Back to Journals » Clinical Ophthalmology » Volume 17
Eye Lesions in Patients After One Year of Kidney Transplantation
Authors Trung NL , Toan PQ , Trung NK, Tuan VA, Huyen NT
Received 26 June 2023
Accepted for publication 18 September 2023
Published 29 September 2023 Volume 2023:17 Pages 2861—2869
DOI https://doi.org/10.2147/OPTH.S424883
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Nguyen Le Trung,1,2 Pham Quoc Toan,3 Nguyen Kien Trung,4 Vu Anh Tuan,1 Nguyen Thu Huyen5
1Vietnam Department of Ophthalmology, Hanoi Medical University, Hanoi, Vietnam; 2Vietnam Department of Ophthalmology, Military Hospital 103, Hanoi, Vietnam; 3Vietnam Department of Nephrology, Military Hospital 103, Hanoi, Vietnam; 4Vietnam Department of Trauma, National Eye Hospital, Hanoi, Vietnam; 5Vietnam Department of Ophthalmology, National Military Hospital 108, Hanoi, Vietnam
Correspondence: Pham Quoc Toan, Email [email protected]
Purpose: Determine the incidence of some eye lesions in kidney transplant patients after one year at Military Hospital 103 and comment on related factors.
Patients and Methods: A cross-sectional study description of 111 kidney transplant patients (222 eyes) at Military Hospital 103. We assessed several eye lesions, including dry eyes, corneal conjunctival calcification, cataracts, and retinopathy.
Results: The rate of retinopathy was 84.7%, dry eye was 59.5%, cataract was 29.7%, and corneal conjunctival calcification was 24.8%, atrophy optic nerve was 9.9%, epiretinal membrane was 1.8%. Post-transplant influence factors associated with cataracts include the dose of prednisolone (OR= 1.6, p < 0.05) and post-transplant diabetes (OR=1.4, p < 0.05). The influence factor related to the atrophy of the optic nerve is systemic infection after transplantation (OR=2.4, p < 0.05).
Conclusion: Retinopathy accounted for the highest rate, followed by dry eye disease; cataracts ranked third; and finally, calcified corneal conjunctiva. Factors that affect cataracts are diabetes mellitus and prednisolone dose. Factors affecting optic nerve atrophy are infections after kidney transplantation.
Keywords: kidney transplant, corneal conjunctival calcification, dry eyes, hypertensive retinopathy
Introduction
With the development of immunosuppression and surgery, kidney transplantation has become the treatment of choice due to its remarkable survival and improved quality of life. Despite the benefits, there are still essential warnings for people vulnerable to eye damage after kidney transplants. However, a comprehensive assessment of eye lesions has yet to be emphasized. The patient himself does not see the real urgency. When a kidney transplant occurs, patients appreciate the role of vision in improving their quality of life. In one study, the most concerning needs after an organ transplant were eye health and the ability to walk.1
Eye lesions have been shown at different rates in studies when using systemic immunosuppressants.2–5 These lesions predate kidney transplantation and then continue to progress. Or it could be new lesions after transplantation. Some of the typical lesions mentioned include cataracts, dry eye conditions, glaucoma, and retinal damage. Less common lesions are bacterial infections of the eyes.
There are several kidney transplant centers in Viet Nam. Still, studies have yet to be seen reporting on the incidence of eye lesions in these subjects and evaluating the effects of post-transplant factors. Therefore, we conducted the study with the objective:
Describe some ocular lesions in patients after a kidney transplant one year and analyze the influence factors of eye lesions with some characteristics in patients after kidney transplantation.
Materials and Methods
Convenient sampling, all qualified patients are examined and evaluated for eye damage. Cross-sectional descriptive research methods have analysis. The study period is from 01/2021-12/2022.
Inclusion Criteria
One hundred eleven patients after a kidney transplant after one year at Military Hospital 103 agreed to participate in the study.
Exclusion Criteria
Systemic with acute pathologies cannot participate.
Process
Patients were measured with best corrected visual acuity using a 5m distance Snellen electronic vision panel. Assess dry eye condition using OSDI questionnaires, setting tear secretion time (Schirmer test) and tear film break time (BUT test)—evaluation of the anterior segment of the cornea, anterior, iris, pupil, and lens. Mydrin-P (Tropicamide + Phenylephrine hydrochloride) pupil dilation evaluates the posterior segment, including the vitreous, optic nerve, and central retina.
Variables
The main outcome variables include signs of corneal calcification according to the Porter-Crombie standard classified into five degrees: degree 0 indicates normal, no residue in the conjunctiva or cornea; grade 1, there is only calcium deposition in the conjunctiva; grade 2 indicates irregular dotted corneal and conjunctival deposition; grade 3 suggests a stream of corneal and conjunctival deposition; grade 4 indicates increased corneal deposition, usually two lines, and deposition in the conjunctiva; Grade 5 indicates extensive corneal deposition, usually three-line and deposit in the conjunctiva.6 Buratto-graded cataracts are divided into morphs: posterior subcapsular cataracts, nucleus, cortex.7 Wong’s graded hypertensive retinopathy is divided into three levels: mild retinopathy with hard exudate and flame hemorrhage. The moderate grade is mild + with soft exudate. Severity is moderate + papilloedema.8 Diagnosis of optic nerve atrophy was observed with a C/D ratio >0.6, and OCT optical tomography showed a decrease in the retinal nerve fiber layer < 85 μm.9 The epiretinal membrane is diagnosed when a macular wrinkle is seen, and an OCT scan shows a membrane. Criteria for diagnosing dry eyes are the Schirmer Test < 10mm, the BUT< test 10 seconds, and OSDI > 10 points. No dry eyes: Schirmer test ≥ 10mm, BUT test ≥ 10 seconds and OSDI ≤ 10 points.10
Independent factors including age group, gender (male or female), diastolic and systolic blood pressure indices are recorded according to the results of the electronic medical record of the post-transplant clinic at the hospital.11 Comorbid systemic diseases (hypertension, diabetes mellitus as diagnosed by an internist after transplant examination and recorded according to electronic medical records at the hospital, systemic infections after kidney transplantation according to electronic medical records in the post-transplant resuscitation department). Drugs for treating systemic diseases (hypertensive drugs, diabetes drugs), doses of medicines (mg/day). Immunosuppressants (drug regimen, drug combination), drug dosage (mg/day), and tacrolimus drug concentration (ng/mL).
Statistics
Data entry has been completed using EpiData 3.1 software (EpiData, Odense, Denmark). Statistical analysis and data cleaning with STATA 16.0 (Stata Corp, College Station, TX, USA). The data are presented as mean, standard deviations, and percentages. Using univariable logistic regression, assess the relationship between outcome variables with independent variables.
Ethical Approval
Our study is approved by the Ethics Council of Hanoi Medical University, Vietnam, according to Decision No. 467/GCN-HDDDNCSYHN-ĐHYHN dated 04/05/2021. All patients provided written informed consent, and this study was conducted following the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. All kidneys were donated voluntarily with written informed consent, and this was conducted in accordance with the Declaration of Istanbul.
Result
This study included 111 patients who had renal transplants between 01 January 2021 and 31 December 2022. Table 1 illustrates a demographic of the characteristics of the study subjects (Table 1).
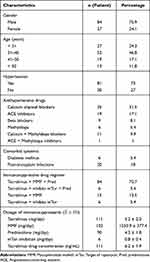 |
Table 1 Characteristics of the Subjects |
Men account for a large proportion of patients of high working age; after transplantation, hypertension is still high and must be treated with antihypertensive drugs with calcium channel blockers mainly; there are six patients with diabetes mellitus after transplant. The immunosuppressive drug regimen consists primarily of 3 drugs tacrolimus + MMF + Prednisolone. Heavily used drugs are tacrolimus, MMF, and Prednisolone.
Figure 1 presents the percentage of eye diseases in patients after kidney transplants (Figure 1).
 |
Figure 1 The proportion of eye lesion. |
The highest proportion of retinal lesions included hypertensive forms of retinal lesions (188 eyes), atrophy of the optic nerve (19 eyes) and the epiretinal membrane (3 eyes), followed by dry eye lesions (132 eyes), cataracts in third place (66 eyes) and finally corneal calcification lesions (55 eyes).
The table below illustrates the proportion of different categories of grades of eye lesions (Table 2).
 |
Table 2 Grades of Ocular Lesions |
Grade 1 corneal conjunctival calcium accounts for the most proportion, posterior subcapsular turbidity predominates, while mild retinal lesions accounts for the majority.
The results of the correlational analysis are shown in Table 3. (Table 3).
 |
Table 3 Influence Factors Post-Renal Transplant Associated with Cataracts: Logistic Regression |
The influence factors associated with cataracts after the 12th month of transplantation are post-transplant diabetes mellitus and the daily dose of prednisolone.
There is a significant difference in the posttransplant infection (OR= 2.4, p <0.05).
Discussion
Our study is the first study conducted on post-kidney transplant subjects in Vietnam. The study provides evidence of the incidence of certain eye lesions and influence factors after transplantation to guide eye treatment for this population.
General Characteristics
One of the issues that emerge from these findings is patients in the advanced working age range, demonstrating that the need for rehabilitation in patients with end-stage renal failure is exceptionally high. To date, the kidney transplant method is still considered the most optimal method. There is a significant difference in the incidence between the sexes, which is thought to be related to lifestyle differences.12 After kidney transplantation, patients who took antihypertensive drugs with different mechanisms accounted for 73%. Several mechanisms explain post-transplant hypertension. In the early post-transplant stages, plasma renin plays a role when elevated in the blood, causing a vasospasm reaction. In the later stages, there is a role of immunosuppressive drugs such as CNI (calcineurin inhibitor) inhibitors and corticosteroids act.13 The drugs used to lower blood pressure were diverse in the study subjects. However, calcium channel blockers are used most significantly due to their rapid and safe hypotensive properties in patients with renal insufficiency.14 After kidney transplantation, this class of drugs is also still preferred over the rest of the group. Six patients developed diabetes mellitus after transplantation. After checking their blood glucose and HbA1c levels, these patients were given the hypoglycemic drug metformin. There were 20 patients with post-transplant systemic infections required longer stays than other patients, receiving high-dose antibiotics for 20 days. Four immunosuppressive regimens were used for the patients in our study at 12 months post-transplant. In the regimen using three immunosuppressants, Tacrolimus + MMF + Prednisolone was used the most, accounting for 75.7%, followed by Tacrolimus + MMF regimen accounting for 13.5%, two regimens Tacrolimus + mTor and Tacrolimus + mTor + Prednisolone was equivalent to 5.4%. 100% of patients receive Tacrolimus and are periodically checked for serum Tacrolimus levels. The dosage of immunosuppressants is presented in Table 1.
Eye Lesions in Patients After Kidney Transplant 1 Year
In our study, the incidence of corneal conjunctival calcification in both eyes was 24.8% (Figure 1). After kidney transplantation, some studies suggest that the rate of corneal conjunctival calcification decreases due to improved kidney function and better control of mineral metabolism. In a study of patients with end-stage kidney disease, corneal conjunctival calcification was found in 50% of patients before transplantation, but this rate decreased significantly after successful transplantation.15,16
Our results found that the prevalence of cataracts was 29.7%, with posterior subcapsular and nucleus, with posterior subcapsular cataracts being the most numerous (Chart 1 and Table 2). The risk of developing cataracts is high in people taking high-dose, long-term steroids and immunosuppressive drugs containing calcineurin inhibitors such as tacrolimus. In addition, people with diabetes after a kidney transplant may also be at higher risk. Previous research has shown that the prevalence of cataracts after kidney transplantation varies from 23% to 58%.17
Our results show that the prevalence of dry eyes after transplantation is 59.4% in both eyes (Figure 1). There are many explanations for this finding: first, it is possible that underlying factors contributing to dry eyes in patients with kidney failure, such as inflammation, drug use, and comorbidities, persist even after a successful kidney transplant. Second, immunosuppressive drugs contribute to dry eyes or worsen pre-existing symptoms. Ginu’s study accounted for only 11.2%, while the author’s study in the post-transplant population lasted from 0.3 to 14 years.3 Sandhu’s study showed a post-transplant dry eye prevalence of 28% in subjects 3.3 ± 2.6 years after transplantation.5 The difference in most dry eyes between our study and Ginu and Sandhu’s study may be due to factors, including patient populations, exam procedures, and methods used to assess dry eyes. Our study specifically investigated the prevalence of dry eyes in patients one year after kidney transplantation. In contrast, Ginu and Sandhu’s study included patients at different post-transplant times (0.3–14 years and unspecified times, respectively). Furthermore, differences in the methods used to assess dry eye also contributed to differences in reported prevalence. Ginu’s research used criteria such as binocular asymmetry, tear film sickle height, Schirmer I and II tests, tear film break-up time, and ocular surface changes. Our study used the Schirmer I test, tear film break-up time (TBUT), and OSDI (Ocular Surface Disease Index) questionnaire.
Documented retinal lesions include the retinal vascular system, optic nerve, and macula lesions. If you have prolonged hypertension, the blood vessels in this area are affected first. These signs are characteristic of chronic lesions.8 These lesions usually have little effect on visual acuity and receive little attention. Only through retinoscopy and by the experience of ophthalmologists can it be discovered. There were 19 cases of optic nerve atrophy, 11 in the right eye and 8 in the left eye (Figure 1). The causes have many reasons. The most common is chronic anemia in people with renal impairment leading to anemia that nourishes the optic nerve head.9,18 In addition, due to vascular system disorders, renin-angiotensin damages the ganglion cell layer, the nerve fiber layer.19 In the post-transplant stage, optic nerve atrophy is thought to be associated with post-transplant infection.2 Another factor is supposed to be persistent glaucoma in patients taking corticosteroids. In our study, the incidence of retinal complications was less than that of author Chillo.20 One possible explanation for this problem may be that our choice of subjects differs from other research. Because kidney transplantation indications are so tight, fewer patients with retinal lesions due to hypertension or diabetes mellitus, while Chillo studied a group of subjects with end-stage renal failure with no or no kidney transplant. In addition, the age of our study group was also lower than in previous studies. In Elon H.C.’s cross-sectional study, van Dijk gave a retinal lesions rate after kidney transplantation of 59% for patients taking low-dose corticosteroids.21 Ginu’s retinal lesions rate of 20.4% is the highest of any lesion, in addition to diabetic retinopathy accounting for 15.1%, papilla edema accounting for 1.7%. The explanation of the scaling difference from our study is due to the author’s classification criteria chosen by Keith Wagner Becker’s classification and the Early Treatment Diabetic Retinopathy Study (ETDRS).3 Sandhu’s study found that the incidence of retinal lesions due to hypertension in patients after kidney transplantation was 40%, diabetic retinopathy was 12%, and other lesions accounted for 10%. Like the Ginu study, Sandhu uses Keith Wagner Becker’s retinal lesions classification system.5
Post-Transplant Influence Factors Associated with Cataracts
When evaluating multivariate regression of post-transplant cataracts with several post-transplant factors, we found that patients receiving anti-rejection drugs with prednisolone with an average dose of prednisolone of 4.3 ± 1.8 mg/day were associated with lesions (Table 3). In previous studies, post-vitreous cataracts in both eyes developed in 45% of subjects with a median duration of 7.4 months, and another cross-sectional study found a prevalence of 87.5% between 6 and 48 months after kidney transplantation.2,22 The risk of cataract formation is thought to be dose-dependent.23 The following relevant factor is post-transplant diabetes, which adds to cataracts. In addition, anti-rejection immunosuppressants have been identified as a risk factor for pre-transplant cataracts. Studies have confirmed that tacrolimus blood levels in kidney transplant patients are associated with cataract conditions. However, this has only been shown in experimental mice when tacrolimus is seen to alter sorbitol, which makes up the protein fibers of the lens.24 Our study found no cataracts associated with tacrolimus concentration and dosage (Table 4).
 |
Table 4 Influence Factors Post-Renal Transplant Associated with Atrophy of Optic Nerve: Logistic Regression |
Post-Transplant Influence Factors Associated with Optic Nerve Atrophy
Tacrolimus blood levels are associated with visual acuity impairment due to neurological optic changes. However, overall clinical presentation and drug concentrations thought to be > 20 ng/mL are likely to cause neurotoxicity.25 In our study, no association with this factor was found. (Table 4) Glaucoma causes optic nerve atrophy. The hypothesis is that prolonged corticosteroid use in patients with renal impairment and post-transplantation may lead to corticosteroid-induced open-angle glaucoma. Previous research has confirmed no association with open-angle glaucoma in kidney transplant patients. At the same time, kidney transplantation reduces the risk of angle-closure glaucoma.26
When antihypertensive drugs such as beta-blockers, or calcium channel blockers, AngII blockers also cause glaucoma. One of the risks thought to cause glaucoma is prolonged post-transplant use of corticosteroids. In our study, when evaluating the association between prednisolone levels after transplantation, there was no association with this factor. (Table 4).
The factor associated with optic nerve atrophy thought to be significant in our study was a post-transplant infection. There were 14 cases of optic nerve atrophy in 20 people with post-transplant systemic infections. Our results are similar to Berindan’s.2
The limitation of our study is the use of cross-sectional description, so it is impossible to conclude the cause-and-effect relationship of some systemic factors that affect the pre-transplant eye, such as hypertension.
Conclusion
The highest prevalence of hypertensive retinopathy was 84.7%, with 9.9% of eyes having atrophy of optic nerve, 1.8% of eyes with epiretinal membranes, followed by dry eye disease accounting for 59.5%, cataract rate of 29.7% and corneal conjunctival calcification of 24.8%.
The post-transplant factors influencing cataract status are those with new post-transplant diabetes mellitus and prednisolone dosage. A predisposing factor for optic nerve atrophy is systemic infection after kidney transplantation.
Abbreviations
MMF, Mycophenolate mofetil; mTor, Target of rapamycin; Pred, prednisolone; Pre-OP, before renal transplant; Post-OP, after renal transplant.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. If you have concerns about sharing the data, please contact [email protected].
Ethical Statements
All participants were dispensed with written informed consents, and the protocol was approved by the Ethical Review Committee of the Hanoi Medical University, Vietnam (Decision no. 467/GCN-HDDDNCSYHN-DHYHN dated 05/04/2021). All patients provided written informed consent, and that this study was conducted following the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Acknowledgments
We would like to sincerely thank the leadership and medical staff at the Department of Nephrology and Ophthalmology, 103 Military Hospital, Military Medical Academy for facilitating the research team to implement the project.
Funding
The authors received no financial support for this article’s research, authorship, and publication.
Disclosure
The authors declare no potential conflicts of interest to this article’s research, authorship, and publication.
References
1. Thomas AG, Ruck JM, Chu NM, et al. Kidney transplant outcomes in recipients with visual, hearing, physical and walking impairments: a prospective cohort study. Nephrol Dial Transplant. 2020;35(7):1262–1270. doi:10.1093/ndt/gfz164
2. Berindán K, Nemes B, Szabó RP, Módis L. Ophthalmic findings in patients after renal transplantation. Transplant Proc. 2017;49(7):1526–1529. doi:10.1016/j.transproceed.2017.06.016
3. Ginu PM, Sati A, Murari T, Kaushik J, Mishra SK, Sharma VK. Ocular manifestations in renal allograft recipients: an Indian perspective. Indian J Ophthalmol. 2021;69(4):900–905. doi:10.4103/ijo.IJO_1120_20
4. Kianersi F, Taheri S, Fesharaki S, et al. Ocular manifestations in hemodialysis patients: importance of ophthalmic examination in prevention of ocular sequels. Int J Prev Med. 2019;10:20. doi:10.4103/ijpvm.IJPVM_464_18
5. Sandhu JS, Kansal S, Bajwa GS, Sandhu J. Ocular changes in renal allograft recipients and patients of chronic kidney disease. Saudi J Kidney Dis Transpl. 2014;25(6):1285–1289. doi:10.4103/1319-2442.144269
6. Porter R, Crombie AL. Corneal and conjunctival calcification in chronic renal failure. Br J Ophthalmol. 1973;57(5):339–343. doi:10.1136/bjo.57.5.339
7. Buratto L, Apple DJ. Phacoemulsification: Principles and Techniques. Slack Incorporated; 2003.
8. Wong TY. Fred Hollows lecture: hypertensive retinopathy - a journey from fundoscopy to digital imaging. Clin Exp Ophthalmol. 2006;34(5):397–400. doi:10.1111/j.1442-9071.2006.01233.x
9. Ab Gani NH, Ibrahim M, Wan Hitam WH, Masnon NA, Hassan A. Bilateral optic atrophy in a young patient with chronic anaemia secondary to end-stage renal disease. Cureus. 2021;13(3):e13969. doi:10.7759/cureus.13969
10. Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II diagnostic methodology report. Ocul Surf. 2017;15(3):539–574. doi:10.1016/j.jtos.2017.05.001
11. Mondal RN, Matin MA, Rani M, et al. Journal of hypertension: open access; 2017.
12. Cobo G, Hecking M, Port FK, et al. Sex and gender differences in chronic kidney disease: progression to end-stage renal disease and haemodialysis. Clin Sci. 2016;130(14):1147–1163. doi:10.1042/cs20160047
13. Popovtzer MM, Pinnggera W, Katz FH, et al. Variations in arterial blood pressure after kidney transplantation. Relation to renal function, plasma renin activity, and the dose of prednisone. Circulation. 1973;47(6):1297–1305. doi:10.1161/01.cir.47.6.1297
14. Ohno S, Ishii A, Yanagita M, Yokoi H. Calcium channel blocker in patients with chronic kidney disease. Clin Exp Nephrol. 2022;26(3):207–215. doi:10.1007/s10157-021-02153-1
15. Kraus MA, Kalra PA, Hunter J, Menoyo J, Stankus N. The prevalence of vascular calcification in patients with end-stage renal disease on hemodialysis: a cross-sectional observational study. Ther Adv Chronic Dis. 2015;6(3):84–96. doi:10.1177/2040622315578654
16. Caldeira JA, Sabbaga E, Ianhez LE. Conjunctival and corneal changes in renal failure. Influence of renal transplantation. Br J Ophthalmol. 1970;54(6):399–404. doi:10.1136/bjo.54.6.399
17. Albert K, Sennesael J, Haentjens P. Incidence and risk factors for posttransplant subcapsular cataract: a long-term retrospective cohort study. Transplant Proc. 2011;43(9):3465–3469. doi:10.1016/j.transproceed.2011.10.007
18. Kolonko P-MJ, Pinocy-Mańdok J, Kocierz M, et al. ”Anemia and erythrocytosis after kidney transplantation: a 5-year graft function and survival analysis”. Transplant Proc. 2009;41(8):3046–3051. doi:10.1016/j.transproceed.2009.07.090
19. Wilkinson-Berka JL, Agrotis A, Deliyanti D. The retinal renin-angiotensin system: roles of angiotensin II and aldosterone. Peptides. 2012;36(1):142–150. doi:10.1016/j.peptides.2012.04.008
20. Chillo P, Ismail A, Sanyiwa A, Ruggajo P, Kamuhabwa A. Hypertensive retinopathy and associated factors among nondiabetic chronic kidney disease patients seen at a tertiary hospital in Tanzania: a cross-sectional study. Int J Nephrol Renovasc Dis. 2019;12:79. doi:10.2147/IJNRD.S196841
21. van Dijk EHC, Soonawala D, Rooth V, et al. Spectrum of retinal abnormalities in renal transplant patients using chronic low-dose steroids. Graefes Arch Clin Exp Ophthalmol. 2017;255(12):2443–2449. doi:10.1007/s00417-017-3823-6
22. Jahadi-hosseini HR, Rahmani B, Karbassi A, Mehrian M, Medghalchi AR. Ocular complications in renal allograft recipients. Transplant Proc. 2003;35(1):309–310. doi:10.1016/s0041-1345(02)03986-6
23. Jobling AI, Augusteyn RC. What causes steroid cataracts? A review of steroid-induced posterior subcapsular cataracts. Clin Exp Optom. 2002;85(2):61–75. doi:10.1111/j.1444-0938.2002.tb03011.x
24. Ishida H, Mitamura T, Takahashi Y, et al. Cataract development induced by repeated oral dosing with FK506 (tacrolimus) in adult rats. Toxicology. 1997;123(3):167–175. doi:10.1016/s0300-483x(97)00102-9
25. Shao X, He Z, Tang L, Gao L. Tacrolimus-associated ischemic optic neuropathy and posterior reversible encephalopathy syndrome after small bowel transplantation. Transplantation. 2012;94(9):e58–60. doi:10.1097/TP.0b013e31826dde21
26. Moon JJ, Kim YW, Oh BL, et al. Nationwide Glaucoma incidence in end stage renal disease patients and kidney transplant recipients. Sci Rep. 2021;11(1):7418. doi:10.1038/s41598-021-86846-3
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
