Back to Journals » International Journal of General Medicine » Volume 13
Extremely Acute-Onset Cerebral Fat Embolism
Received 1 August 2020
Accepted for publication 1 October 2020
Published 14 October 2020 Volume 2020:13 Pages 833—837
DOI https://doi.org/10.2147/IJGM.S274803
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Shota Tsuru, Hiroshi Adachi
Department of Intensive Care Medicine, Aso Iizuka Hospital, Fukuoka 820-8505, Japan
Correspondence: Shota Tsuru Department of Intensive Care Medicine
Aso Iizuka Hospital, 3-83yoshio-Machi Iizuka-City, Fukuoka 820-8505, Japan
Tel +81-90-7538-4416
Fax +81-948-29-5744
Email [email protected]
Abstract: Cerebral fat embolism (CFE) causes the neurological involvement observed in fat embolism syndrome, which is a post-traumatic complication seen mostly after long bone fractures and usually presents 24– 72 h after the injury. An early 80s female who sustained an isolated traumatic fracture of the left distal femur without dislocation was alert on admission but fell into a coma 55 min after the injury. Brain computed tomography showed no abnormalities. Brain magnetic resonance imaging was performed approximately 5 h after the accident, and diffusion-weighted images revealed hyperintense, dot-like lesions disseminated in a “starfield” pattern in the brain. The patient was diagnosed with CFE and admitted to the intensive care unit. The day after the injury, the patient developed petechiae on the palpebral conjunctiva and was still comatose 4 months after the trauma. The current patient developing CFE in less than 1 h after a traumatic injury illustrates that CFE should be considered in patients with sudden deterioration of consciousness within 1 h after long bone fractures.
Keywords: emergency medicine, trauma, radiology (diagnostics)
Introduction
Fat embolism syndrome (FES) is a potentially fatal complication and develops most frequently after long bone fractures1–6 and usually presents 24–72 h after the injury, with an average time of 48.5 h from injury to symptom presentation.1 FES is diagnosed clinically by respiratory distress, neurological impairment, and petechial rash. Cerebral fat embolism (CFE) causes the neurological involvement observed in FES. The majority of case reports on CFE report that cerebral dysfunction associated with CFE is reversible.1,2,4–9
The risk factors for FES are young age, multiple closed long bone fractures, crush injury, and conservative therapy for fractures or delayed stabilization.10–12 We herein present the rare case of CFE that occurred in <1 h after the injury with no obvious risk factor and was associated with a poor neurological outcome.
Case Report
The current study was approved by the Institutional Ethics Committee at Aso Iizuka Hospital. Written informed consent was obtained from the patient’s family for publication of this case report and accompanying images. An early 80s healthy female was involved in a car accident at a parking lot. Her right leg was compressed between a wall and a parked car and extracted within a few minutes. She called an ambulance by herself and was conscious upon arrival at another hospital 45 min after the accident. However, her consciousness suddenly deteriorated 55 min after the injury; she became drowsy and developed a single generalized tonic–clonic seizure. Brain computed tomography (CT) was normal, but her neurological status did not recover; therefore, she was transferred to our hospital.
At the time of arrival approximately 2 h after the injury, her Glasgow Coma Scale score was 4/15 (eye-opening, 1; verbal response, 1; and motor response, 2). Her vital signs were as follows: blood pressure, 136/86 mmHg; heart rate, 86 beats/min; respiratory rate, 30 breaths/min; body temperature, 37.1°C; and oxygen saturation, 100%. The patient was intubated for airway protection. Plain X-ray showed a fracture in the left distal femur without dislocation (Figure 1). Whole-body CT did not show any other organ injuries, and brain CT did not show cerebral abnormalities. Her hemoglobin level and platelet count were 10.9 g/dL and 242,000 cells/dL, respectively. No petechial rash was present. Brain magnetic resonance imaging (MRI) was performed approximately 3 h after the presentation to our hospital (approximately 5 h after the injury), and diffusion-weighted imaging (DWI) revealed disseminated, hyperintense dot-like lesions in the brain, exhibiting the “starfield” pattern (Figure 2). The patient was diagnosed with CFE and admitted to the intensive care unit.
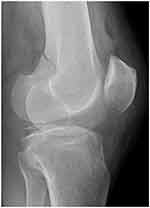 |
Figure 1 Plain X-ray. Plain X-ray showing a fracture in the left distal femur without dislocation. |
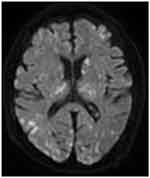 |
Figure 2 Brain magnetic resonance imaging. Diffusion-weighted imaging showing disseminated, hyperintense dot-like lesions in the brain, demonstrating the “starfield” pattern. |
We started the treatment with corticosteroids (hydrocortisone 300 mg/day) and whole-body cooling therapy. Then, cefazolin was administered as prophylactic antibiotics without using any sedative or anti-epileptic drugs. Petechiae developed on the palpebral conjunctiva on the second day after injury (Figure 3) and an electroencephalograph (EEG) revealed a wide range of low voltage, and obvious epileptiform discharges were not found. Tracheostomy was performed on day 3, and the patient was withdrawn from the ventilator on day 4. Brain MRI on day 4 demonstrated CFE exacerbation on DWI (Figure 4). She was transferred to a rehabilitation hospital on day 125 without neurological improvement. During hospitalization, electrocardiogram showed no abnormality, and transthoracic echocardiography and transesophageal echocardiography (including microbubble test) showed no evidence of arteriovenous malformation (AVM) or cardiac shunting, such as a patent foramen ovale, atrial septal defect, or ventricular septal defect. Outcome and follow-up after the transfer, she remained comatose and finally died 436 days after the accident.
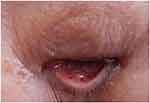 |
Figure 3 Physical findings second day after injury. Petechiae developed on the palpebral conjunctiva. |
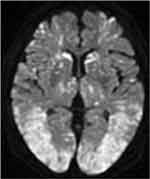 |
Figure 4 Follow-up brain magnetic resonance imaging. Diffusion-weighted imaging showing the exacerbation of disseminated, hyperintense dot-like lesions in the brain. |
Discussion
FES is a post-traumatic complication associated with long bone fractures. The clinical diagnosis of FES is based on the presence of at least two major or one major plus a minimum of four Gurd’s criteria.13 The major criteria include hypoxia, cerebral involvement, and petechial rash, whereas the minor criteria comprise tachycardia, fever, anemia, and thrombocytopenia. The current case exhibited only the major Gurd’s criterion of cerebral involvement on day 1, especially within the first hour after the trauma; therefore, the FES diagnosis based on clinical examination is challenging. Conversely, the definitive diagnosis of CFE depends on imaging findings. After the publication of the original Gurd’s criteria, it was modified because of the existing association between clinical symptoms and neuroimaging; altered mentality was observed with multiple cerebral white matter lesions on brain MRI.14 CT may show generalized cerebral edema or high-density spots but is frequently unremarkable in CFE. MRI is more sensitive and recommended as the initial imaging modality in patients with clinically suspicious CFE.6,15–17 On DWI, CFE presents typically with the starfield pattern of scattered bright spots on a dark background; thus, DWI should be performed in all patients with deteriorated mental status after a long bone fracture.15,16
There are two widely accepted mechanisms of CFES: the mechanical and biochemical theories.1,3,9,14–19 The mechanical theory states that fat particles released from the bone marrow of damaged bone escape to the systemic circulation through a damaged vein and finally cause obstruction of cerebral blood vessel. This theory is based on the existence of AVM or cardiac shunting as the pathway of fat particles to venous circulation. The biochemical theory is that inflammatory mediators, such as lipase, lead to the release of free fatty acids (FFAs) and aggregate fat globules, which have histotoxic effects, resulting in tissue edema and ischemic change. There is the latent phase or symptom-free interval before the activation of this theory because neutral fats of the damaged bone marrow are hydrolyzed to FFAs.9 The specific duration is unknown, but it takes more time than the mechanical theory.1 In our case, no echocardiographic evidence of AVM and right-to-left shunt led us to believe the biochemical theory. However, the latency period <1 h after the accident would support the mechanical theory. We could be in favor of both theories; however, there was no conclusive evidence.
Studies have reported nonconvulsive status epilepticus (NCSE) secondary to FES.14,20,21 NCSE shows only the presence of an altered consciousness but lacks convulsive motor activity; thus, EEG should be diagnosed. Our patient had been in coma without focal motor or generalized tonic–clonic seizure; however, EEG showed no epileptiform discharges even without the use of any sedative or antiepileptic drugs. Therefore, we concluded that the patient did not develop NCSE but a condition similar to severe hypoxic encephalopathy.
A typical case of FES has a latency period of 24–72 h, with an average of 48.5 h, after the injury,1 whereas the most recent study reported that FES manifested between 16 h and 7 days, with an average of 56 h, after the insult.2 Our review of the literature identified only three cases of FES with a latency period of less than 3 h3,18,19 (Table 1).
 |
Table 1 Demographic Profile of the Reported CFE Patients Whose Latent Period Was <6 h |
Age, sex, and the type of trauma were different, but the common point was that the patient whose latency period was <3 h had a poor neurological outcome. The onset in our patient was earlier than that observed in previously reported cases of hyperacute CFE, and we failed to identify other cases of extremely acute CFE within the first hour after injury. The majority of the previous case reports illustrate that cerebral dysfunction associated with CFE is diverse, ranging from headache to confusion, seizure, rigidity, and coma. However, almost all the cases were reversible and had good neurological recovery.1,2,4–9,22 In contrast, the previously reported patients with acute-onset CFE3,18,19 as well as our patient exhibited poor neurological outcomes, raising the possibility that the rapid onset of CFE might be a peculiar risk factor for worse neurological outcomes. Future studies and further education of trauma physicians are necessary for the diagnosis of CFE to improve prognosis.
Conclusion
The current report is the first to illustrate that CFE can develop in less than 1 h after injury, suggesting that hyperacute-onset CFE is a risk factor for poor neurological prognosis. CFE should be considered in patients who sustained long bone fractures with sudden deterioration of consciousness within 1 h after the injury. Brain MRI should be included in the initial workup of CFE.
Acknowledgments
The authors would like to thank Enago (www.enago.jp) for the English language review.
Funding
The authors have disclosed that they have no financial support.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Tsai IT, Hsu CJ, Chen YH, Fong YC, Hsu HC, Tsai CH. Fat embolism syndrome in long bone fracture–clinical experience in a tertiary referral center in Taiwan. J Chin Med Assoc. 2010;73:407–410. doi:10.1016/S1726-4901(10)70088-5
2. Aggarwal R, Banerjee A, Soni KD, Kumar A, Trikha A. Clinical characteristics and management of patients with fat embolism syndrome in level I apex trauma centre. Chin J Traumatol. 2019;22(3):172–176. doi:10.1016/j.cjtee.2019.01.007
3. Chen P-C, Hsu C-W, Liao W-I, Chen Y-L, Ho C-H, Tsai S-H. Hyperacute cerebral fat embolism in a patient with femoral shaft fracture. Am J Emerg Med. 2013;31(9):e1–e3. doi:10.1016/j.ajem.2013.05.003
4. Cronin KJ, Hayes CB, Moghadamian ES. Early-onset fat embolism syndrome: a case report. JBJS Case Connect. 2018;8:e44. doi:10.2106/JBJS.CC.17.00175.
5. Hermann B, Brisson H, Langeron O, et al. Unexpected good outcome in severe cerebral fat embolism syndrome. Ann Clin Transl Neurol. 2018;5(8):988–995. doi:10.1002/acn3.596
6. Shacklock E, Gemmell A, Hollister N. Neurological effects of fat embolism syndrome: a case report. J Intensive Care Soc. 2017;18:339–341. doi:10.1177/1751143717718664
7. Aravapalli A, Fox J, Lazaridis C. Cerebral fat embolism and the “starfield” pattern: a case report. Cases J. 2009;2:212. doi:10.1186/1757-1626-2-212
8. Bethany R, Reynolds HN, Bodanapally UK, Dreizin D. Cerebral fat embolism syndrome: diagnostic state of the art: with and without intra-medullary fixation, with and without long bone fractures. Int J Crit Care Emerg Med. 2014;1:1.
9. Mellor A, Soni N. Fat embolism. Anaesthesia. 2001;56(2):145–154. doi:10.1046/j.1365-2044.2001.01724.x
10. Konstantinos P, Michael K, Paul Z, et al. Fat embolism due to bilateral femoral fracture: a case report. Int J Gen Med. 2012;5:59–63.
11. Dillerud E. Abdominoplasty combined with suction lipoplasty: a study of complications, revision and risk factors in 487 cases. Ann Plast Surg. 1990;25:333. doi:10.1097/00000637-199011000-00001
12. Schonfeld SA, Ploysongsang Y, DiLisio R, et al. Fat embolism prophylaxis with corticosteroid: a prospective study in high-risk patients. Ann Int Med. 1983;99:438–443. doi:10.7326/0003-4819-99-4-438
13. Gurd AR. Fat embolism: an aid to diagnosis. J Bone Joint Surg Br. 1970;52:732–737. doi:10.1302/0301-620X.52B4.732
14. Scarpino M, Lanzo G, Lolli F, Grippo A. From the diagnosis to the therapeutic management: cerebral fat embolism, a clinical challenge. Int J General Med. 2019;12:39–48. doi:10.2147/IJGM.S177407
15. You JS, Kim SW, Lee HS, Chung SP. Use of diffusion-weighted MRI in the emergency department for unconscious trauma patients with negative brain CT. Emerg Med J. 2010;27(2):131–132. doi:10.1136/emj.2008.066399
16. Lin KY, Wang KC, Chen YL, Lin PY, Lin KH. Favorable outcome of cerebral fat embolism syndrome with a glasgow coma scale of 3: a case report and review of the literature. Indian J Surg. 2015;77:S46–S48. doi:10.1007/s12262-014-1109-3
17. Bollineni VR, Gelin G, Van Cauter S. Cerebral fat embolism syndrome. J Belgian Soc Radiol. 2019;103:1–2. doi:10.5334/jbsr.1781
18. Berlot G, Bussani R, Shafiei V, Zarrillo N. Fulminant cerebral fat embolism: case description and review of the literature. Case Rep Crit Care. 2018;7813175. doi:10.1155/2018/7813175
19. Eriksson EA, Schultz SE, Cohle SD, Post KW. Cerebral fat embolism without intracardiac shunt: a novel presentation. J Emerg Trauma Shock. 2011;4:309–312. doi:10.4103/0974-2700.82233
20. Fernández-Torre JL, Burgueño P, Ballesteros MA, Hernández-Hernández MA, Villagrá-Terán N, de Lucas EM. Super-refractory nonconvulsive status epilepticus secondary to fat embolism: a clinical, electrophysiological, and pathological study. Epilepsy Behav. 2015;49:184–188. doi:10.1016/j.yebeh.2015.04.045
21. Olivera Arencibia Y, Vo M, Kinaga J, et al. Fat embolism and nonconvulsive status epilepticus. Case Rep Neurol Med. 2018:5057624. doi:10.1155/2018/5057624
22. Scarpino M, Lanzo G, Cappelli F, et al. Cerebral fat embolism after video-assisted thoracic surgery. Ann Thorac Surg. 2016;102(5):e409–e411. doi:10.1016/j.athoracsur.2016.04.073
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
