internal diameter and cross-sectional area of the distal radial artery and proximal radial artery were smaller in the PRAO group than in the non-PRAO group, and the differences were statistically significant (p < 0.05). Multifactorial model analysis showed that the puncture approach, radial artery diameter and procedure type were predictive factors of PRAO, and the receiver operating characteristic curve showed a good predictive value.
Conclusion: A larger radial artery diameter and DTRA may reduce the incidence of PRAO. Preoperative radial artery ultrasound can guide the clinical selection of appropriate arterial sheath and puncture approach.
Keywords: proximal radial artery occlusion, coronary intervention, ultrasound, influencing factors, the distal transradial approach
Introduction
The puncture path is constantly updated and developed with the continuous maturation of coronary intervention. In current practice, radial artery puncture sites include both the proximal radial artery (PRA) and the distal radial artery (DRA). These two distinct locations are commonly utilized to access the radial artery during various interventional procedures. The proximal transradial approach (PTRA) is a conventional puncture approach, which was recommended by experts as the preferred method for coronary intervention in 2018.1 The distal transradial approach (DTRA) is an emerging puncture approach in recent years. Its puncture site is located in the snuffbox segment of the radial artery. However, this approach is still in the trial stage. Current studies on the DTRA penetration path have confirmed that this approach has a relatively high puncture success rate2 and feasibility.3,4 However, postoperative proximal radial artery occlusion (PRAO) remains the most important clinical concern for both DTRA and PTRA. The occurrence of PRAO limits the repeated use of the operative radial artery, which has a great impact on patients who need coronary angiography (CAG) review or percutaneous coronary intervention (PCI), uremic patients with operational venous fistula and patients with coronary artery bypass grafting. Therefore, identifying the factors influencing PRAO is crucial to developing approaches to reduce the incidence of PRAO as much as possible. So far, most studied have only reported the factors influencing PRAO in the PTRA path.5–7 Herein, patients undergoing coronary intervention were enrolled to analyze the factors influencing PRAO and determine the best strategy to reduce PRAO and provide important information for procedural decision-making.
Materials and Methods
Study Subjects
A total of 628 patients with suspected coronary heart disease who were admitted to the Department of Cardiology of Xuzhou central hospital from May 2021 to November 2022, and who underwent CAG or PCI through the right PRA or DRA were included. A 6F sheath was used.Exclusion criteria were as follows: (1) patients with anatomical variations of the radial artery(n=14); (2) preoperative complications such as right radial artery stenosis or occlusion(n=51); (3) patients who failed to undergo PRA puncture and switched to other puncture paths(n=22), such as brachial artery or ulnar artery; (4) patients who failed to undergo DRA puncture and switched to other puncture paths(n=31), such as PRA, brachial artery or ulnar artery; (5) incomplete clinical and imaging data(n=50). According to the exclusion criteria, 460 patients were selected. Patients were divided into the PRAO group and the non-PRAO group based on postoperative ultrasound diagnosis of radial artery occlusion. There were 42 cases in the PRAO group and 418 cases in the non-PRAO group (Figure 1). The work was in line with the Declaration of Helsinki.This study was approved by the Ethics committees of Xuzhou Central hospital, and all participants provided informed consent to participate.
 |
Figure 1 Study flow diagram. |
Ultrasonic Examination
A color Doppler ultrasound diagnostic system Philips EPIQ 7C, linear array probe L12-3 or Philips IE33, and linear array probe L9-3 were used. The patient was placed in a supine position with the arms naturally abducted. Before procedure, the route of the right radial artery was analyzed, and the lumen diameter and transverse area of the PRA (2–3cm below the metacarpal line) and the DRA (snuff pit) with a normal anatomical structure were measured (Figure 2). After the procedure, the forearm segment of the right radial artery was examined for occlusion and other complications were recorded. All data were measured three times and averaged. All cases were examined 1 day before procedure and 1–4 days after procedure by two experienced physicians specializing in cardiovascular ultrasound. Color Doppler ultrasound showed no significant blood flow signal in the lumen of the right radial artery by definition of radial artery occlusion.
 |
Figure 2 Ultrasound of distal radial artery at the snuff pit of right hand. |
Puncture Procedure and Hemostasis
The procedure was performed by an experienced operator (over 2000 PTRA and 200 DTRA operations). The patient was placed in a supine position on the operating table with the arm naturally abducted by the side. The operator stood on the right side of the patient. Local anesthesia was performed at the place where the DRA or PRA pulsated most strongly and the Seldinger method was used for puncture. After successful puncture of the DRA or PRA with a 20G puncture trocar (Terumo Corp., Tokyo, Japan), 0.021″ hydrophilic coated guide wire was pushed into the artery as the guiding track. A 6F Radifocus Introducer II (Terumo Corp., Tokyo, Japan) sheath was delivered along the guide wire. Approximately 500 μg of nitroglycerin and heparin were routinely injected along the arterial sheath. CAG was performed with 5000 IU of heparin, while PCI was performed by adding additional heparin (100 μ/Kg),8 in the arterial sheathing based on the patient’s body weight to maintain the activated clotting time (ACT) at 250–300 seconds.9 The radial artery sheath tube was removed after operation, a gauze gasket and elastic tape were used for cross-compression dressing at the DRA entry path puncture, and a TR BandTM radial artery hemostatic device (Terumo Corp., Tokyo, Japan) was used for compression hemostasis at the PRA entry path puncture. After 2 h of CAG and 4 h of PCI, decompression was performed to check whether bleeding continued. If there was no bleeding, the compression was completely removed. If there was bleeding, the compression was fixed again with gauze pressure or an inflatable compressor balloon, and the pressure was checked every 10 minutes until the bleeding stopped.
Statistical Analysis
All data were analyzed using SPSS version 24 statistical software. The measurement data were expressed as mean ± standard deviation. A t-test of two independent samples was used for comparison between groups, The numerical value (rate) was used for count data. The chi-square test was used for comparison between groups. The risk factors of anterior radial artery occlusion were analyzed using conditional logistic regression, and a prediction model was established. The receiver operating characteristic (ROC) curve of the sample was analyzed using the prediction model, and the area under the ROC curve (AUC) was calculated. P<0.05 was considered statistically significant.
Results
Comparison of Baseline Data Between the Two Groups
The PRAO of 460 patients was 9.1%. Gender, body weight, body mass index (BMI), procedure type and puncture approach statistically differed between the two groups (Table 1). Female, low body weight, low body mass index (BMI), CAG and PTRA were more likely to cause PRAO. No statistically significant differences in age, comorbidities(hypertension, diabetes, hyperlipidemia, smoking, prior myocardial infarction, prior myocardial infarction, atrial fibrillation), heart Failure with EF < 40%, creatinine clearance (mL/min) < 50% and medications (1–4days after CAG/PCI) were found between the two groups.
 |
Table 1 The Baseline Clinical Characteristic of Participants According to PRAO Status |
Comparison of Ultrasonic Indexes Between the Two Groups
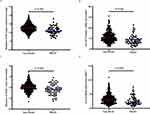
The internal diameter and cross-sectional area of proximal and distal radial arteries were smaller in the PRAO group than in the non-PRAO group, and the differences were statistically significant (Figure 3).
Multivariate Analysis
Multiple linear regression analysis showed that the type of procedure, the puncture approach and the internal diameter of PRA were the main influencing factors for PRAO (Table 2). The degree of occlusion in patients who underwent CAG was 3.494 times higher than that in patients who received PCI. PRA puncture was 3.24 times that of DRA puncture. The PRA diameter affected the degree of occlusion, and the occlusion occurrence was reduced by 0.187 times when the diameter increased by 1 mm. The ROC curve was plotted based on the three risk factors, which revealed a high predictive value for PRAO (AUC = 0.788, Figure 4).
 |
Table 2 Multivariate Analysis to Explore the Risk Factor of PRAO |
 |
Figure 4 The ROC curve to predict the risk of PRAO. |
Early Radial Artery Complications
In addition to PRAO, early postoperative radial artery ultrasound also revealed early radial artery hematoma in 8 cases, pseudoaneurysm in 4 cases and DRA occlusion in 8 cases, with an incidence of 1.7, 0.8 and 1.7%, respectively. No arteriovenous fistula or osteofascial compartment syndrome was observed. DRA occlusion occurred in DTRA.
Discussion
Radial artery occlusion is a common complication after coronary intervention,7 which can be divided into early and late occlusion. The primary mechanism of early occlusion is acute arterial thrombosis, which is caused by endothelial and vascular injury, local hypercoagulability and slow blood flow during pressure hemostasis. Liu et al10 reported that the initial occurrence rate of early occlusion was 14.5%. Furthermore, they highlighted the potential for re-occlusion even after 1 month and 3 months. Moreover, the incidence of RAO decreased to 12.5% after 1 month and further dropped to 4.1% after 3 months. In our study, radial artery ultrasound was reviewed 1–4 days after procedure, PRAO patients were recorded, a target was indicated for subsequent review of radial artery ultrasound and the influencing factors of PRAO were analyzed. The results showed that DTRA, PRA diameter and procedure type were the main influencing factors of PRAO.
DRA is the dorsal branch of the wrist in the snuff pit area. To determine the most effective ways to reduce PRAO, Kiemenij11 first proposed the DTRA approach in 2017. Theoretically, the special anatomical structure of DRA can help reduce the incidence of PRAO.12 It has small blood vessels, shallow location, deep bone structure, easy compression and short compression time, which can reduce the risk of PRAO. Besides, the radial artery of the forearm sends out the superficial palm branch in addition to the DRA in the wrist. Even when the puncture site of the DTRA is occluded, the downstream flow of the radial artery of the forearm to the superficial palmar branch continues, which reduces the possibility of PRAO caused by slow blood flow or even stasis of the radial artery of the forearm. Comparative studies on DTRA and PTRA13–15 also confirmed that the DTRA approach is more advantageous in reducing the incidence of PRAO in addition to improving the comfort level of operators and patients. Notably, a recent international, multicenter, randomized controlled trial16 revealed the occurrence of PRAO in the DTRA group was remarkably low, but there were no significant difference in the incidence of PRAO between DTRA and PTRA. In our study, the incidence of PRAO in the DTRA approach was low (3.8%), which was significantly lower than that in PTRA (12.7%). However, it is higher than the 0.9% obtained by Fu et al17 which may be due to the different evaluation methods of PRAO. In our study, the ultrasonic evaluation method was used in all cases, which can visualize the patency of radial artery blood flow and reduce the false negative rate compared with the touch method.18
As the DTRA approach has been implemented by multiple interventionalists, there has been a growing understanding of the internal diameter characteristics of both the PRA and the DRA. It has been observed that the internal diameter of the DRA tends to be smaller in comparison to that of the PRA, and furthermore, a correlation between the internal diameters of the two arteries has been identified. Meo et al19 reported that the internal diameter of DRA was 19% smaller than that of PRA. The mismatch between the radial artery diameter and the sheath diameter is one of the influencing factors of PRAO. Saito et al20 found that the probability of PRAO is significantly increased when the ratio of the radial artery diameter to the sheath diameter is <1, compared with that when the ratio is ≥1. With the appearance of the hydrophilic coated sheath, it is feasible for the radial artery diameter to be slightly smaller than the sheath size. However, in cases where successful puncture and catheterization of an excessively small radial artery are achieved, the radial artery may remain in a prolonged state of passive over-dilation. This condition can increase friction between the sheath and the artery, posing potential risks such as radial artery spasm,21 and eventually lead to the damage and occlusion of the radial artery. In our study, 6F sheath tubes were used, and the results showed that the internal diameter and cross-sectional area of DRA and PRA were smaller in the PRAO group than in the non-PRAO group, and the differences were statistically significant. The internal diameter of the radial artery is an uncontrollable factor. Therefore, it is crucial to accurately evaluate the DRA diameter and the PRA diameter by ultrasonography before procedure. For thinner radial arteries, smaller arterial sheath tubes may be selected to reduce the incidence of PRAO.
The differences between CAG and PCI primarily manifest in several key aspects, namely catheterization time, heparin dosage, and postoperative compression duration. Theoretically, the incidence of PRAO in CAG with short operation time, catheterization time and compression time and a low dose of heparin may be lower than that of PCI. However, we found that the PRAO of patients undergoing CAG was higher than that of patients receiving PCI, which was consistent with the results of a recent meta-analysis.7 This could be because of the intraoperative use of heparin. Hahalis et al22 compared the incidence of PRAO in patients who received CAG with 2500 IU or 5000 IU of heparin and found that 5000 IU heparin could maintain the patency of the radial artery. In a multi-center randomized study, patients who received CAG were randomly given 100 U/kg or 50 U/kg heparin before operation based on individual differences The results showed that compared with 50 U/kg heparin, 100 U/kg heparin significantly reduced the incidence of PRAO.23 In the present study, individual differences in the administration of PCI patients and monitoring ACT maintained at 250–300 seconds ensured adequate heparin dosage to the maximum extent. However, although patients receiving CAG were all given heparin at a high dose of 5000 IU, they were not administered based on individual differences, which may have led to inadequate dosing in some patients, thus causing radial artery occlusion.
In addition, the role of postoperative anticoagulant and antiplatelet therapy in the prevention and treatment of thrombosis cannot be ignored. Kaya et al24 reported that apixaban treatment has a good effect on the treatment of thrombosis. Qin et al25 conducted a study demonstrating that dual antiplatelet therapy for one month after the procedure could prevent PRAO.In this study, there was no significant difference in the use of postoperative anticoagulant and antiplatelet therapy between the groups, which may be due to the observation of early occlusion rate.
Recently, it has been reported that the angle of puncture in femoral access influences the complications.26 Thus, whether the radial artery occlusion rate after puncture varied with different angles remain to be clarified. Ultrasound technology can well show the relationship between the radial artery and the surrounding soft tissue and bone.In future, we shall use ultrasound technology to explore the appropriate radial artery puncture angle to prevent PRAO.
Limitations
This study is a single-center prospective observational study with a small sample size and a short follow-up time. Thus, multi-center studies with a large sample size and longer follow-up time should be conducted to further validate our findings.
Conclusion
In summary, a larger PRA diameter and the DTRA approach may reduce the incidence of PRAO. Radial ultrasound plays a very important role in DTRA or PTRA coronary intervention, which may guide the clinical selection of the appropriate arterial sheath and puncture path.
Data Sharing Statement
All data generated or analyzed during this study are included in this article.
Ethics Approval and Consent to Participate
The study was reviewed and approved by the ethics committee of the Xuzhou central hospital. The NO. of ethics committee approval is XZXY-LJ-20211110-060.
Acknowledgments
We acknowledge and thank all participants for their cooperation and sample contributions.
Funding
This research was funded by Scientific research project of Xuzhou Medical Reserve Talents project (No.XWRCHT20220025).
Disclosure
All authors declare that there is no conflict of interest associated with this article.
References
1. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87–165. doi:10.1093/eurheartj/ehy394
2. Li SS, Li JM, Liu LL, Liu W, Yang H, Feng CG. Analysis of the risk factors related to the success rate of distal transradial artery access in patients with coronary heart disease. Risk Manag Healthc Policy. 2022;15:657–663. doi:10.2147/rmhp.S357780
3. Mizuguchi Y, Izumikawa T, Hashimoto S, et al. Efficacy and safety of the distal transradial approach in coronary angiography and percutaneous coronary intervention: a Japanese multicenter experience. Cardiovasc Interv Ther. 2020;35(2):162–167. doi:10.1007/s12928-019-00590-0
4. Valsecchi O, Vassileva A, Cereda AF, et al. Early clinical experience with right and left distal transradial access in the anatomical snuffbox in 52 consecutive patients. J Invasive Cardiol. 2018;30(6):218–223.
5. Wang J, Yi C, Zhang J. Study on influencing factors of radial artery occlusion after repeated right radial artery coronary intervention. Contrast Media Mol Imaging. 2022;2022:9624339. doi:10.1155/2022/9624339
6. Pancholy SB, Ahmed I, Bertrand OF, Patel T. Frequency of radial artery occlusion after transradial access in patients receiving warfarin therapy and undergoing coronary angiography. Am J Cardiol. 2014;113(2):211–214. doi:10.1016/j.amjcard.2013.09.043
7. Rashid M, Kwok CS, Pancholy S, et al. Radial artery occlusion after transradial interventions: a systematic review and meta-analysis. J Am Heart Assoc. 2016;5(1). doi:10.1161/jaha.115.002686
8. Schulz S, Mehilli J, Neumann FJ, et al. Intracoronary Stenting and Antithrombotic Regimen: rapid Early Action for Coronary Treatment (ISAR-REACT) 3A Trial Investigators. ISAR-REACT 3A: a study of reduced dose of unfractionated heparin in biomarker negative patients undergoing percutaneous coronary intervention. Eur Heart J. 2010;31(20):2482–2491. doi:10.1093/eurheartj/ehq330
9. Brener SJ, Moliterno DJ, Lincoff AM, Steinhubl SR, Wolski KE, Topol EJ. Relationship between activated clotting time and ischemic or hemorrhagic complications: analysis of 4 recent randomized clinical trials of percutaneous coronary intervention. Circulation. 2004;110(8):994–998. doi:10.1161/01.CIR.0000139868.53594.24
10. Liu X, Zhu H, Rui L, et al. Occurrence of radial artery occlusion after transradial interventional therapy in patients with coronary artery disease. JiangSuYiYao. 2019;45(7):3.
11. Kiemeneij F. Left Distal Transradial Access in the Anatomical Snuffbox for Coronary Angiography (ldTRA) and Interventions (ldTRI). EuroIntervention. 2017;13(7):851–857. doi:10.4244/eij-d-17-00079
12. Sgueglia GA, Di Giorgio A, Gaspardone A, Babunashvili A. Anatomic basis and physiological rationale of distal radial artery access for percutaneous coronary and endovascular procedures. JACC Cardiovasc Interv. 2018;11(20):2113–2119. doi:10.1016/j.jcin.2018.04.045
13. Koutouzis M, Kontopodis E, Tassopoulos A, et al. Distal versus traditional radial approach for coronary angiography. Cardiovasc Revascular Med. 2019;20(8):678–680. doi:10.1016/j.carrev.2018.09.018
14. Eid-Lidt G, Rivera Rodríguez A, Jimenez Castellanos J, Farjat Pasos JI, Estrada López KE, Gaspar J. Distal radial artery approach to prevent radial artery occlusion trial. JACC Cardiovasc Interv. 2021;14(4):378–385. doi:10.1016/j.jcin.2020.10.013
15. Tsigkas G, Papageorgiou A, Moulias A, et al. Distal or traditional transradial access site for coronary procedures: a single-center, randomized study. JACC Cardiovasc Interv. 2022;15(1):22–32. doi:10.1016/j.jcin.2021.09.037
16. Aminian A, Sgueglia GA, Wiemer M, et al. Distal Versus Conventional Radial Access for Coronary Angiography and Intervention: the DISCO RADIAL Trial. JACC Cardiovasc Interv. 2022;15(12):1191–1201. doi:10.1016/j.jcin.2022.04.032
17. Fu Y, Zuo K, Yang Y, et al. Distal transradial access: a safe and feasible approach for coronary catheterization in cases of total radial artery occlusion. J Cardiovasc Transl Res. 2022;15(5):1203–1211. doi:10.1007/s12265-022-10238-9
18. Qin H, Yang Y, Sun H. Diagnostic value of ultrasonography on acute occlusion of radial artery after percutaneous coronary intervention. Linchuang Chaosheng YiXue. 2012;14(9):610–612.
19. Meo D, Falsaperla D, Modica A, et al. Proximal and distal radial artery approaches for endovascular percutaneous procedures: anatomical suitability by ultrasound evaluation. Radiol Med. 2021;126(4):630–635. doi:10.1007/s11547-020-01299-4
20. Saito S, Ikei H, Hosokawa G, Tanaka S. Influence of the ratio between radial artery inner diameter and sheath outer diameter on radial artery flow after transradial coronary intervention. Catheter Cardiovasc Intervent. 1999;46(2):173–178. doi:10.1002/(sici)1522-726x(199902)46:2<173::Aid-ccd12>3.0.Co;2-4
21. Fukuda N, Iwahara S, Harada A, et al. Vasospasms of the radial artery after the transradial approach for coronary angiography and angioplasty. Jpn Heart J. 2004;45(5):723–731. doi:10.1536/jhj.45.723
22. Hahalis G, Xathopoulou I, Tsigkas G, et al. A comparison of low versus standard heparin dose for prevention of forearm artery occlusion after 5 French coronary angiography. Int J Cardiol. 2015;187:404–410. doi:10.1016/j.ijcard.2015.03.366
23. Hahalis GN, Leopoulou M, Tsigkas G, et al. Multicenter Randomized Evaluation of High Versus Standard Heparin Dose on Incident Radial Arterial Occlusion After Transradial Coronary Angiography: the SPIRIT OF ARTEMIS Study. JACC Cardiovasc Interv. 2018;11(22):2241–2250. doi:10.1016/j.jcin.2018.08.009
24. Kaya A, Hayıroğlu Mİ, Keskin M, Tekkeşin Aİ, Alper AT. Resolution of left ventricular thrombus with apixaban in a patient with hypertrophic cardiomyopathy. Turk Kardiyol Dern Ars. 2016;44(4):335–337. doi:10.5543/tkda.2015.68054
25. Qin Z, Yang X, Cheng W, Wang J, Jin Z. Different antiplatelet strategies for radial artery protection after transradial coronary angiography-a prospective observational cohort study. Front Cardiovasc Med. 2022;9:913008. doi:10.3389/fcvm.2022.913008
26. Hayıroğlu Mİ, Çınar T, Bıçakçı B, et al. Predictors of femoral hematoma in patients undergoing elective coronary procedure: a trigonometric evaluation. Int J Cardiovasc Imaging. 2018;34(8):1177–1184. doi:10.1007/s10554-018-1339-8
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.