Back to Journals » Clinical Epidemiology » Volume 13
Extremely and Very Preterm Deliveries in a Maternity Unit of Inappropriate Level: Analysis of Socio-Residential Factors
Authors Roussot A, Goueslard K, Cottenet J, Von Theobald P, Rozenberg P, Quantin C
Received 21 October 2020
Accepted for publication 4 March 2021
Published 14 April 2021 Volume 2021:13 Pages 273—285
DOI https://doi.org/10.2147/CLEP.S288046
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Vera Ehrenstein
Adrien Roussot,1,2 Karine Goueslard,1,2 Jonathan Cottenet,1– 4 Peter Von Theobald,5 Patrick Rozenberg,6,7 Catherine Quantin1– 4,8
1Biostatistics and Bioinformatics (DIM), University Hospital, Dijon, France; 2Bourgogne Franche-Comté University, Dijon, France; 3Inserm, CIC 1432, Dijon, France; 4Clinical Investigation Center, Clinical Epidemiology/Clinical Trials Unit, Dijon University Hospital, Dijon, France; 5Department of Gynecology and Obstetrics, Hospital Felix Guyon, CHU La Reunion, France; 6EA 7285, Versailles Saint Quentin University, Versailles, France; 7The Department of Obstetrics and Gynecology, Poissy-Saint Germain Hospital, Poissy, France; 8High-Dimensional Biostatistics for Drug Safety and Genomics, CESP, Inserm, Université Paris-Saclay, UVSQ, Univ. Paris-Sud, Villejuif, France
Correspondence: Catherine Quantin
Biostatistics and Bioinformatics (DIM), University Hospital, Dijon, BP 77908, Dijon, 21079, France
Tel +33 3 8029 3629
Fax +33 3 8029 3973
Email [email protected]
Purpose: To analyze the socio-residential factors associated with extremely and very preterm deliveries occurring in non-level 3 maternity units in France.
Materials and Methods: This is a population-based observational retrospective study using national hospital data from 2012 to 2014. A generalized estimating equations regression model was used to study the characteristics of women who delivered very preterm and the socio-residential risk factors for not delivering in a level 3 maternity unit at 24– 31+6d weeks of gestation.
Results: Among deliveries resulting in live births and without contraindication to in-utero transfer, we identified 9198 extremely or very preterm deliveries; 2122 (23.1%) of these were managed in a non-level 3 unit. Our study showed that young maternal age (women under 20 years at delivery) was associated with the risk of giving birth prematurely in a non-level 3 maternity, and particularly in a level 1 maternity unit (adjusted relative risk, 1.53; 95% CI 1.09– 2.16). Living more than 30 minutes away from the closest level 3 unit increased the risk of delivering very preterm in a level 1 or 2 unit. Living in an urban area or urban periphery increased the risk of giving birth in a level 2 maternity unit (adjusted relative risk, 1.53; 95% CI 1.28– 1.83 and 1.42; 95% CI 1.17– 1.71, respectively).
Conclusion: This study shows that young pregnant women living more than 30 minutes from a level 3 hospital have an increased risk of delivering in a maternity unit that is not equipped to deal with premature births. The risk also increases with an urban place of residence when the delivery occurs in a level 2 unit. A clearer understanding of the population at risk of delivering prematurely in a non-level 3 maternity could lead to improvements in structuring healthcare to encourage earlier management and better support.
Keywords: preterm delivery, hospital claims data, neonatal intensive care unit, NICU, maternity level, socio-residential factors
Introduction
Diverse models for organizing perinatal care have been developed over time in the aim of improving the management of at-risk deliveries. Among the types of at-risk deliveries, preterm births (before a term of 37 weeks of gestation [WG]) have increased significantly over the past two decades in most high-income countries, and in 2010 there were an estimated 8.6 premature births per 100 live births (95% CI 8.3–9.4).1,2 Concerning women, it is well known that a preterm delivery is more likely when they also have severe morbidities.3–5 In addition, a very premature delivery also causes severe complications in both women6 and children.7–9 Maternity wards of varying levels provide support for expectant mothers with different care needs according to the degree of risk presented by each pregnancy. Depending on the term, estimated fetal weight and maternal characteristics, delivery must take place in a maternity hospital with appropriate equipment and adequately qualified professionals.
In France, the percentage of preterm births increased from 7.4% in 2010 to 8.5% in 2016 (1.4% up to 32WG in 2010 to 2.3% in 2016).10,11 Pregnancy follow-up and the levels of perinatal care are strictly supervised by the French health authorities.12,13 Premature deliveries up to 32 WG should take place in a level 3 maternity, which is the highest level of care and contains a neonatal intensive care unit (NICU), in order to limit the risks of severe morbidity14 and mortality15,16 in preterm or low-birth-weight newborns and their mothers.17
However, this organization is not entirely dysfunction-free. Some premature births do not occur in the recommended level of maternity unit, which increases the risk of neonatal mortality,18 vascular and respiratory syndromes, and nosocomial infections.19,20 In 2003, Zeitlin et al showed that women delivering up to 32 WG in a deprived environment were more likely to give birth in a non-level 3 maternity unit, thereby deviating from the recommendation.21 However, the study by Zeitlin et al was conducted in a single region with particular characteristics (an urban region including the city of Paris, which has a better geographic access to perinatal care than other French regions). We therefore aimed to investigate the risk factors for spontaneous delivery up to 32 WG outside of a recommended level 3 maternity unit in all of France, including both rural and urban territories.
We hypothesized that: a) deliveries that occur in a maternity unit whose level does not correspond to national recommendations for best practice applied to local contexts cannot be considered the sole responsibility of the maternity staff who may have failed to transfer the patient, or of erroneous antenatal counseling; and b) such events are the result of a series of socio-spatial circumstances related to the home environment of the mother and the localization of the maternity department.
Our objectives were thus to identify from the French hospital medico-administrative database (Programme de Médicalisation des Systèmes d’Information [PMSI]) all premature deliveries at 24–31+6d WG that occurred outside a level 3 maternity unit and to examine possible socio-residential factors that would explain the inappropriate hospital care of premature newborns.
Materials and Methods
Study Population
All Deliveries 2012–2014
We identified all deliveries in the French PMSI national database from 2012 to 2014. The data was collected from anonymous discharge abstracts (DAs) from metropolitan facilities for mothers living in France with a valid geographical residence code from delivery procedures coded according to the French Classification of Medical Procedures and an associated diagnosis ICD-10 code Z37 (hospital births) or Z3900 (non-hospital births). Stillbirths and medical abortions were excluded. We also excluded abstracts when the term was less than 24 WG or greater than 31+6d WG, or when the mother was younger than 11 or older than 55 years.
Extremely and Very Preterm Deliveries
Extremely preterm and very preterm deliveries were defined according to the WHO classification.22 Extremely preterm deliveries occur up to 28 WG and very preterm deliveries occur between 28 and 31+6d WG. In our study, extremely and very preterm deliveries were identified according to the gestational age recorded in the mother’s DA at the time of delivery.
Preterm Deliveries in an Appropriate Maternity Unit
Maternity units in France are classified into levels according to the care they are able to provide. Since 1998, the French ministry of health has defined four levels as follows.13
Level 1: maternity able to provide postnatal care for newborns with no particular complications.
Level 2A: maternity affiliated with a neonatal unit that can provide constant monitoring and specialized care for high-risk newborns or for newborns whose condition deteriorated after birth, whether they were born in the facility or not.
Level 2B: maternity affiliated with a neonatal unit that can provide constant monitoring and specialized care for high-risk newborns or for newborns whose condition deteriorated after birth, whether they were born in the facility or not. These maternities have an intensive care unit for neonates.
Level 3: maternity affiliated with a neonatal unit or neonatal intensive care unit (NICU) that can provide constant monitoring and specialized care for newborns suffering from severe illness or in critical condition and who require intensive care, whether they were born in the facility or not. These maternities are equipped for the most serious maternal and neonatal complications.
The French Health Authority (HAS) recommendations state that all deliveries between 24 WG and 31+6d WG must take place in a level 3. This recommendation is of course not applicable for women who present a contraindication to in-utero transfer (IUT). Contraindications include eclampsia, retroplacental hematoma, abnormal fetal heart rate, Benckiser’s hemorrhage, hemorrhagic placenta praevia, acute pulmonary edema and uterine rupture.
Contraindications to antenatal transfer were identified according to ICD-10 codes registered in delivery/birth DAs, which were then excluded from the analysis. We detected all birth DAs linked to extremely or very preterm deliveries thanks to their valid anonymous mother/newborn key number. Eclampsia was identified with ICD-10 code O15 (mothers’ DAs); retroplacental hematoma was identified with ICD-10 codes O45 (mothers’ DAs) or P021 (newborns’ DAs); abnormal fetal heart rate was identified with codes O680 and O682 (mothers’ DAs); Benckiser’s hemorrhage was identified with codes O694 and O695 (mothers’ DAs); hemorrhagic placenta praevia was identified with codes O44 (mothers’ DAs) and P020 (newborns’ DAs); acute pulmonary edema was identified with code J81 (mothers’ DAs), and uterine rupture was identified with code O71. Women who delivered prematurely in an appropriate facility (level 3) were attributed to group A, and those who delivered prematurely in an inappropriate facility (level 1, 2A or 2B) were attributed to group B. Deliveries with an ICD-10 code O603 (Preterm delivery without spontaneous labor) were excluded from group B. Indeed, group B only includes women with spontaneous labor because we considered that an induced preterm delivery in a non-level 3 maternity unit would be linked to a medical condition preventing antenatal transfer. All of our selection criteria are presented in Figure 1.
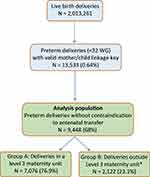 |
Figure 1 Flowchart of the study population. Note: *Excluding women with induced preterm deliveries (N = 250). |
Variables of Interest
Confounding Variables
Five explanatory variables are used in the models relating to socio-residential status: maternal age at delivery, travel time to the closest level 3 maternity unit, gestational age in WG, deprivation index and level of urbanization.
Maternal Age at Delivery
Maternal age at delivery is available in the anonymous DAs. The variable was divided into three classes: younger than 20 years, 20 to 40 years, older than 40 years.
Material and Social Deprivation Index
Variables relative to socio-residential factors were generated from French census data obtained from the INSEE (Institut National de la Statistique et des Etudes Economiques)23 and household income data from 2013.24 We set our scores according to the average of the data sourced from the municipalities that make up each residence code in the PMSI.
The deprivation index was created from the combination of two measures of the socio-residential environment:
- Social deprivation, measured with standardized scores for unemployment, blue collar workers, people without a diploma or only a middle school diploma, and immigrants;
- Material deprivation, measured with a standardized score for non-taxable households.
Each of the two scores were divided into three classes, from the least deprived to the most deprived, taking −1 and +1 standard deviation as the borders of the distribution, and were then crossed to create a bi-dimensional scale following Pampalon’s “material and social deprivation index” model.25 Five levels of population deprivation were identified according to the geographical code ranking for the two scores: level 1 designates the most advantaged population (class 1 for the two scores), and level 5 designates the most deprived population (class 3 for the two scores). Figure S1 shows the five levels according to the distribution of the two scores (see Figure S1).
Levels of Urbanization
We categorized the geographical codes found in the PMSI data according to the geographical areas established by the INSEE,26 which classifies urban areas according to the level of urbanization and the number of jobs held in the area. We determined four levels of urbanization: major urban centers, the suburbs of major urban centers, small and mid-sized centers and rural areas. Geographical codes classified as major urban centers were areas with 10,000 or more employed persons. The suburbs of major urban centers were located in peri-urban areas and connected to these attractive urban centers through various transport networks. Small and mid-sized centers were the least populated urban areas with 1500 to 5000 employed persons. Rural areas were located outside the major or small urban centers.
Access Time to the Closest Level 3 Unit
The maternity units were identified with their postal address. The time required to access the closest level 3 maternity unit was calculated using the zip code of the woman’s residence and the maternity unit’s postal address with Chronomap© by MapInfo© software and the IGN Route500© vectorial network (expressed in minutes).
Statistical Analysis
Descriptive Analyses and Tests of Significance
Qualitative variables were described as numbers and percentages and compared using a Chi-squared test.
Means (±SD) for maternal age at delivery and the travel time to the closest level 3 maternity were computed and compared with a Student’s t-test. Table 1 presents the comparison between group A (women without contraindication to antenatal transfer who delivered very preterm in a level 3) and group B (women without contraindication to antenatal transfer and without induced labor who delivered very preterm in a level 1 or in a level 2).
 |
Table 1 Characteristics of Women Who Delivered Extremely or Very Preterm in or Outside an Appropriate Level of Maternity |
Multivariate Regression Analysis
The outcome of interest was delivering in a non-level 3 maternity unit (yes or no).
In order to analyze how socio-residential environment influences premature delivery in level 1 or 2 maternity units, data were integrated into a marginal generalized estimating equations (GEE) regression model, using a log function and a negative binomial distribution to reflect the correlated nature of the data (geographical codes and years) and their over-dispersion. Two models were fitted, firstly on the population of women who gave birth in level 1 compared with women who gave birth in level 3 maternity units, secondly on those who gave birth in level 2 compared with women who gave birth in level 3 maternity units. The analyses focused on the women’s individual variables (age: 11–20/21-40/41-55; travel time to the closest level 3 maternity unit: 0–30 mins/31-60 mins/>60 mins; number of WG) and the contextual variables characterizing their residential environment (deprivation index: privileged/national average/material deprivation/social deprivation/deprived; level of urbanization: major urban centers/suburbs of major centers/small and mid-sized centers/rural areas). The results of GEE regressions are expressed in crude and adjusted relative risks (aRR) and are presented in Table 2 with their 95% confidence interval (CI). Analyses were performed with the GENMOD PROC of SAS® software version 9.4 (SAS Institute Inc., Cary, NC, USA).
 |
Table 2 Extremely and Very Preterm Deliveries in a Non-Level 3 Maternity Unit, Crude and Adjusted Risks for Delivering in a Level 1 or in a Level 2 |
Results
Characteristics of the Study Populations
After reducing the analysis population to the 9198 extremely and very preterm deliveries without contraindication to antenatal transfer, and without induced labor for group B, (see Figure 1 and Table 1), we identified 7076 (76.9%) deliveries that were managed in level 3 maternity units (group A), and 2122 deliveries (23.1%) that were not managed in level 3 maternity units (group B). Group B deliveries were mainly managed in level 2B (43.4%) and 2A maternities (39.2%), and around 17% were managed in level 1. The youngest woman was 14 years old, the oldest was 54.
On average, the women in group B were younger than in group A (28.8 vs 29.9 years). Mean travel time to the closest level 3 maternity unit was significantly higher in group B than in group A (46.7 vs 29.2 min).
The women in group B lived in a more deprived environment than the women in group A. They resided in areas affected more acutely by material deprivation (4.0% vs 3.0% for group A), and social deprivation (18.9% vs 15.7% for group A) or in a context of general deprivation with both low economic resources and social disadvantage (10.8% vs 9.9% for group A). Conversely, 7.6% of group A women were living in a privileged environment compared with only 6.5% of group B.
Our analysis relative to the level of urbanization showed that 68.6% of women in group A were living in major urban centers compared with only 61.0% of women in group B. Significantly more women in group B were living in the suburbs (21.0% vs 18.5%), in small or mid-sized centers (8.7% vs 6.3%), and in rural areas (9.3% vs 6.6%).
Effect of Socio-Residential Environment on Delivery at an Inappropriate Maternity Unit
The results of the marginal model (GEE) show that the adjusted relative risk (aRR) of premature delivery in a non-level 3 maternity increased with the travel time to the nearest level 3 maternity in women who gave birth in level 1 or 2 (see Table 2). In both cases, the longer the travel time, the greater the risk (for >60 minutes, delivering in level 1: aRR=2.66; 95% CI, [2.03–3.50], delivering in level 2: aRR=3.08 [2.72–3.49]; for 30–60 minutes, delivering in level 1: aRR=2.10 [1.56–2.83]; delivering in level 2: aRR=2.84 [2.49–3.24]).
The risk also increased for women younger than 20 years (level 1: aRR=1.53 [1.09–2.16]; level 2: aRR=1.30 [1.12–1.50]).
Concerning maternal home environment, the risk of delivering in a level 2 maternity unit was higher for women living in a socially deprived socio-residential environment (aRR=1.41 [1.24–1.61]) or for women living in the most privileged environment (aRR=1.42 [1.20–1.68]) when compared with the national average.
The risk of giving birth in a level 2 maternity was also higher for women living in major urban centers (aRR=1.53 [1.28–1.83]) or their suburbs (aRR=1.42 [1.17–1.71]) than for women living in small and mid-sized centers. Conversely, living in a major urban center was protective for women who gave birth in level 1 (aRR=0.59 [0.42–0.81]), as was living in the suburbs (aRR=0.63 [0.45–0.90]). Women were more likely to deliver in a non-level 3 at 24 WG, whatever the level of maternity (level 1: aRR=1.87 [1.14–3.06]; level 2: aRR=1.89 [1.55–2.31]) and at 31 WG for women who delivered in level 2 (aRR=1.53 [1.30–1.81]).
Discussion
Study Population and Impact of Socio-Residential Context
Our population of parturient women was divided into two groups: those who delivered in a maternity unit that was the appropriate level for their gestational age (group A), and those who delivered in a maternity unit that was not the appropriate level for their gestational age (group B). We found that, in France, 2122 deliveries were not managed in an appropriate maternity during the 2012–2014 study period. This represents almost one quarter (23.1%) of all live deliveries at 24–31+6d WG without contraindication to antenatal transfer (9198 deliveries) identified in the PMSI national database. We also showed that a longer travel time to the closest level 3 maternity unit, young maternal age, and certain socio-residential factors (such as social deprivation or living in a privileged area) increased the risk of giving birth prematurely in a non-level 3 maternity unit.
Our findings indicate that antenatal transfers are not systematically done in accordance with the guidelines for perinatal care, as shown by the relatively high proportion (23.1%) of women who gave birth outside of a maternity unit equipped to care for their premature newborn, excluding contraindications available in the PMSI database. In France, maternity units are organized in networks so that professionals can make arrangements to ensure that women deliver in adequate facilities,27,28 and almost 75% of extremely or very premature children are born in an appropriate facility. Our results show that the risk of delivering in an inappropriate facility is higher in specific populations.
To our knowledge, this is the first time that the travel time between a woman’s geographical code of residence and the closest level 3 maternity has been shown to be a risk factor for delivering prematurely in a non-level 3 maternity unit. Unlike in our study, Zeitlin et al did not find that a long distance to the closest level 3 maternity unit increased the risk of delivering in a non-level 3 maternity unit, after adjustment on the contextual and individual characteristics.21 However, their study was conducted in the Ile-de-France region, which has the largest urban population and highest population density in France, and the distances to level 3 maternity units are shorter than in most of the country. Moreover, this French region offers one of the highest densities of intensive neonatal care services in Europe.29
The second risk factor for delivering prematurely in a non-level 3 maternity unit identified in our study is young maternal age. It is well known that young maternal age increases the risk of premature delivery,30–32 and this risk increases further in young women living in precarious socio-economic conditions and with a low level of education.33,34 We would therefore expect that this small population would be managed appropriately. It can therefore be assumed that some young women experience a series of difficulties that lead to premature delivery in an inappropriate facility: lack of information, social isolation, inadequate transport, and difficulties in accessing care.
Furthermore, similar to previous studies, our study suggests a protective effect for older women, though the effect was not significant for women who delivered in a level 2 maternity unit. It is well known that advanced maternal age is associated with a risk of prematurity in some developing35 and high-income countries.36–38 Our study suggests that women older than 40 are less likely to give birth prematurely outside an appropriate facility. This protective factor may be explained by older women’s more favorable socio-economic status and higher level of education. Moreover, several studies conducted in developed countries have shown that the risk of complications linked to advanced maternal age also promotes better monitoring during pregnancy and more scheduled deliveries in appropriate facilities.39,40
Our third novel finding is that women living in a context of social deprivation have a higher risk of giving birth in a level 2 maternity, ie outside level 3 maternities. This corroborates with previous findings on the negative impact of a disadvantaged socio-economic environment on extremely and very preterm delivery and access to perinatal care.41–43 This precarious environment is often associated with a lower level of education among mothers44–46 and fathers.47 Zeitlin et al showed a significantly higher risk of delivery in a non-level 3 maternity unit for women in the poorest quintile of their precarity indicator,21 but they also found that residing in a privileged context did not have a significant effect on giving birth in an appropriate facility. Conversely, our results show that women residing in the least deprived territories (level 1 of deprivation index) had an increased risk of extremely and very preterm delivery in a level 2 maternity unit. The results reported by Zeitlin et al may be explained by the fact that the study was conducted only in the Ile-de-France region, where both level 3 and level 2 maternity units are more accessible (see Figure 2). Similarly, better access to level 2 maternity units in large urban centers and their suburbs may explain the significantly higher risk of giving birth in level 2 facilities for women residing in these areas. In fact, level 2 maternity hospitals are often located in urban centers, and in France most urban centers have as many deprived populations as they do affluent populations. Premature delivery in a level 2 maternity therefore seems to affect mostly women living in urban areas, whether in privileged or disadvantaged neighborhoods, and too far from the nearest level 3 maternity hospital to be cared for in a timely and appropriate manner.
 |
Figure 2 Travel time to the closest maternity unit by level of maternity unit and type of area. |
Our findings also indicate that women delivering at 24 WG had a higher risk of being managed in a non-level 3 maternity unit. This may suggest that women at risk of preterm birth at 24WG have insufficient time for a transfer to a level 3 maternity, and therefore remain in the initial maternity unit. Newborns are not systematically revived at 24WG in France, which may explain why women remained in the maternity unit closest to their home. Zeitlin and al. also showed that the risk of not being managed at level 3 was significantly higher at 24–25WG, but also at 31WG,21 which may point to a threshold effect between premature delivery management at level 3 and level 2B (from 32WG). We also found a slightly increased and significant risk at 31WG for women who delivered in a level 2 maternity unit, but the risk was not significant for level 1 units. Therefore, we can assume that improvements in the regionalization of care and the organization of maternal transfers within perinatal networks have contributed to increasingly appropriate management of women since the publication of the research conducted in Ile-de-France in 2003.
While the organization of health care appears to be efficient, our findings suggest a need for adjustments in the care of women at risk of premature delivery, especially in the youngest age group. It may be possible to improve the management of younger women, as it has already been done for older women, taking into account the risk of complications linked to these two particular classes of young and advanced maternal age. Because women’s health needs are not isolated events, continuity of care during pregnancy may be proposed through a single care provider who has the opportunity to establish a trusting relationship, particularly for vulnerable women (deprivation, young maternal age), and provide timely and relevant information. Concerning the territorial distribution of care structures, the impact of distance and the proportion of extremely and very premature births occurring in both level 1 and 2 maternity units should be considered by health-care planners when discussing the reorganization of care. Our study showed that the regionalization of perinatal care could take into account the distance to the nearest level 3 maternity unit, whether it is located in same the region or not, in order to limit the risks of overly long intra-regional transfers and to promote early care for at-risk pregnant women.48,49 While antenatal hospitalization seem to be a possible solution for preventing very premature deliveries outside of an appropriate facility, there is currently no consensus on this practice.50–54 However, a Danish study showed that the risk of antenatal hospitalization increased with a low and a high maternal age.55 This study also showed that antenatal hospitalization was strongly associated with the risk of very premature delivery.
Further qualitative surveys could be conducted among health professionals and women in order to better understand what leads to premature delivery outside an appropriate maternity facility. These factors could then be used as levers to implement better monitoring and referral strategies at the time of childbirth.
Weaknesses
The first weakness is related to the nature of the PMSI, which is a medico-administrative database that does not contain detailed information regarding the status of women presenting to maternity wards. We were therefore unable to distinguish between women for whom a high risk of delivery during transport contraindicated transfer and those for whom delivery was not imminent. Nevertheless, using the ICD10 code O603 (Preterm delivery without spontaneous labour), we were able to identify and remove from group B the 250 women whose premature delivery took place in a non-level 3 maternity unit but who could not be transferred due to complications. The discharge abstract of 148 of these women indicated a diagnosis of emergency caesarean section, implying that they could not have been transferred to a level 3 maternity unit in any case. The others had various conditions complicating pregnancy or childbirth, so it can be assumed that a transfer was not possible and the delivery therefore took place in the first-line maternity hospital. Concerning contraindication to antenatal transfer, a recent validation study showed that the sensitivity of eclampsia recording in PMSI data is only 50%, but this event remains rare.56
Moreover, our study may suffer from a lack of data regarding several known risk factors for preterm delivery. We assumed that PMSI data do not provide information about marital status or women’s foreign origin for example. Concerning the marital status, several studies highlighted that there is a decreasing risk of preterm delivery among married or in-couple women.57–60 Another important risk factor seems related to the immigrant status, even if there is no consensus about the influence of a foreign origin on obstetrical outcomes. In Germany for example, a study showed that immigrant status leads to an increasing risk of preterm delivery61 but other German studies concluded conversely and underlined that the risk of preterm delivery or poor neonatal outcomes varied depending on the women’s origin country.62,63 In other countries, the link between immigrant status and increase in risk of preterm delivery is clearer,59,64 but many authors insist on the importance of the entanglement between socio-economic status, level of education, parity and mother’s origin in the risk of preterm birth, each of these variables having its importance,55,65–67 but certain may vary during women’s life. Regarding parity, a high parity is a potential risk factor of preterm delivery. However, this information is only partially available in the PMSI data. Delivery procedures registered in the PMSI data contains information about the status of women (primiparous or multiparous), but are not available for caesarian section. Thus, we have chosen to not include this variable, since we would have had the information only for vaginal preterm deliveries.
Another limit is related to the French classification of maternity units in four levels, which is based on specific criteria. We categorized the maternity units according to the official French classification. In France, the level of maternity is based foremost on neonatal criteria, such as weeks of gestation, estimated fetal weight, and possible fetal comorbidities. In the United States, the classification is based on obstetric and maternity care provisions.68 We therefore assume that our results are not necessarily generalizable everywhere, considering that each national classification of perinatal care is different. However, we believe that our results can provide valuable insights into the identification of certain vulnerable populations.
The main strength of our study is that it is based on comprehensive nationwide PMSI data. The French national hospital information system makes it possible to identify all hospital stays for individuals covered by the national health insurance system. It was thus possible to identify all deliveries registered in hospitals within this database. Because very few deliveries take place outside of hospital facilities in France (0.3%), this data was particularly complete. A previous validation study has confirmed the quality and the exhaustiveness of PMSI data, especially for the recording of gestational age, with a concordance rate between 92% and 98%. Concerning maternal age, the concordance rate of maternal age at delivery was 94.8%.56 These data are also a valuable resource for the evaluation of perinatal care and management because the PMSI database allows the linkage of consecutive hospital discharge abstracts and, for singleton pregnancy, mothers’ and children’s abstracts are linked by a shared anonymous key used for both since 2012. Finally, our data source can be used to follow up on the children who are born outside appropriate facilities and to assess their state of health.
Conclusions
When a parturient woman decides to go to a particular maternity hospital or clinic, her choice may have been influenced by numerous factors. When faced with premature delivery, the time needed to get to a level 3 maternity for suitable management may hinder access to appropriate care. Despite satisfying results in terms of health care organization, this study shows that young pregnant women living more than 30 minutes from a level 3 maternity unit and in a context of socio-residential deprivation have an increased risk of delivering at a maternity unit that is not equipped to deal with premature births. The understanding gained here about risk factors can be used to make improvements in maternal health education and structuring healthcare to include earlier and more carefully planned management of at-risk populations of pregnant women. The deleterious effect of these factors should prompt policy makers to ensure that the women who fit this at-risk profile are identified in a timely manner and followed up carefully during their pregnancy.
Ethics Approval and Informed Consent
This study was approved by the National Committee for data protection (registration number 1576793) and therefore was conducted in accordance with the Declaration of Helsinki. Written consent was not needed for this study. The PMSI database was transmitted by the national agency for the management of hospitalization data (ATIH number 2015-111111-47-33).
Acknowledgments
The authors warmly thank Dr Evelyne Combier for her wise advice and her great help in carrying out this work. The authors thank Mrs. Amandine Dufour for her contribution to the survey of the perinatal networks. This study is part of the nation-wide French TANGO project (L’offre territoriale de soins hospitaliers en France métropolitaine: de la prise en charge hospitalière des grossesses pathologiques à celle des nourrissons de moins d’un an, analyse des bases nationales du PMSI pour les hospitalisations se rapportant aux naissances 2012 – 2015). The authors are also thankful to Suzanne Rankin, Harbajan Chadha-Boreham and Gwenaelle Periard for reviewing and building the manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Funding
This study received support from the “Direction Générale de la Santé” (DGS), the “Caisse Nationale de l’Assurance Maladie des Travailleurs Salariés” (CNAMTS), and from the Inserm and the INSERM Plan Cancer in France, within the framework of the call for projects launched by the IRESP in 2014.
Disclosure
Jonathan Cottenet reports grants from IRESP during the conduct of the study. The authors declared that they have no other potential conflicts of interest for this work.
References
1. Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet Lond Engl. 2012;379(9832):2162–2172. doi:10.1016/S0140-6736(12)60820-4
2. Blencowe H, Cousens S, Chou D, et al. Born too soon: the global epidemiology of 15 million preterm births. Reprod Health. 2013;10(Suppl1):S2. doi:10.1186/1742-4755-10-S1-S2
3. Kilpatrick SJ, Abreo A, Gould J, Greene NI, Main EK. Confirmed severe maternal morbidity is associated with high rate of preterm delivery. Am J Obstet Gynecol. 2016;215(2):
4. Jiang M, Mishu MM, Lu D, Yin X. A case control study of risk factors and neonatal outcomes of preterm birth. Taiwan J Obstet Gynecol. 2018;57(6):814–818. doi:10.1016/j.tjog.2018.10.008
5. Cobo T, Kacerovsky M, Jacobsson B. Risk factors for spontaneous preterm delivery. Int J Gynaecol Obstet. 2020;150(1):17–23. doi:10.1002/ijgo.13184
6. Reddy UM, Rice MM, Grobman WA, et al. Serious maternal complications after early preterm delivery (24–33 weeks’ gestation). Am J Obstet Gynecol. 2015;213(4):
7. Luu TM, Mian MO, Nuyt AM. Long-term impact of preterm birth: neurodevelopmental and physical health outcomes. Clin Perinatol. 2017;44(2):305–314. doi:10.1016/j.clp.2017.01.003
8. Chehade H, Simeoni U, Guignard JP, Boubred F. Preterm birth: long term cardiovascular and renal consequences. Curr Pediatr Rev. 2018;14(4):219–226. doi:10.2174/1573396314666180813121652
9. Platt MJ. Outcomes in preterm infants. Public Health. 2014;128(5):399–403. doi:10.1016/j.puhe.2014.03.010
10. Blondel B, Lelong N, Kermarrec M, Goffinet F; Coordination nationale des Enquêtes Nationales Périnatales. [Trends in perinatal health in France between 1995 and 2010: results from the National Perinatal Surveys]. J Gynecol Obstet Biol Reprod (Paris). 2012;41(2):151–166. French. doi:10.1016/j.jgyn.2011.11.008
11. Coulm B, Bonnet C, Blondel B. Enquête Nationale Périnatale. Rapport 2016, Les Naissances; 2017. Available from: http://www.epopé-inserm.fr/wp-content/uploads/2017/11/ENP2016_rapport_complet.pdf.
12. MOH. Typologie Des Établissements de Soins En Périnatalité Définie Par Les Décrets 98-899 et 98-900 Du 9 Octobre 1998 et Par Les Articles R. 712.84 à R. 712.88 Du Code de La Santé Publique. 1998.
13. Haute Autorité de Santé. Femmes enceintes ayant une complication au cours de leur grossesse: transferts en urgence entre les établissements de santé; 2012. Available from: https://www.has-sante.fr/portail/jcms/c_1339442/fr/femmes-enceintes-ayant-une-complication-au-cours-de-leur-grossesse-transferts-en-urgence-entre-les-etablissements-de-sante.
14. Jensen EA, Lorch SA. Effects of a birth hospital’s neonatal intensive care unit level and annual volume of very low-birth-weight infant deliveries on morbidity and mortality. JAMA Pediatr. 2015;169(8):e151906. doi:10.1001/jamapediatrics.2015.1906
15. Watson SI, Arulampalam W, Petrou S, et al. The effects of designation and volume of neonatal care on mortality and morbidity outcomes of very preterm infants in England: retrospective population-based cohort study. BMJ Open. 2014;4(7):e004856. doi:10.1136/bmjopen-2014-004856
16. Wehby GL, Lopez-Camelo J, Castilla EE. Hospital volume and mortality of very low-birthweight infants in South America. Health Serv Res. 2012;47(4):1502–1521. doi:10.1111/j.1475-6773.2012.01383.x
17. Clapp MA, James KE, Kaimal AJ. The effect of hospital acuity on severe maternal morbidity in high-risk patients. Am J Obstet Gynecol. 2018;219(1):
18. Lasswell SM, Barfield WD, Rochat RW, Blackmon L. Perinatal regionalization for very low-birth-weight and very preterm infants: a meta-analysis. JAMA. 2010;304(9):992–1000. doi:10.1001/jama.2010.1226
19. Chien LY, Whyte R, Aziz K, et al. Improved outcome of preterm infants when delivered in tertiary care centers. Obstet Gynecol. 2001;98(2):247–252. doi:10.1016/s0029-7844(01)01438-7
20. Shah PS, Shah V, Qiu Z, Ohlsson A, Lee SK; Canadian Neonatal Network. Improved outcomes of outborn preterm infants if admitted to perinatal centers versus freestanding pediatric hospitals. J Pediatr. 2005;146(5):626–631. doi:10.1016/j.jpeds.2005.01.030
21. Zeitlin J, Gwanfogbe CD, Delmas D, et al. Risk factors for not delivering in a level III unit before 32 weeks of gestation: results from a population-based study in Paris and surrounding districts in 2003. Paediatr Perinat Epidemiol. 2008;22(2):126–135. doi:10.1111/j.1365-3016.2007.00921.x
22. Howson CP, Kinney MV, Lawn JE. March of dimes, PMNCH, save the children, WHO. Born too soon: the global action report on preterm birth. Geneva; 2012. Available from: http://www.who.int/pmnch/media/news/2012/201204_borntoosoon-report.pdf.
23. INSEE. Recensement 2013: résultats sur un territoire, bases de données et fichiers détail | insee. 2017. Available from: https://www.insee.fr/fr/information/2409289.
24. impots.gouv.fr. French general direction of public finances. Income tax per municipalities; 2014. Available from: https://www.impots.gouv.fr/portail/statistiques.
25. Pampalon R, Raymond G. Indice de défavorisation matérielle et sociale: son application au secteur de la santé et du bien-être. Santé Société Solidar. 2003;2(1):191–208. doi:10.3406/oss.2003.932
26. Brutel C, Levy D Le nouveau zonage en aires urbaines de 2010 - Insee Première – 1374; 2011. Available from: https://www.insee.fr/fr/statistiques/1281191.
27. Lecoeur C, Thibon P, Prime L, et al. Frequency, causes and avoidability of outborn births in a French regional perinatal network. Eur J Obstet Gynecol Reprod Biol. 2014;179:22–26. doi:10.1016/j.ejogrb.2014.05.009
28. Martin I, Roussel A, Olieric M-F, et al. [Analysis of obstetric-pediatric care in the perinatal period. Are births before 31 weeks’ gestation in level 2B maternity units avoidable?]. Arch Pediatr Organe off Soc Francaise Pediatr. 2017;24(12):1188–1196. French. doi:10.1016/j.arcped.2017.09.025
29. Pilkington H, Blondel B, Papiernik E, et al. Distribution of maternity units and spatial access to specialised care for women delivering before 32 weeks of gestation in Europe. Health Place. 2010;16(3):531–538. doi:10.1016/j.healthplace.2009.12.011
30. Blomberg M, Birch Tyrberg R, Kjølhede P. Impact of maternal age on obstetric and neonatal outcome with emphasis on primiparous adolescents and older women: a Swedish Medical Birth Register Study. BMJ Open. 2014;4(11):e005840. doi:10.1136/bmjopen-2014-005840
31. Khashan AS, Baker PN, Kenny LC. Preterm birth and reduced birthweight in first and second teenage pregnancies: a register-based cohort study. BMC Pregnancy Childbirth. 2010;10:36. doi:10.1186/1471-2393-10-36
32. Marvin-Dowle K, Kilner K, Burley VJ, Soltani H. Impact of adolescent age on maternal and neonatal outcomes in the Born in Bradford cohort. BMJ Open. 2018;8(3):e016258. doi:10.1136/bmjopen-2017-016258
33. Chen X-K, Wen SW, Fleming N, Demissie K, Rhoads GG, Walker M. Teenage pregnancy and adverse birth outcomes: a large population based retrospective cohort study. Int J Epidemiol. 2007;36(2):368–373. doi:10.1093/ije/dyl284
34. Wong SPW, Twynstra J, Gilliland JA, Cook JL, Seabrook JA. Risk factors and birth outcomes associated with teenage pregnancy: a Canadian sample. J Pediatr Adolesc Gynecol. 2020;33(2):153–159. doi:10.1016/j.jpag.2019.10.006
35. Laopaiboon M, Lumbiganon P, Intarut N, et al. Advanced maternal age and pregnancy outcomes: a multicountry assessment. BJOG Int J Obstet Gynaecol. 2014;121(Suppl 1):49–56. doi:10.1111/1471-0528.12659
36. Carolan M, Frankowska D. Advanced maternal age and adverse perinatal outcome: a review of the evidence. Midwifery. 2011;27(6):793–801. doi:10.1016/j.midw.2010.07.006
37. Kenny LC, Lavender T, McNamee R, O’Neill SM, Mills T, Khashan AS. Advanced maternal age and adverse pregnancy outcome: evidence from a large contemporary cohort. PLoS One. 2013;8(2):e56583. doi:10.1371/journal.pone.0056583
38. Heazell AEP, Newman L, Lean SC, Jones RL. Pregnancy outcome in mothers over the age of 35. Curr Opin Obstet Gynecol. 2018;30(6):337–343. doi:10.1097/GCO.0000000000000494
39. Schummers L, Hutcheon JA, Hacker MR, et al. Absolute risks of obstetric outcomes risks by maternal age at first birth: a population-based cohort. Epidemiol Camb Mass. 2018;29(3):379–387. doi:10.1097/EDE.0000000000000818
40. Ancel P, Saurel‐Cubizolles M, Renzo CDG, Papiernik E, Bréart G. Very and moderate preterm births: are the risk factors different? BJOG Int J Obstet Gynaecol. 2005;106(11):1162–1170. doi:10.1111/j.1471-0528.1999.tb08142.x
41. Auger N, Park AL, Gamache P, Pampalon R, Daniel M. Weighing the contributions of material and social area deprivation to preterm birth. Soc Sci Med. 2012;75(6):1032–1037. doi:10.1016/j.socscimed.2012.04.033
42. Bonet M, Smith LK, Pilkington H, Draper ES, Zeitlin J. Neighbourhood deprivation and very preterm birth in an English and French cohort. BMC Pregnancy Childbirth. 2013;13:97. doi:10.1186/1471-2393-13-97
43. Kramer MS, Séguin L, Lydon J, Goulet L. Socio-economic disparities in pregnancy outcome: why do the poor fare so poorly? Paediatr Perinat Epidemiol. 2000;14(3):194–210. doi:10.1046/j.1365-3016.2000.00266.x
44. Oftedal A-M, Busterud K, Irgens LM, Haug K, Rasmussen S. Socio-economic risk factors for preterm birth in Norway 1999–2009. Scand J Public Health. 2016;44(6):587–592. doi:10.1177/1403494816653288
45. Tamura N, Hanaoka T, Ito K, et al. Different risk factors for very low birth weight, term-small-for-gestational-age, or preterm birth in Japan. Int J Environ Res Public Health. 2018;15(2):369. doi:10.3390/ijerph15020369
46. Zhang X, Zhou M, Chen L, Hao B, Zhao G. Risk factors for preterm birth: a case-control study in rural area of western China. Int J Clin Exp Med. 2015;8(3):4527–4532.
47. Meng Y, Groth SW. Fathers count: the impact of paternal risk factors on birth outcomes. Matern Child Health J. 2018;22(3):401–408. doi:10.1007/s10995-017-2407-8
48. Brantley MD, Davis NL, Goodman DA, Callaghan WM, Barfield WD. Perinatal regionalization: a geospatial view of perinatal critical care, United States, 2010–2013. Am J Obstet Gynecol. 2017;216(2):
49. Clerc J, Doret M, Decullier E, Claris O, Picaud J-C, Dupuis O. [Is it possible to prevent preterm births outside of level-3 maternity wards? Experience of Greater Lyon perinatal network]. Gynecol Obstet Fertil. 2011;39(7–8):412–417. French. doi:10.1016/j.gyobfe.2011.02.020
50. Maloni JA. Antepartum bed rest for pregnancy complications: efficacy and safety for preventing preterm birth. Biol Res Nurs. 2010;12(2):106–124. doi:10.1177/1099800410375978
51. Sosa CG, Althabe F, Belizán JM, Bergel E. Bed rest in singleton pregnancies for preventing preterm birth. Cochrane Database Syst Rev. 2015;(3):CD003581. doi:10.1002/14651858.CD003581.pub3
52. Martins I, Pereira I, Clode N. A pilot randomized controlled trial of complete bed rest versus activity restriction after preterm premature rupture of the membranes. Eur J Obstet Gynecol Reprod Biol. 2019;240:325–329. doi:10.1016/j.ejogrb.2019.07.037
53. Shibata M, Kaji T, Yonetani N, et al. Effect of prolonged hospitalization on fetal growth in threatened preterm labor. J Med Investig. 2019;66(1.2):153–156. doi:10.2152/jmi.66.153
54. Berger R, Rath W, Abele H, Garnier Y, Kuon R-J, Maul H. Reducing the risk of preterm birth by ambulatory risk factor management. Dtsch Arzteblatt Int. 2019;116(50):858–864. doi:10.3238/arztebl.2019.0858
55. Bendix J, Hegaard HK, Langhoff-Roos J, Bergholt T Changing prevalence and the risk factors for antenatal obstetric hospitalizations in Denmark 2003–2012. Clinical epidemiology; 2016. Available from: https://www.dovepress.com/changing-prevalence-and-the-risk-factors-for-antenatal-obstetric-hospi-peer-reviewed-article-CLEP.
56. Goueslard K, Cottenet J, Benzenine E, Tubert-Bitter P, Quantin C. Validation study: evaluation of the metrological quality of French hospital data for perinatal algorithms. BMJ Open. 2020;10(5):e035218. doi:10.1136/bmjopen-2019-035218
57. Zeitlin JA, Saurel-Cubizolles M-J, Ancel P-Y; EUROPOP Group. Marital status, cohabitation, and risk of preterm birth in Europe: where births outside marriage are common and uncommon. Paediatr Perinat Epidemiol. 2002;16(2):124–130. doi:10.1046/j.1365-3016.2002.00396.x
58. Shapiro GD, Bushnik T, Wilkins R, et al. Adverse birth outcomes in relation to maternal marital and cohabitation status in Canada. Ann Epidemiol. 2018;28(8):503–509.e11. doi:10.1016/j.annepidem.2018.05.001
59. Hidalgo-Lopezosa P, Jiménez-Ruz A, Carmona-Torres JM, Hidalgo-Maestre M, Rodríguez-Borrego MA, López-Soto PJ. Sociodemographic factors associated with preterm birth and low birth weight: a cross-sectional study. Women Birth J Aust Coll Midwives. 2019;32(6):e538–e543. doi:10.1016/j.wombi.2019.03.014
60. Granese R, Gitto E, D’Angelo G, et al. Preterm birth: seven-year retrospective study in a single centre population. Ital J Pediatr. 2019;45(1):45. doi:10.1186/s13052-019-0643-9
61. Scholaske L, Brose A, Spallek J, Entringer S. Perceived discrimination and risk of preterm birth among Turkish immigrant women in Germany. Soc Sci Med 1982. 2019;236:112427. doi:10.1016/j.socscimed.2019.112427
62. David M, Borde T, Brenne S, et al. Obstetric and perinatal outcomes among immigrant and non-immigrant women in Berlin, Germany. Arch Gynecol Obstet. 2017;296(4):745–762. doi:10.1007/s00404-017-4450-5
63. Zolitschka KA, Miani C, Breckenkamp J, et al. Do social factors and country of origin contribute towards explaining a “Latina paradox” among immigrant women giving birth in Germany? BMC Public Health. 2019;19(1):181. doi:10.1186/s12889-019-6523-9
64. Khanolkar AR, Wedrén S, Essén B, Sparén P, Koupil I. Preterm and postterm birth in immigrant- and Swedish-born parents: a population register-based study. Eur J Epidemiol. 2015;30(5):435–447. doi:10.1007/s10654-014-9986-0
65. El-Sayed AM, Tracy M, Galea S. Life course variation in the relation between maternal marital status and preterm birth. Ann Epidemiol. 2012;22(3):168–174. doi:10.1016/j.annepidem.2012.01.002
66. Bushnik T, Yang S, Kaufman JS, Kramer MS, Wilkins R. Socioeconomic disparities in small-for-gestational-age birth and preterm birth. Health Rep. 2017;28(11):3–10.
67. Margerison CE, Luo Z, Li Y. Economic conditions during pregnancy and preterm birth: a maternal fixed-effects analysis. Paediatr Perinat Epidemiol. 2019;33(2):154–161. doi:10.1111/ppe.12534
68. American College of Obstetricians and Gynecologists. Levels of maternal care. Obstetric care consensus no. 2: levels of maternal care. Obstet Gynecol. 2015;125:502–515. doi:10.1097/01.AOG.0000460770.99574.9f
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
