Back to Journals » International Medical Case Reports Journal » Volume 10
Extraordinary and prolonged Erlotinib-induced clinical response in a patient with EGFR wild-type squamous lung cancer in third-line therapy: a case report
Authors Gambale E, Carella C, Amerio P , Buttitta F, Patea RL, Natoli C, De Tursi M
Received 17 February 2017
Accepted for publication 16 March 2017
Published 23 May 2017 Volume 2017:10 Pages 173—175
DOI https://doi.org/10.2147/IMCRJ.S134944
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ronald Prineas
Elisabetta Gambale,1 Consiglia Carella,1 Paolo Amerio,2 Fiamma Buttitta,3 Rosa Lucia Patea,4 Clara Natoli,1 Michele De Tursi1
1Medical Oncology Unit, Department of Medical, Oral and Biotechnological Sciences, 2Department of Dermatology and Venereology, University G. d’Annunzio, Chieti, 3Department of Medicine and Aging Sciences, University of Chieti-Pescara, Chieti, 4Department of Radiologic Sciences, University of Chieti-Pescara, Chieti, Italy
Abstract: Several small molecules, epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors (TKIs), such as gefitinib, erlotinib and afatinib, have been demonstrated to significantly improve clinical outcomes in patients with advanced EGFR-mutated non-small cell lung cancer (NSCLC), but erlotinib activity in EGFR wild-type squamous carcinoma is still highly debated. Here, we describe a prolonged and unexpected clinical response to erlotinib in a male former heavy cigarette smoker with wild-type EGFR squamous-cell cancer.
Keywords: erlotinib, squamous NSCLC, EGFR wild type
Introduction
Specific mutations of epidermal growth factor receptor (EGFR) play a key role in the development of non-small cell lung cancer (NSCLC), primarily adenocarcinomas, and specific EGFR-targeted therapy has revolutionized the treatment of the subset of adenocarcinomas harboring an EGFR-activating mutation.1,2
Several small molecules, EGFR-tyrosine kinase inhibitors (TKIs), such as gefitinib, erlotinib and afatinib, have been demonstrated to significantly improve clinical outcomes (response rate [RR], progression-free survival [PFS] and overall survival) in patients with advanced EGFR-mutated NSCLC, when used in the first-,3 second- or third-line treatment.4,5 Erlotinib is not only approved as a single agent in NSCLC that has EGFR-activating mutations, but it is also approved in chemorefractory NSCLC regardless of EGFR mutation status and maintenance therapy after platinum-based doublets. It is known that, in addition to the mutation in the EGFR gene, patient characteristics of being Asian, having an adenocarcinoma, being female and being a nonsmoker give an increased response to EGFR inhibitors, but erlotinib activity in EGFR wild-type squamous carcinoma is still highly debated.6
Here, we describe a prolonged and unexpected clinical response to erlotinib in a male former heavy cigarette smoker with wild-type EGFR squamous-cell cancer.
Case report
We report a case of a 65-year-old Caucasian man, a former heavy smoker, with a medical history of arterial hypertension, cardiac arrhythmia and chronic obstructive pulmonary disease (COPD). A whole-body computed tomography (CT) scan showed an area of parenchymal consolidation in the anterior segment of the right upper lobe and infiltration of the pulmonary vein. In October 2009, the patient underwent surgical right pneumonectomy and ipsilateral hilar-mediastinal lymphadenectomy. Histopathology and mutation testing (PCR in real time) showed an EGFR wild-type squamous non-small cell lung cancer (sqNSCLC) involving the cava vein and the mediastinum with no metastasis in 13 lymph nodes removed; whole-body CT scan did not show evidence of metastasis (TNM 2009: pT4 pN0). Post-operative course was complicated by stroke and transient hemiparesis. The first post-surgery CT scan evaluation performed in January 2010 revealed a solid nodule of 2.6 cm in diameter in the mediastinum, a paraesophageal lesion and spleen metastases. The patient received a first-line chemotherapy regimen, four cycles of carboplatin and gemcitabine, but subsequent CT scan evidenced disease progression. The patient was started on a second-line weekly docetaxel chemotherapy; this treatment was interrupted after the first cycle for grade 4 neutropenia and atrial fibrillation. The clinical history of this patient (short disease progression-free interval and limiting bone marrow toxicity) ruled out the use of a new chemotherapy regimen. Therefore, erlotinib, 150 mg/day, was started in March 2011. The patient presented a grade 3 skin rash within 1 month from the beginning of erlotinib treatment. Skin toxicity was treated with topic and systemic therapies, and an erlotinib dose reduction was needed (100 mg/die). The first posttreatment evaluation CT scan showed a stable disease according to RECIST criteria (reduction of paraesophageal lesion from 50 mm to 40 mm). The patient continued erlotinib 100 mg once daily with a persistent grades 2–3 skin toxicity. No other adverse events were reported. Four months later, a new CT scan highlighted a persistent stable disease with continuing reduction of the paraesophageal lesion (35 mm vs 40 mm). CT scans were performed every 4 months with confirmed stable disease. The paraesophageal lesion continued to decrease: a CT scan performed on May 2, 2013, showed a reduction of paraesophageal lesion (18 mm [Figure 1B] vs 35 mm [Figure 1A]). Several CT scans performed every 4 months confirmed stable disease, but due to G4 skin toxicity, erlotinib treatment was stopped in March 2016, 5 years after the beginning of the therapy. However, 2 months later, in May 2016, the first posttreatment CT scan documented lung disease progression. Written informed consent has been provided by the patient to publish the case and the image.
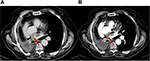  | Figure 1 Left paraesophageal lesion (red arrow): 35 mm (A) and 18 mm (B). |
Discussion
Approximately 10% of Caucasian and up to 50% of Asian patients with NSCLC are positive for EGFR mutations, and this is predictive for response to the EGFR-TKIs.1 Several randomized trials showed that TKIs provide an improved RR and PFS, with a good tolerability of treatment and a better quality of life (QoL), when compared with cytotoxic treatment as a first-line chemotherapy in EGFR-mutated advanced NSCLC patients.3 Conversely, for the first-line treatment of locally advanced or metastatic NSCLC with wild-type or unknown EGFR status, platinum-based doublet chemotherapy (with agents such as taxanes, gemcitabine, vinorelbine and etoposide) remains the standard of care.7
Regarding the second- or third-line therapies, the BR.21 trial demonstrated that erlotinib is superior to the best supportive care for the treatment of patients with EGFR wild-type NSCLC, including squamous-cell cancer.8 These results were confirmed by a Phase IV study (Tarceva Lung Cancer Survival Treatment [TRUST]).9 However, a randomized trial comparing erlotinib and docetaxel as a second-line treatment for EGFR wild-type NSCLC (TArceva Italian Lung Optimization tRial [TAILOR]) demonstrated the superiority of using docetaxel in a second-line therapy.10 Similarly, a Japanese randomized Phase III trial of erlotinib versus docetaxel as a second- or third-line therapy (Docetaxel and Erlotinib Lung Cancer Trial [DELTA]) indicated the superiority of using chemotherapy in an EGFR wild-type subpopulation.11 However, despite the evidence of the TAILOR and DELTA trials, for patients in whom no alternative is recommended, erlotinib might be considered.12 Furthermore, in the BR.21 trial, the survival benefit with erlotinib was maintained in male smokers with squamous histology and in the TAILOR trial, there was similar OS in the sqNSCLC patients treated with erlotinib or docetaxel.
Conclusion
Although activating mutations in the EGFR gene are the most important predictive biomarkers for EGFR-TKI treatment, the clinical benefits cannot only be explained by these mutations.13,14
In patients with progressive EGFR wild-type sqNSCLC after failure of standard chemotherapy, comorbidities and a low probability of a response to a chemotherapy, EGFR-TKIs are a valid treatment option over placebo or best supportive care. In fact, although in the second-line setting, survival outcomes are comparable between cytotoxic treatment and EGFR-TKI treatment in a meta-analysis, the latter is more tolerable as a second-line therapy.15 In the present case report, the choice of erlotinib exhibited an unexpected prolonged response, unexpected because the clinical characteristics of our patient did not correlate with those predictive of response. In fact, despite the absence of EGFR mutation, squamous histology, male sex and heavy smoking history, the clinical efficacy of TKI continued for 5 years. Our patient has already reached a PFS of 62 months and OS not yet reached, with a global objective response of 64%. Moreover, our case report shows how important it is to maintain EGFR inhibition overtime. In fact, when skin toxicity forced to stop therapy, the disease has quickly progressed.
In conclusion, this case report confirms that EGFR-TKI therapy may confer benefit in terms of PFS and OS, regardless of the mutation status, histological subgroup and other clinical characteristics.
Disclosure
The authors report no conflicts of interest in this work.
References
Dearden S, Stevens J, Wu YL, Blowers D. Mutation incidence and coincidence in non-small-cell lung cancer: meta-analyses by ethnicity and histology (mutMap). Ann Oncol. 2013;24(9):2371–2376. | ||
Bronte G, Franchina T, Alù M, et al. The comparison of outcomes from tyrosine kinase inhibitor monotherapy in second- or third-line for advanced non-small-cell lung cancer patients with wild-type or unknown EGFR status. Oncotarget. 2016;7(24):35803–35812. | ||
Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicenter, open-label, randomised phase 3 study. Lancet Oncol. 2012;13(3):239–246. | ||
Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. National Cancer Institute of Canada Clinical Trials Group. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353(2):123–132. | ||
Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell-cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010;11(6):521–529. | ||
Zeng Z, Chen HJ, Yan HH, Yang JJ, Zhang XC, Wu YL. Sensitivity to epidermal growth factor receptor tyrosine kinase inhibitors in males, smokers, and non-adenocarcinoma lung cancer in patients with EGFR mutations. Int J Biol Markers. 2012;28(3):249–258. | ||
Peters S, Adjei AA, Gridelli C, et al. Metastatic non-small-cell lung cancer (NSCLC): ESCMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(suppl 7):56–64. | ||
Bezjak A, Tu D, Seymour L, et al. Symptom improvement in lung cancer patients treated with erlotinib: quality of life analysis of the National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Clin Oncol. 2006;24(24):3831–3837. | ||
Reck M, van Zandwijk N, Gridelli C, et al. Erlotinib in advanced non-small cancer lung cancer: efficacy and safety findings of the global phase IV Tarceva Lung Cancer Survival Treatment study. J Thorac Oncol. 2010;5(10):1616–1622. | ||
Garassino MC, Martelli O, Broggini M, et al. Erlotinib versus docetaxel as second-line treatment of patients with advanced non-small cell lung cancer and wild-type EGFR tumors (TAILOR): a randomised controlled trial. Lancet Oncol. 2013;14(10):981–988. | ||
Kawaguchi T, Ando M, Asami K, et al. Randomized Phase III Trial of erlotinib versus docetaxel as second-or third line therapy in patients with advanced non-small-cell lung cancer: docetaxel and erlotinib lung cancer trial (DELTA). J Clin Oncol. 2014;32(18):1902–1908. | ||
Vale CL, Burdett S, Fisher DJ, et al. Should Tyrosine Kinase Inhibitors be considered for advanced non-small-cell lung cancer patients with wild type EGFR? Two systematic reviews and meta-analyses of randomized trials. Clin Lung Cancer. 2015;16(3):173–174. | ||
Wang F, Fu S, Shao Q, et al. High EGFR copy number predicts benefits from tyrosine kinase inhibitor treatment for non-small cell lung cancer patients with wild type EGFR. J Transl Med. 2013;11:U1–U10. | ||
Bell DW, Lynch TJ, Haserlat SM, et al. Epidermal growth factor receptor mutations and gene amplification in non-small cell lung cancer: molecular analysis of the IDEAL/INTACT gefitinib trails. J Clin Oncol. 2005;23(31):8081–8092. | ||
Goss GD, Spaans JN. Epidermal growth factor receptor inhibition in the management of squamous cell carcinoma of the lung. Oncologist. 2016;21(2):205–213. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
