Back to Journals » Clinical Interventions in Aging » Volume 18
Factors Affecting Mortality in Elderly Hypertensive Hospitalized Patients with COVID-19: A Retrospective Study
Received 20 July 2023
Accepted for publication 7 November 2023
Published 20 November 2023 Volume 2023:18 Pages 1905—1921
DOI https://doi.org/10.2147/CIA.S431271
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Maddalena Illario
Qun Xu,1 Fangzhou Li,2 Xujiao Chen2,3
1School of Medicine, Zhejiang University, Hangzhou, Zhejiang Province, People’s Republic of China; 2Department of Geriatrics, Zhejiang Provincial Hospital of Chinese Medicine, Hangzhou, Zhejiang Province, People’s Republic of China; 3Zhejiang Hospital, Hangzhou, Zhejiang Province, People’s Republic of China
Correspondence: Xujiao Chen, Department of Geriatrics, Zhejiang Provincial Hospital of Chinese Medicine, Hangzhou, Zhejiang, 310003, Email [email protected]
Purpose: Corona Virus Disease 2019 (COVID-19) endangers the health and survival of the elderly. We tried to explore factors especially kidney function which affected mortality in elderly hypertensive patients with COVID-19.
Methods: We conducted a retrospective research of 748 COVID-19 elderly patients (≥ 65 years old) at Zhejiang Hospital. This study compared demographic data, laboratory values, comorbidities, treatments, and clinical outcomes of hypertension and non-hypertension participants, and subgroup analysis of age and frailty was conducted in the hypertension population. Survival analysis was used to determine risk factors for death in elderly patients with COVID-19.
Results: Our study revealed that the elderly hypertensive patients with COVID-19 had higher blood urea nitrogen (BUN), serum uric acid (UA), serum creatinine (Scr), lower estimated glomerular filtration rate (eGFR), higher incidence of severity, admission to intensive care unit (ICU) and death, and longer in-hospital stay than non-hypertensive patients, which also occurred in the very elderly hypertensive patients compared with younger hypertensive patients and frail hypertensive patients compared with no-frail hypertensive patients. In addition, the prevalence of acute kidney injury (AKI) was higher in the oldest old hypertensive patients and frail hypertensive patients. Multivariate survival analysis indicated that the independent risk factors for death from COVID-19 were age ≥ 80 years, heart failure, antiviral therapy, calcium channel blocker (CCB) therapy, mechanical ventilation, AKI, and eGFR< 60 mL/min per 1.73 m2.
Conclusion: The results of the present study suggested that the elderly hypertensive patients with COVID-19 would have more serious kidney injury, more serious disease progression and higher mortality, which also occurred in very elderly and frailty subgroup. Kidney dysfunction was closely related to mortality in elderly patients with COVID-19.
Keywords: mortality, kidney dysfunction, frailty, elderly, hypertensive hospitalized patients with COVID-19
Introduction
In late 2019, a highly transmissible and pathogenic coronavirus triggered a pandemic of acute respiratory disease, threatening human health and public safety. On February 11, the International Committee on Taxonomy of Viruses named the novel coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the World Health Organization named the disease “Corona Virus Disease 2019 (COVID-19)”.1 It appears that people of all ages are susceptible to SARS-CoV-2 infection, but the clinical presentation varies by age. In general, most young people and children have only mild symptoms (non-pneumonia or mild pneumonia) or no symptoms, while elderly men (>60 years) with comorbidities are more likely to develop severe respiratory disease that requires hospitalization or even death.2 In fact, a large number of studies well demonstrated that the in-hospital death in patients with COVID-19 was associated with older age.3–6 As the high mortality of elderly patients compared with other populations, it is necessary to explore the risk factors of death of elderly patients with COVID-19.
Some pre-existing diseases which included lung disease, cardiovascular disease, kidney disease, type 2 diabetes and hypertension greatly increased the risk of severe symptoms and death in patients with COVID-19.7–9 Epidemiological studies have found that hypertension is the most common comorbidity of patients with COVID-19.7–9 It is estimated that the prevalence of hypertension in Chinese patients with COVID-19 is between 15% and 25%. About 49.7% of hospitalized patients with COVID-19 in the United States suffer from hypertension.10 Among patients over 65 years old, the high comorbidity rate of hospitalized patients with COVID-19 is as high as 69%.11 Hypertension as a comorbidity has been found to be associated with an increased risk of serious disease and mortality with SARS-CoV-2 infection.8,12 The relationship between COVID-19 and hypertension does not seem surprising, nor does it necessarily imply a causal relationship, given the high prevalence of hypertension worldwide.11,13 In some studies, hypertension was not an independent factor for the mortality of COVID-19 according to multivariate adjusted analysis, in spite of the fact that it was identified as a risk factor through univariate5,14 or bivariate survival analysis.15 Hypertension patients are usually accompanied by a variety of comorbidities, such as diabetes, hypertension mediated heart injury and other cardiovascular related complications, which increase with age and are associated with COVID-19 severity and mortality. Frailty is also a complex age-related clinical disease characterized by a decrease in the physiological capacity of multiple organ systems, which increases susceptibility to stressors and increases the risk of adverse consequences.16 A meta-analysis showed a linear relationship between an increase in clinical frailty scale scores and an increase in mortality.17 Therefore, the influence of hypertension on the course of COVID-19 may be affected by the interaction of age and other risk factors.11,18,19
Kidney damage ranging from a benign to a malignant represents a frequent event in the course of hypertension.20 The function and morphology of the kidney change markedly with age. Those can be observed in the kidneys of the elderly including structural changes such as renal vascular changes, glomerulosclerosis, tubular atrophy and interstitial fibrosis, as well as functional changes such as a decrease in glomerular filtration rate (GFR) and ultrafiltration coefficient, an increase in capillary pressure, changes in renal vasoconstriction and vasodilator sensitivity, and reduced autoregulation, and functional reserve. Hypertension as an age-related disease can accelerate these changes and have random deleterious effects on the kidneys.21–23 It has been indicated that the prevalence of hypertension in COVID-19 patients with AKI is significantly higher than that in non-AKI patients, which suggests that hypertension is related to renal function in patients with COVID-19.3,24–26 A recent research reported that frailty was also a risk factor for AKI development in elderly patients.27 This study analyzed the differences in kidney function and outcomes of elderly hypertensive patients with COVID-19 during hospitalization and carried out a subgroup analysis of age and frailty, followed by the survival analysis in order to clarify the risk factors of death in elderly patients.
Materials and Methods
Study Design and Participants
This single-center retrospective study was conducted in Zhejiang Hospital during the COVID-19 pandemic period from December, 2022 to January, 2023. All elderly patients (age ≥ 65 years) who tested positive for polymerase-chain reaction in nasopharyngeal samples for neocrown pneumonia and received inpatient treatment from December 8, 2022, to January 7, 2023, were eligible. Patients with chronic kidney disease, renal insufficiency, uremia, renal failure, and hemodialysis status were excluded. Patients were also excluded if they were with duplicate data and missing important data. The total sample size depends on the number of cases admitted to our hospital in this study. The study complied with the Declaration of Helsinki and was approved by Zhejiang Hospital Ethics Review Committee (2022-31J). Participants who took part in screening for geriatric syndrome were given written informed consent before participating in the study.
Data Collection
Clinical data and vital signs were recorded on electronic medical records by clinicians in their daily work. The electronic medical records of each patient were reviewed and the following data were collected: age, gender, cigarette smoking, alcoholism, comorbidities including hypertension, coronary atherosclerotic heart disease, arrhythmia, heart failure, cerebral infarction, diabetes, chronic obstructive pulmonary disease (COPD), malignancy, Alzheimer’s disease (AD) and sleep disorders; treatments during hospitalization including thymalfasin, antiviral therapy (molnupiravir and paxlovid), angiotensin receptor blocker (ARB), calcium channel blocker (CCB), statins, anticoagulant drugs (heparin, clopidogrel, aspirin, rivaroxaban, dabigatran and warfarin), nutrition supplement (enteral nutrition, parenteral nutrition, and whey protein powder), endotracheal intubation and mechanical ventilation; geriatric syndrome including dystrophy, frailty and sarcopenia; laboratory parameters including blood urea nitrogen (BUN), serum uric acid (UA), serum creatinine (Scr), serum potassium, serum sodium, serum chlorine, estimated glomerular filtration rate (eGFR) and urine tests (proteinuria, glycosuria and ketone); time of hospitalization and discharge or death; severe or intensive care unit (ICU) admission.
Definitions
According to the Kidney Disease: Improving Global Output (KDIGO) guidelines, acute kidney injury (AKI) is defined as either an increase in serum creatinine from baseline by at least 50% to the highest creatinine level during hospitalization, or a 0.3 mg/dL increase in serum creatinine within 48 hours.28 Due to a lack of baseline creatinine levels (defined as the average creatinine value from 7 to 365 days prior to hospitalization), the minimum creatinine value during hospitalization was used as baseline creatinine. Dystrophy, frailty and sarcopenia were determined through screening for geriatric syndrome. Meeting any one of the two criteria is defined as malnutrition, which means that Participants who lost more than 3 kg of weight without intentional weight loss or had experienced loss of appetite in the past 3 months. The calf circumference of participants was measured and the maximum value was taken; men <34 cm and women <33 cm were considered to have sarcopenia, excluding paralysis and edema. Frailty was assessed by the FRAIL scale, which include five questions: 1) Do you feel tired? 2) Can you walk a distance of one block (500 meters)? 3) Can you go up a staircase? 4) Do you suffer from more than 5 diseases? 5) Have you lost more than 5% of your weight in the past 6 months? Scale score ≥3 was defined as frailty.
Statistical Analysis
All data in this study were statistically analyzed by SPSS IBM® SPSS Statistics, version 26 or Prism 8 (GraphPad). The primary analysis compared the renal function, ICU occupancy, severity, mortality, and length of stay of elderly hospitalized patients with COVID-19 during hospitalization. All continuous variables were presented as mean ± SD or median (interquartile range [IQR]), while categorical variables were expressed as frequency or percentages (%). The normality of distribution of continuous variables was assessed with Kolmogorov–Smirnov’s test. Continuous variables conforming to normal distribution were compared by independent t-test, and other numerical variables were compared by Mann–Whitney U-test. Distributions of categorical variables were compared using the Chi-square test or Fisher exact test. Participants were categorized into AKI and no AKI group according to serum creatinine level during hospitalization or eGFR ≥ 60 mL/min per 1.73 m2 or eGFR < 60 mL/min per 1.73 m2 group according to eGFR level during hospitalization. Survival curves were plotted by the Kaplan–Meier method in AKI patients and non-AKI patients, as well as in eGFR subgroups, using a logarithmic rank test. Multivariate Cox regression analysis was used to analyze the correlation between AKI or eGFR and in-hospital death, in which covariates included variables with p-values <0.05 in univariate analysis. All statistical tests were 2-sided, and a P value <0.05 was considered statistically significant.
Results
Patients
During the period from December 8, 2022, to January 7, 2023, a total of 1096 elderly patients with COVID-19 were admitted to the hospital. Fifty-five patients were excluded due to data duplication. One hundred and seventy-three patients were excluded from the present study due to chronic kidney disease (n = 64), renal insufficiency (n = 125), uremia (n = 15), renal failure (n = 31), and receiving long-term dialysis (n = 46). One hundred and twenty patients were excluded due to missing important data. Eventually, 748 eligible patients were included in the study (Figure 1).
 |
Figure 1 Flowchart of the study. |
The clinical characteristics of the patients included in the study are shown in Table 1. Median age was 76 (IQR 69–85) years, ranging from 65 years to 99 years. Of these, 416 (55.6%) were men, 158 (21.1%) had history of cigarette smoking and 116 (15.5%) had history of alcoholism. The median length of hospital stay was 8 (IQR 4–18) days. Hypertension is the most common comorbidity (422, 56.4%), followed by diabetes (199, 26.6%), malignancy (167, 22.3%), arrhythmia (146, 19.5%), cerebral infarction (146, 19.5%) and coronary atherosclerotic heart disease (136, 18.2%). Sixty-three of 590 patients (8.4%) were assessed as malnutrition, 119 of 536 patients (15.9%) were with frailty and 233 of 540 patients (31.1%) had sarcopenia. During the hospitalization, these patients were treated with thymalfasin (58, 7.8%), antiviral therapy (68, 9.1%), ARB (120, 16.0%), CCB (180, 24.1%), statins (181, 24.2%), anticoagulant drugs (273, 36.5%), nutrition supplement (55, 7.4%), endotracheal intubation (42, 5.6%), mechanical ventilation (64, 8.6%), respectively. The laboratory data of patients are also shown in Table 1. The median admission BUN, Scr, UA, and eGFR for these patients were 6.00 (IQR 4.70–7.70) mmol/L, 72.50 (IQR 60.00–88.00) μmol/L, 285.00 (IQR 230.00–363.00) μmol/L, and 80.15 (IQR 65.00–89.88) mL/min/1.73 m2. The median values of Peak BUN, Scr and UA for these patients were 6.60 (IQR 5.02–8.80) mmol/L, 76.00 (IQR 63.00–95.00) μmol/L and 301.00 (IQR 243.00–384.00) μmol/L. The median value of minimum eGFR was 76.45 (59.80–88.68) mL/min/1.73 m2 and 589 patients (78.7%) had a glomerular filtration rate below 90 mL/min/1.73 m2. According to definitions, 94 of 748 patients (12.6%) developed AKI during their hospitalization.
 |
Table 1 Clinical Characteristics of All Patients |
Inpatient Characteristics by Hypertension Status
As shown in Table 2, compared with study participants without hypertension, those with hypertension were older (median 78 vs 73, p < 0.001) and were more likely to be with frailty (19.2% vs 11.7%, p = 0.018) and had the longer length of hospital stay (9(5–23.25) vs 7(4–13), p < 0.001). With the exception of COPD, all pre-existing comorbidities were significantly elevated in patients with hypertension. There was no statistically significant difference in gender, smoking status, alcoholism and treatment received during hospitalization.
 |
Table 2 Inpatient Characteristics by Hypertension Status |
As to the Kidney function, patients with hypertension were featured by higher levels of BUN (median 6.29 vs 5.69 mmol/L, p = 0.002), UA (median 295.00 vs 274.00 μmol/L, p = 0.011) and Scr (median 75.00 vs 68.00 μmol/L, p < 0.001) and lower levels of eGFR (median 76.45 vs 85.80 mL/min/1.73 m2, p < 0.001) on admission. The peak levels of BUN (median 7.05 vs 6.20 mmol/L, p < 0.001), UA (median 319.50 vs 282.00 μmol/L, p < 0.001) and Scr (median 80.50 vs 70.50 μmol/L, p < 0.001) were significantly elevated and the minimum levels of eGFR (median 71.95 vs 83.20 mL/min/1.73m2, p < 0.001) were significantly decreased in patients with hypertension compared with patients without hypertension during hospitalization. The proportions of study participants with eGFR ≥ 90 mL/min per 1.73 m2, eGFR 60–89.9 mL/min per 1.73 m2, eGFR 30–59.9 mL/min per 1.73 m2, eGFR 15–29.9 mL/min per 1.73 m2 and eGFR<15 mL/min per 1.73 m2 were 14.7%, 55.2%, 23.5%, 4.7% and 1.9% among those who developed hypertension, while the corresponding proportions were 29.8%, 51.8%, 15.0%, 1.5% and 1.8% among those without hypertension, respectively.
Clinical Features of Very Elderly Patients with Hypertension
Table 3 shows that among patients with hypertension, the oldest old (≥80 years old) were more likely to suffer from comorbidities such as coronary atherosclerotic heart disease (31.4% vs 19.9%, p = 0.007), cerebral infarction (34.0% vs 18.6%, p < 0.001), diabetes (32.9% vs 18.4%, p < 0.001), COPD (14.7% vs 3.9%, p < 0.001) and AD (24.6% vs 2.6%, p < 0.001) and were more likely to be with frailty (35.1% vs 6.1%, p < 0.001) and sarcopenia (39.8% vs 19.5%, p < 0.001). Furthermore, the very elderly (≥80 years old) group contained a higher proportion of patients who required mechanical ventilation (16.2% vs 5.2%, p < 0.001) or endotracheal intubation (11.0% vs 2.6%, p < 0.001). All serum kidney function laboratory findings were significantly worse except for UA among ≥80-year-old patients with hypertension compared with <80-year-old patients with hypertension on admission. Older participants (≥80 years old) had higher levels of peak BUN (median 8.60 vs 6.20 mmol/L, p <0.001), peak Scr (median 89.00 vs 76.00 μmol/L, p < 0.001) and peak UA (median 333.00 vs 313.00 μmol/L, p = 0.019) and lower level of minimum eGFR (median 61.60 vs 81.30 mL/min per 1.73 m2, p < 0.001) with all minimum eGFR below normal during hospitalization. Among the oldest old,41 of 191 (21.5%) developed AKI compared with 19 of 231 (8.2%) in younger patients. Finally, older participants were more likely to have a longer hospital stay (median 15 vs 7, p < 0.001).
 |
Table 3 Clinical Features of Very Elderly Patients with Hypertension |
Clinical Features of Frail Patients with Hypertension
This is shown in Table 4 that frail patients with hypertension were older (median 90 vs 73, p < 0.001) and more likely to have history of coronary atherosclerotic heart disease (37.0% vs 21.5%, p = 0.006), arrhythmia (38.3% vs 23.8%, p = 0.012), cerebral infarction (34.6% vs 21.1%, p = 0.016), COPD (17.3% vs 5.4%, p = 0.001), AD (30.9% vs 5.4%, p < 0.001) and sleep disorders (14.8% vs 7.2%, p = 0.042) and more likely to receive mechanical ventilation (13.6% vs 4.9%, p = 0.010) and have a longer hospital stay (median 27 vs 7, p < 0.001). There were no statistically significant difference in kidney function laboratory findings except for BUN (median 7.18 vs 6.00 mmol/L, p = 0.002) between frailty group and no-frailty group among patients with hypertension on admission. Frail patients with hypertension had higher levels of peak BUN (median 8.63 vs 6.49 mmol/L, p < 0.001), peak Scr (median 89.00 vs 79.00 μmol/L, p =0.008) and peak UA (median 358.00 vs 308.00 μmol/L, p = 0.008) and lower level of minimum eGFR (median 63.20 vs 74.50 mL/min per 1.73 m2, p < 0.001) compared with no-frail patients with hypertension during hospitalization. 24.7% patients in frail group developed AKI compared with 9.4% patients in no-frail group. The proportion of participants with proteinuria (27.2% vs 9.8%) and ketone (11.1% vs 3.1%) were significantly higher in patients who were frail than those who were not.
 |
Table 4 Clinical Features of Frail Patients with Hypertension |
Outcomes of Elderly Patients with COVID-19
As shown in Table 5, among the 748 patients, 189 became severely ill (25.3%), 59 were admitted to the ICU (7.9%), and 39 died (5.2%). Compared with those without hypertension, patients with hypertension, high incidence of severe illness (28.2% vs 21.5%, p = 0.036), higher ICU admission rate (9.7% vs 5.5%, p = 0.035) and higher in-hospital mortality (6.9% vs 3.1%, p = 0.020). In the elderly population with hypertension, very elderly participants were more likely to be admitted to the ICU (15.7% vs 4.8%, p < 0.001), develop severe illness (42.9% vs 16.0%, p < 0.001) or death (13.6% vs 1.3%, p < 0.001). The proportion of participants with severe illness (48.1% vs 14.8%, p < 0.001), admission to ICU (12.3% vs 2.2%, p = 0.001), and death (14.8% vs 1.3%, p < 0.001) during hospital stay were significantly higher in patients who were frail than those who were not.
 |
Table 5 Outcomes of Elderly Patients with COVID-19 |
Kidney Function and Mortality
Kaplan–Meier analysis revealed a higher in-hospital death for patients with eGFR<60 mL/min per 1.73 m2 and for those developing AKI during hospital stay (Figure 2). Table 6 displays the results of Cox regression for in-hospital mortality. By univariate analysis, age ≥80 years (HR = 5.275, p < 0.001), heart failure (HR = 3.637, p = 0.002), antiviral therapy (HR = 2.397, p = 0.038), CCB treatment (HR = 2.056, p = 0.030), endotracheal intubation (HR = 9.659, p < 0.001), mechanical ventilation (HR = 13.200, p < 0.001), AKI (HR = 6.805, p < 0.001) and eGFR < 60 mL/min per 1.73 m2 (HR = 6.559, p < 0.001) were predictors of in-hospital death. As there is a correlation between Scr and eGFR, we constructed two multivariate Cox regression models, one including AKI and the other including eGFR. Multivariate Cox regression analysis including eGFR indicated that heart failure (HR = 2.801, p = 0.017), antiviral therapy (HR = 3.322, p = 0.006), CCB therapy (HR = 2.087, p = 0.034), mechanical ventilation (HR = 10.871, p <0.001), and eGFR < 60 mL/min per 1.73 m2 (HR = 3.625, p = 0.001) were independent predictors of in-hospital mortality. Independent predictors, by another multivariate Cox analysis included age ≥80 years (HR = 3.110, p = 0.013), heart failure (HR = 2.534, p = 0.033), CCB treatment (HR = 2.719, p = 0.004), mechanical ventilation (HR = 7.780, p < 0.001) and AKI (HR = 2.347, p = 0.033).
 |
Table 6 Multivariable Cox Regression Analysis on the Risk Factors Associated with Death in Older Patients with COVID-19 |
Discussion
This is a retrospective study of elderly patients with COVID-19. The prevalence of AKI in the present study was 12.7%, and the proportion of patients with eGFR <60 mL/min per 1.73 m2 was 25%. Compared with non-hypertensive patients, hypertensive patients had more pronounced kidney dysfunction and a higher proportion of patients with eGFR <60 mL/min per 1.73 m2 after SARS-CoV-2 infection, but there was no difference in the prevalence of AKI. In the hypertensive population, kidney function was more abnormal after SARS-CoV-2 infection in patients aged ≥80 years and in patients with frailty than in controls, with a significantly higher prevalence of AKI and a significantly higher proportion of patients with eGFR <60 mL/min per 1.73 m2. On the other hand, the overall mortality rate of COVID-19 was 5.2%. Factors associated with a higher risk of death included age ≥80 years, heart failure, antiviral therapy, CCB therapy, mechanical ventilation, AKI, and eGFR <60 mL/min per 1.73 m2.
Hypertension is the most common comorbidity in patients with COVID-19, and its association with the more severe course of COVID-19 and COVID-19-related deaths remains controversial. Early reports suggested that the prevalence of hypertension in patients with severe COVID-19 was significantly higher than that in patients without severe COVID-19,29,30 which also occurred in patients admitted to ICU31 and died of COVID-19.5 Similar results were obtained in our study that the prevalence of severity, admission to ICU, and death in the hypertensive group are significantly higher than those in the non-hypertensive group. Abdalla et al proposed that the increased risk of hospitalization of hypertensive patients with COVID-19 was due to the poor cholesterol output of macrophages caused by ATP Binding Cassette Subfamily G Member 1 depletion and the inflammatory effects of advanced glycation end product-‐bovine serum albumin.32 However, further multivariable analysis revealed that hypertension is not an independent risk factor for death in elderly patients with COVID-19. Our findings were supported by a prospective multi-center observational cohort study which reported that hypertension alone does not increase the mortality rate of COVID-19 or the hospitalization risk of patients receiving treatment.33 Nevertheless, a previous meta-analysis came to an inconsistent conclusion: those combined with hypertension had a significantly higher risk of in-hospital deaths, admission to ICU, and need for invasive ventilation among COVID-19 patients.34 In addition, older age and other potential risk factors are mostly observed in patients with hypertension. Subgroup analyses were performed considering that the association could be confounded by age and other comorbidities. It can be found that among patients with hypertension, the oldest old and frail patients had a more severe course of COVID-19 and higher mortality. The further survival analysis indicated that age and heart failure were important risk factors for the increased in-hospital mortality of COVID-19. In Japan, the mortality in patients aged 70–79 years and ≥80 years was 6.8% and 14.8%, respectively, higher than that in all age groups which was 2.6%.18 A large prospective observational cohort study in the UK reported that increasing age is a strong predictor of in-hospital mortality, and the hazard ratio for death compared to younger subjects age <50 years old escalated from 2.63 (95% CI 2.06–3.35) in patients aged between 50 and 59 years and to 11.09 (95% CI 8.93–13.77) in patients of at least 80 years old.35 Although our study did not find frailty as an independent risk factor, Sara et al reported that the frailty level was the strongest prognostic factor for death in patients aged 65 years or older.36 Several studies indicated that frailty was strongly associated with adverse outcomes and outperformed age as a predictor in patients with COVID-19 so that its detection should not be neglected.37,38 Overall, mortality is predicted by age and the presence of other comorbidities, whereas hypertension has no significant intervention on COVID-19 lethality, which was consistent with previous studies.35,39,40
The main finding of this study is that the renal dysfunction in the COVID-19 hypertensive group and its subgroups was more severe compared with the control group, which was independently associated with COVID-19 in-hospital mortality. Although the lungs are considered the site of replication of SARS-CoV-2, infected patients often report other symptoms and multiple-organ failure, indicating involvement in the gastrointestinal tract, heart, cardiovascular system, kidneys, and other organs.41 After lung infection, the virus may accumulate in the kidneys through blood and cause damage to resident kidney cells.4 It has been confirmed that SARS-CoV2 has robust replication ability in human kidney cell lines (293T)42 and human kidney organoid models.43 Indeed, microscopic analyses of renal tissue revealed the accumulation of viral RNA in the tubules, accompanied by tubular isometric vacuolation and formation of double-membrane vesicles containing vacuoles, resulting in moderate to severe renal tubular injury.44,45 The incidence of renal abnormalities in patients with covid-19 pneumonia is high, with a sizeable fraction of patients presenting with proteinuria, hematuria, increased serum creatinine (Scr) and blood urea nitrogen (BUN).4,46 Cheng et al reported that the prevalence of kidney disease and the development of AKI during hospitalization in patients with COVID-19 are very high, which were related to the in-hospital mortality.4 There are many factors that contribute to renal dysfunction in patients with COVID-19, among which hypertension, age and frailty have been found to be risk factors for renal function impairment in patients with COVID-19.24,27 Kidney disease has also been proved to be an important risk factor for mortality in elderly patients with COVID-19. A retrospective investigation of hospitalized older patients with confirmed COVID-19 at Zhongnan Hospital of Wuhan University at the beginning of 2020 reported that the proportion of patients at least 65 years old with an elevated baseline serum creatinine was 21.8% compared with 8.8% in younger patients, and elevated baseline serum creatinine levels are associated with mortality in elderly patients with COVID-19.47 Yan et al conducted a retrospective observational cohort study of elderly patients in a large tertiary nursing university hospital in Wuhan, China, and led to a similar conclusion: the incidence of AKI in elderly patients with COVID-19 is 13%, higher than that of COVID-19 in the general population and AKI is associated with a high risk of death.48
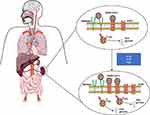
The mechanism of kidney injury in COVID-19 patients is still unclear, among which SARS-CoV-2 direct infection of renal tissue is the most comprehensive mechanism (Figure 3). SARS-CoV-2 binds to sensitive cells expressing ACE2 after contacting with the airway surface and has potential toxic effects on type II alveolar epithelial cells, leading to lung injury and acute respiratory failure. However, ACE2 is not only expressed in the lungs but also in the liver, spleen, brain, intestine, and heart, with the highest expression in the kidneys, cardiovascular system, and gas gut system.45,49 Human tissue RNA sequencing data show that the expression level of angiotensin-converting enzyme 2 (ACE2) in the urinary organs (kidneys), which mainly widely expressed in renal tubular is nearly 100 times higher than that in the respiratory organs (lungs).4,45 In addition, transmembrane protease serine 2 (TMPRSS2) and cathepsin L (cat L), two facilitators of SARS coronavirus type 2 infection, were highly expressed in multiple cell types of the kidney.50 Therefore, the virus has a strong affinity for the kidney, mainly leading to acute tubular injury, while the glomerulus remaining intact, except for mild glomerulosclerosis found in some cases of coexisting disease, which suggests that other diseases such as hypertension and diabetic nephropathy may have been involved.45 ACE2 represents a key enzyme component of the renin angiotensin aldosterone system (RAAS), and commonly used antihypertensive drugs, such as angiotensin-converting enzyme inhibitor (ACEI) and ARB, were reported to induce upregulation of ACE2 membrane expression, increasing the chances of virus entry into organs.18 An RNA sequencing study targeting 436 patients indicated that the expression of ACE2 increased with age in both the lungs and kidneys of humans, which increase the risk of SARS-CoV-2 infection.51 Aging-related structural and functional changes also contribute to AKI in the aging kidney.22 These all provide a good explanation for the causes of renal function damage in hypertension and elderly COVID-19 patients. Other possible mechanisms of kidney injury include microvascular dysfunction caused by endothelial injury, as well as the potential role of cytokine storms in AKI immunopathology. Renal injury in the acute phase of COVID-19 is closely related to the longitudinal decline of renal function and the post acute state.25 Therefore, it is necessary to pay close attention to the kidney function during and after hospitalization.
There were some limitations to our study. First, the incidence of AKI might not be accurate because baseline creatinine values were missing and replaced with minimum creatinine values, and urine volume data were missing from medical records and not collected to define AKI. Secondly, due to our complete reliance on electronic health information systems as data sources, which are with missing data as some patients did not undergo certain tests, so there might be other potential confounding factors that were not included. Finally, this is a single-center study, and a larger cohort of medical centers is needed to ensure its generalizability.
Conclusion
Kidney dysfunction is very common in hospitalized hypertensive elderly patients with COVID-19, which is more prominent in the elderly aged ≥80 years and the frail population, and is associated with higher in-hospital mortality. Therefore, early detection and treatment of renal dysfunction in elderly patients with COVID-19 is necessary to reduce mortality and achieve better prognosis.
Abbreviations
BUN, blood urea nitrogen; UA, serum uric acid; Scr, serum creatinine; eGFR, estimated glomerular filtration rate; ICU, intensive care unit; AKI, acute kidney injury; CCB, calcium channel blocker; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; COPD, chronic obstructive pulmonary disease; AD, Alzheimer disease; ARB, angiotensin receptor blocker; IQR, interquartile range; ACE, angiotensin-converting enzyme; ACEI, angiotensin-converting enzyme inhibitor; cat L, cathepsin L; TMPRSS2, transmembrane protease serine 2.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethical Approval
The Ethics Committee of Zhejiang Hospital approved this study (2022–31J). Participants who took part in screening for geriatric syndrome were given written informed consent before participating in the study. All methods were carried out in accordance with relevant guidelines and regulations.
Acknowledgments
We sincerely thank the staff from the Information Department of Zhejiang Hospital for their positive involvement in this study.
Funding
This study was financially supported by the Major Social Welfare Project of Zhejiang Science and Technology Department (No. 2023C03162), the China National Key R&D Program (No. 2020YFC2009001), and theZhejiang Provincial Program for the Cultivation of High‐Level Innovative Health Talents (2022-1).
Disclosure
The authors have no conflicts of interest to declare that are relevant to the content of this article.
References
1. Gorbalenya AE, Baker SC, Baric RS, et al. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5(4):536–544. doi:10.1038/s41564-020-0695-z
2. Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19(3):141–154. doi:10.1038/s41579-020-00459-7
3. Rostami Z, Mastrangelo G, Einollahi B, et al. A prospective study on risk factors for acute kidney injury and all-cause mortality in hospitalized COVID-19 patients from Tehran (Iran). Front Immunol. 2022;13:874426 doi:10.3389/fimmu.2022.874426.
4. Cheng Y, Luo R, Wang K, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–838. doi:10.1016/j.kint.2020.03.005
5. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi:10.1016/S0140-6736(20)30566-3
6. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574. doi:10.1001/jama.2020.5394
7. Patel AB, Verma A. COVID-19 and angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: what is the evidence? JAMA. 2020;323(18):1769–1770. doi:10.1001/jama.2020.4812
8. Nashiry A, Sarmin Sumi S, Islam S, Quinn JMW, Moni MA. Bioinformatics and system biology approach to identify the influences of COVID-19 on cardiovascular and hypertensive comorbidities. Brief Bioinform. 2021;22(2):1387–1401. doi:10.1093/bib/bbaa426
9. Guan W, Liang W, Zhao Y, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55(5):2000547. doi:10.1183/13993003.00547-2020
10. Peng M, He J, Xue Y, Yang X, Liu S, Gong Z. Role of hypertension on the severity of COVID-19: a review. J Cardiovasc Pharmacol. 2021;78(5):e648–e655. doi:10.1097/FJC.0000000000001116
11. Gallo G, Calvez V, Savoia C. Hypertension and COVID-19: current evidence and perspectives. High Blood Press Cardiovasc Prev. 2022;29(2):115–123. doi:10.1007/s40292-022-00506-9
12. Bepouka B, Situakibanza H, Sangare M, et al. Mortality associated with COVID‐19 and hypertension in sub‐Saharan Africa. A systematic review and meta‐analysis. Jl Clin Hypertens. 2022;24(2):99–105. doi:10.1111/jch.14417
13. Schiffrin EL, Flack JM, Ito S, Muntner P, Webb RC. Hypertension and COVID-19. Am J Hypertens. 2020;33(5):373–374. doi:10.1093/ajh/hpaa057
14. Simonnet A, Chetboun M, Poissy J, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obes. 2020;28(7):1195–1199. doi:10.1002/oby.22831
15. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. doi:10.1001/jamainternmed.2020.0994
16. Dent E, Martin FC, Bergman H, Woo J, Romero-Ortuno R, Walston JD. Management of frailty: opportunities, challenges, and future directions. Lancet. 2019;394(10206):1376–1386. doi:10.1016/S0140-6736(19)31785-4
17. Pranata R, Henrina J, Lim MA, et al. Clinical frailty scale and mortality in COVID-19: a systematic review and dose-response meta-analysis. Arch Gerontol Geriatr. 2021;93:104324. doi:10.1016/j.archger.2020.104324
18. Shibata S, Arima H, Asayama K, et al. Hypertension and related diseases in the era of COVID-19: a report from the Japanese Society of Hypertension Task Force on COVID-19. Hypertens Res. 2020;43(10):1028–1046. doi:10.1038/s41440-020-0515-0
19. Shah H, Khan MSH, Dhurandhar NV, Hegde V. The triumvirate: why hypertension, obesity, and diabetes are risk factors for adverse effects in patients with COVID-19. Acta Diabetol. 2021;58(7):831–843. doi:10.1007/s00592-020-01636-z
20. Mennuni S, Rubattu S, Pierelli G, Tocci G, Fofi C, Volpe M. Hypertension and kidneys: unraveling complex molecular mechanisms underlying hypertensive renal damage. J Hum Hypertens. 2014;28(2):74–79. doi:10.1038/jhh.2013.55
21. Chronopoulos A, Cruz DN, Ronco C. Hospital-acquired acute kidney injury in the elderly. Nat Rev Nephrol. 2010;6(3):141–149. doi:10.1038/nrneph.2009.234
22. Anderson S, Eldadah B, Halter JB, et al. Acute kidney injury in older adults. J Am Soc Nephrol. 2011;22(1):28–38. doi:10.1681/ASN.2010090934
23. Martin JE, Sheaff MT. Renal ageing. J Pathol. 2007;211(2):198–205. doi:10.1002/path.2111
24. Hirsch JS, Ng JH, Ross DW, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209–218. doi:10.1016/j.kint.2020.05.006
25. Gu X, Huang L, Cui D, et al. Association of acute kidney injury with 1-year outcome of kidney function in hospital survivors with COVID-19: a cohort study. Ebiomedicine. 2022;76:103817. doi:10.1016/j.ebiom.2022.103817
26. Ng JH, Hirsch JS, Hazzan A, et al. Outcomes among patients hospitalized with COVID-19 and acute kidney injury. Am J Kidney Dis. 2021;77(2):204–215. doi:10.1053/j.ajkd.2020.09.002
27. Küçük M, Ergün B, Yakar MN, et al. The effect of frailty on the development of acute kidney injury in critically-ill geriatric patients with COVID-19. Turk J Med Sci. 2022;52(5):1495–1503. doi:10.55730/1300-0144.5488
28. Kellum JA, Romagnani P, Ashuntantang G, Ronco C, Zarbock A, Anders HJ. Acute kidney injury. Nat Rev Dis Primers. 2021;7(1):52. doi:10.1038/s41572-021-00284-z
29. Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146(1):110–118. doi:10.1016/j.jaci.2020.04.006
30. Shi Y, Yu X, Zhao H, Wang H, Zhao R, Sheng J. Host susceptibility to severe COVID-19 and establishment of a host risk score: findings of 487 cases outside Wuhan. Crit Care. 2020;24(1):108. doi:10.1186/s13054-020-2833-7
31. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061. doi:10.1001/jama.2020.1585
32. Abdalla M, El Arabey AA, Gai Z. Hypertension is still a moving target in the context of COVID‐19 and post‐acute COVID‐19 syndrome. J Med Virol. 2023;95(1). doi:10.1002/jmv.28128
33. Shalaeva EV, Shadmanov KA, Azizova FL, et al. Is lone hypertension a risk factor for more severe COVID-19 outcomes? Glob Heart. 2022;17(1):17. doi:10.5334/gh.1099
34. Qian Z, Li Z, Peng J, Gao Q, Cai S, Xu X. Association between hypertension and prognosis of patients with COVID-19: a systematic review and meta-analysis. Clin Exp Hypertens. 2022;44(5):451–458. doi:10.1080/10641963.2022.2071914
35. Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ. 2020;369(m 1985). doi:10.1136/bmj.m1985
36. Tehrani S, Killander A, Astrand P, Jakobsson J, Gille-Johnson P. Risk factors for death in adult COVID-19 patients: frailty predicts fatal outcome in older patients. Int J Infect Dis. 2021;102:415–421. doi:10.1016/j.ijid.2020.10.071
37. Majumder J, Minko T. Recent developments on therapeutic and diagnostic approaches for COVID-19. Aaps J. 2021;23(1):14. doi:10.1208/s12248-020-00532-2
38. Simon NR, Jauslin AS, Rueegg M, et al. Association of frailty with adverse outcomes in patients with suspected COVID-19 infection. J Clin Med. 2021;10(11):2472. doi:10.3390/jcm10112472
39. Mancusi C, Grassi G, Borghi C, et al. Determinants of healing among patients with coronavirus disease 2019: the results of the SARS-RAS study of the Italian Society of Hypertension. J Hypertens. 2021;39(2):376–380. doi:10.1097/HJH.0000000000002666
40. Iaccarino G, Grassi G, Borghi C, Ferri C, Salvetti M, Volpe M. Age and multimorbidity predict death among COVID-19 patients: results of the SARS-RAS study of the Italian society of hypertension. Hypertens. 2020;76(2):366–372. doi:10.1161/HYPERTENSIONAHA.120.15324
41. Synowiec A, Szczepanski A, Barreto-Duran E, Lie LK, Pyrc K. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): a Systemic Infection. Clin Microbiol Rev. 2021;34(2). doi:10.1128/CMR.00133-20
42. Chu H, Chan JF, Yuen TT, et al. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: an observational study. Lancet Microbe. 2020;1(1):e14–e23. doi:10.1016/S2666-5247(20)30004-5
43. Monteil V, Kwon H, Prado P, et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181(4):905–913. doi:10.1016/j.cell.2020.04.004
44. Farkash EA, Wilson AM, Jentzen JM. Ultrastructural evidence for direct renal infection with SARS-CoV-2. J Am Soc Nephrol. 2020;31(8):1683–1687. doi:10.1681/ASN.2020040432
45. Diao B, Wang C, Wang R, et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 infection. Nat Commun. 2021;12(1). doi:10.1038/s41467-021-22781-1
46. Naicker S, Yang C, Hwang S, Liu B, Chen J, Jha V. The Novel Coronavirus 2019 epidemic and kidneys. Kidney Int. 2020;97(5):824–828. doi:10.1016/j.kint.2020.03.001
47. Chen T, Dai Z, Mo P, et al. Clinical characteristics and outcomes of older patients with coronavirus disease 2019 (COVID-19) in Wuhan, China: a single-centered, retrospective study. J Gerontol: Series A. 2020;75(9):1788–1795 doi:10.1093/gerona/glaa089.
48. Yan Q, Zuo P, Cheng L, et al. Acute kidney injury is associated with in-hospital mortality in older patients with COVID-19. J Gerontol: Series A. 2021;76(3):456–462 doi:10.1093/gerona/glaa181.
49. Oussalah A, Gleye S, Clerc Urmes I, et al. Long-term ACE Inhibitor/ARB use is associated with severe renal dysfunction and acute kidney injury in patients with severe COVID-19: results from a referral center cohort in the Northeast of France. Clin Infect Dis. 2020. doi:10.1093/cid/ciaa677
50. Puelles VG, Lutgehetmann M, Lindenmeyer MT, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383(6):590–592. doi:10.1056/NEJMc2011400
51. Jiang X, Eales JM, Scannali D, et al. Hypertension and renin-angiotensin system blockers are not associated with expression of angiotensin-converting enzyme 2 (ACE2) in the kidney. Eur Heart J. 2020;41(48):4580–4588. doi:10.1093/eurheartj/ehaa794
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.