Back to Journals » OncoTargets and Therapy » Volume 11
Extramedullary relapse of acute lymphoblastic leukemia after allogeneic hematopoietic stem cell transplantation treated by CAR T-cell therapy: a case report
Authors Wang D, Shi R, Wang Q, Li J
Received 2 February 2018
Accepted for publication 25 June 2018
Published 28 September 2018 Volume 2018:11 Pages 6327—6332
DOI https://doi.org/10.2147/OTT.S164430
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Samir Farghaly
Dongmei Wang,1 Rui Shi,1 Qinglong Wang,2 Jianqiang Li2
1Department of Hematology, Harrison International Peace Hospital, Hengshui, Hebei 053000, People’s Republic of China; 2Hebei Senlangbio Technology Co., Ltd, Shijiazhuang, Hebei 050000, People’s Republic of China
Abstract: Philadelphia chromosome-positive (Ph-positive) acute leukemia (ALL) accounts for around one quarter of adult cases of ALL and is usually associated with poor prognosis. The patients still encounter a high rate of relapse even after they receive hematopoietic stem cell transplantation (HSCT). HSCT is considered the established therapy and best option for many malignant ALL cases, however, disease relapse remains the main reason of failure. In recent years, chimeric antigen receptor (CAR) T-cell therapy has become a promising treatment for patients with advanced blood cancers. Here, we report a rare case of a Ph-positive ALL patient with extramedullary relapse in her cervix after receiving allogeneic HSCT. Given the unsatisfactory response to chemotherapy, tyrosine kinase inhibitor (TKI) treatment, and HSCT transplantation, she had received CD19+ CAR T-cell therapy 8 months earlier. The following ultrasound check indicated that her cervix relapse went through significant remission with almost undetectable tumor mass. This case strongly supports the efficacy of CAR T-cell therapy on adult ALL with extramedullary relapse.
Keywords: acute lymphoblastic leukemia, allogeneic hematopoietic stem cell transplantation, CAR T-cell therapy, cervix relapse
Introduction
Philadelphia chromosome-positive (Ph-positive) acute lymphoblastic leukemia (ALL) accounts for 20%–30% of adult leukemia cases and is the most common cytogenetic abnormality in adult ALL patients.1,2 Patients have been associated with poor prognosis and more relapses have been associated with patients on combination chemotherapy regimens. The Philadelphia chromosome results from a reciprocal translocation between the ABL1 oncogene on chromosome 9 and a breakpoint cluster region (BCR) gene on chromosome 22 and this translocation results in an oncogenic BCR-ABL gene fusion that encodes tyrosine kinase signaling protein.3 Thus, tyrosine kinase inhibitors (TKIs) targeting this kinase are incorporated into regimins and widely used as first-line therapy, which significantly improves response rate and disease-free survival.1,4 Furthermore, allogeneic hematopoietic stem cell transplantation (HSCT) provides the best opportunity of a cure for a majority of eligible patients.5 However, disease relapse remains the main cause of the failure. For those without transplantation, the emergence of resistance to TKIs means that patients are faced with the dangers of disease recurrence after treatment with TKIs therapy.6 Treatment options become extremely limited for Ph-positive ALL patients after they relapse from transplantation.
Chimeric antigen receptors (CARs) are fusion proteins that incorporate a tumor cell antigen-recognition portion with a T-cell activation domain. Thus, T-cells can be modified through virus vectors to express anti-tumor CARs recognizing tumor surface markers and exert cytotoxicity to eliminate malignant cells.6 It has been shown that anti-CD19 CAR T-cells rapidly induced remission of B-cell malignancies in patients.7,8 Meanwhile, anti-CD19 CAR T-cell therapy has also been reported to provide partial remission on bone marrow relapse in Ph-positive ALL patients previously receiving HSCT therapy.9,10 Here, we describe a Ph-positive ALL patient with extramedullary relapse after HSCT therapy, who received anti-CD19 CAR T-cells infusions.
Case report
The patient was a 52-year-old woman who reported frequent micturition, urgent micturition, and dysuria for 1 week and was admitted to our hospital on April 5, 2017. Previously she had presented to Peking University People’s Hospital (People’s Rebulic of China) with dizziness in 2011 and was diagnosed with Ph-positive ALL with BCR/ABL210 fusion gene positive, revealed by a routinely used, RT-qPCR-based method. The patient was given chemotherapy immediately for three courses and the symptoms were alleviated. Afterward, she received haploidentical allogeneic HSCT without obvious rejection and was discharged after recovery. Two years after the therapy, bone marrow examination showed no relapse and minimal residual disease (MRD) reached negative status. In July 2015, she was admitted to Beijing Cancer Hospital with a complaint of vaginal bleeding. Cervical biopsy and immunohistochemistry demonstrated B lymphoblastic lymphoma. Then she underwent stereotactic radiosurgery using Gamma Knife in combination with Chinese traditional medicine; however, the response was not satisfactory. In January 2016, the patient was admitted to our hospital with abdominal distention and pain in her left lower extremities. Gynecologic ultrasonography revealed a hypoechoic signal in her pelvis and diffuse appearance of lower uterus, cervix, and upper vagina with a 79×47 mm sized hypoechoic mass. Bone marrow examination indicated reduced proliferation without lymphoblasts. Doppler ultrasonography indicated suspected deep vein thrombosis in her left lower extremity. On December 20, 2016, she was officially diagnosed as having a relapse of ALL. The patient was given VP-16 chemotherapy and low-molecular-weight heparins, in combination with oral administration of dasatinib (100 mg/day). With partial remission, she was further treated with bortezomib-cyclophosphamide-dexamethasone (VCD) chemotherapy and the deep vein thrombosis was invisible. Ultrasound examination indicated the size of the pelvic mass was decreased to 21×24 mm. However, on February 16, 2017, the patient presented to our hospital with a chief complaint of dysuria, frequent micturition, and urgent micturition due to the compression from the enlarged mass. Pelvic ultrasound showed a 59×26 mm solid mass (mainly located in the anterior cervix) with extension into the posterior wall of the urinary bladder and the border was poorly defined. Mass extension to the vagina was also observed and color doppler flow imaging (CDFI) revealed weak blood flow signal around. Due to the lack of response to her previous chemotherapy protocol, the patient was given induction chemotherapy with the vincristine (4 mg/day, day +1, +8, +15, and +22), daunorubicin (40 mg/day, day +1, +8 and +15), cyclophosphamide (1 mg/day, day +1 and +15), and dexamethasone (10 mg/day, day +1 to +7 and +15 to +21) (VDCD) protocol. After the treatment, she still exhibited symptoms of urinary tract obstruction and was admitted to our hospital on April 5, 2017. At that time, her physical examination showed the vital signs as: blood pressure 135/85 mmHg, pulse 67/min, respiration 20/min and body temperature 36.3°C. The patient appeared conscious, oriented and able to communicate. No lymphadenectasis was palpable in the superficial lymph node all over the body and no swelling was observed in either tonsil. No abnormality was noted in either the chest or abdomen. Pelvic ultrasound showed smooth surface of anterior and posterior uterine wall. Echo signal was evenly distributed in myometrium. Hypoechoic units were observed around both cervix and urinary tract with the size of 57×31 and 47×35 mm, respectively. The border was poorly defined and the mass shape was irregular. Both sides of the ovary were unclear. Blood routine examination: white blood cell, 5.79×109/L; neutrophils, 3.36×109/L; hemoglobin, 106 g/L; platelet, 336×109/L. Bone marrow smear examination showed hyperplasia without detection of lymphoblasts. Accordingly, the patient was diagnosed with extramedullary relapse of Ph-positive ALL after haploidentical allogeneic HSCT and four courses of chemotherapy treatment. The catheter was retained since the patient could not urinate.
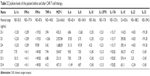
Pathology results from July 2017 indicated strong positive of CD19 (CD19+++) in the cervix. Due to the lack of response to traditional therapy and in combination with all the examination results, the patient agreed to receive anti-CD19 CAR T-cell therapy with the permission from the ethics committee in the hospital and her family. Before the treatment, organ functions, T-cell functions, and tumor condition were evaluated to decide if the patient was able to receive CAR T-cell therapy directly. Ultrasound results indicated an enlarged mass (57×31 mm) in the cervix. The patient was given chemotherapy with liposomal doxorubincin (40 mg/day, day +1) in combination with dexamethasone (10 mg/day, day +1 to +4) on April 8, 2017, to reduce the tumor burden. After this treatment, the symptoms were alleviated and parameters met the CAR T-cell therapy criteria. On day −14, blood from the patient’s peripheral vein was collected and sent for CAR T-cell culture (Senlang Biotechnology Co., Ltd, Shijiazhuang, Hebei). In brief, T-cells were enriched from collected peripheral blood and activated for CAR virus transduction. With certain days of proliferative expansion, engineered CAR-T-cells were further manufactured ready for administration. After 14 days culture, T-cells were expended to 118-fold with infection rate of 12.6%. The cell quality parameters are listed in Table 1.
  | Table 1 T-cell quality control |
Lymphodepleting chemotherapy with the FC regimen (cyclophosphamide 900 mg/m2, days −2 and −1; fludarabine 25 mg/m2, days −4 to −2) was given to the patient before the infusion. On April 21, 2017, the patient received a single dose infusion of 2×106 CAR T-cells/kg over 40–60 minutes. Some 30 minutes before the infusion, she was given 25 mg promethazine through intramuscular injection and vital signs were closely monitored throughout the whole process until 2 hours after the infusion, which included: 1) Any clinical symptom change; 2) vital signs (temperature, blood pressure, blood oxygen, respiratory, heart rate); 3) C-reactive protein (CRP); 4) blood routine examination; 5) ferritin; 6) blood biochemical examination; 7) BNP; 8) troponin; 9) coagulation tests plus D-dimer; 10) detection of CAR T-cell number in peripheral circulation through flow cytometry and quantitative polymerase chain reaction (qPCR); 11) access the tumor size by ultrasound; 12) cytokine levels. Before infusion, the patient’s axillary temperature was 36.8°C and reached 37.2°C 2 hours after infusion as the highest temperature on that day, which dropped back to normal level afterwards. She developed febrile syndrome from day +1 to +5 and her body temperature reached a peak of 40.7°C at 16:00 on day +2 (April 23) (Figure 1), indicating a low-grade cytokine release syndrome (CRS) (Figure 2 and Table 2). On day +5 (April 26), urinary incontinence was observed which was likely due to neurotoxicity side effect from CAR-T therapy. After treatment with dexamethasone (10 mg/day) for 3 days, her fever and urinary incontinence symptoms were alleviated. On day +14, pelvic ultrasound indicated shrunken hypoechoic mass of 45×26 mm in the cervix and vagina; on day +28, no mass was detected. After the therapy, the patient followed a monthly check for 3 months until now and no sign of tumor recurrence was observed. Physiological parameters of the patient before and after the therapy were listed as following: body temperature (Figure 1), CRP (Figure 2), CAR T-cell expansion (Figure 3), ultrasound results (Figure 4), and cytokine levels (Table 2). As shown in Figure 4A, on day −7 before CAR T-cell therapy, the ultrasound indicated a smooth surface of anterior and posterior uterine wall. Echo signal was evenly distributed in myometrium. Hypoechoic unit was observed around both cervix and urinary tract with the size of 75×48 mm. The border was poorly defined and the mass shape was irregular. Echo signal inside was uneven and blood signal could be observed inside and around the mass. On day +5 (April 26, 2017), bedside ultrasound showed hypoechoic signal around the cervix and vagina, indicating a mass of 70×40 mm size with irregular shape and blood flow signal. After the first phase of CAR T-cell therapy (day +28), ultrasound revealed a normal-sized uterus with even echo signal in myometrium and smooth anterior and posterior uterine wall as shown in Figure 4B. Figure 4C exhibited the post-therapy 2-month follow-up check and ultrasound indicated normal-sized uterus with even echo signal in myometrium and smooth anterior and posterior uterine. Figure 4D and E shows ultrasound images of normal sized uterus on day +90 and day +120 post-treatment, respectively. The most recent 12-month (day +360) follow-up check (April 26, 2018) did not show significant restrictive echo with abnormality, nor obvious blood flow signal (Figure 4F).
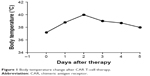  | Figure 1 Body temperature change after CAR T-cell therapy. |
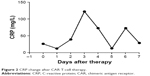  | Figure 2 CRP change after CAR T-cell therapy. |
  | Table 2 Cytokine levels of the patient before and after CAR T-cell therapy |
  | Figure 3 Expansion of CAR T-cell from peripheral blood after therapy. |
Discussion
Extramedullary relapse of Ph-positive ALL after allogeneic HSCT are usually reported in the central neural system11–15 and recurrence in the cervix is rare.16–18 CAR T-cell therapy specifically targets cancer cells through recognizing specific tumor antigen. So far, it is the most promising and effective therapy curing CD19-positive leukemia. It is reported that anti-CD19 CAR-T therapy is beneficial for certain extramedullary tissues relapse with or without allogeneic-HSCT, such as breast, kidney, CNS, pancreas, pelvic fascia, subcutaneous adipose tissue of chest, bone, liver, lung, and muscle tissue.19–23 Herein, we reported a case of an adult patient receiving CD19+ CAR T-cell therapy to treat the relapse of Ph-positive ALL after allogeneic HSCT with an extremely rare location in the cervix, which has never been reported. After the failure of chemotherapy and TKI treatment, the patient underwent CD19+ CAR T-cell therapy and has acquired 8-month full recovery until now with significantly remission of cervix tumor growth and improved life quality. Learning from this case, we believe that CAR T-cell therapy not only can be applied to treat hematopoietic malignancies, but also shows great potential in the treatment of rare extramedullary relapse ALL case (eg, in the cervix) with significant clinical efficacy and safety. In order to fully evaluate the efficacy of this treatment and eventually fulfill this strategy, we will keep following up with the patient recording her remission and survival time and include larger numbers of similar cases.
Acknowledgment
Written informed consent was acquired from the patient with approval of case detail and related patient information for publication use.
Disclosure
The authors report no conflicts of interest in this work.
References
Liu-Dumlao T, Kantarjian H, Thomas DA, O’Brien S, Ravandi F. Philadelphia-positive acute lymphoblastic leukemia: current treatment options. Curr Oncol Rep. 2012;14(5):387–394. | ||
Wetzler M, Dodge RK, Mrózek K, et al. Prospective karyotype analysis in adult acute lymphoblastic leukemia: the cancer and leukemia Group B experience. Blood. 1999;93(11):3983–3993. | ||
Rowley JD. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973;243(5405):290–293. | ||
Ottmann OG, Pfeifer H. First-line treatment of Philadelphia chromosome-positive acute lymphoblastic leukaemia in adults. Curr Opin Oncol. 2009;21(Suppl 1):S43–S46. | ||
Fielding AK. How I treat Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2010;116(18):3409–3417. | ||
Jabbour EJ, Cortes JE, Kantarjian HM. Resistance to tyrosine kinase inhibition therapy for chronic myelogenous leukemia: a clinical perspective and emerging treatment options. Clin Lymphoma Myeloma Leuk. 2013;13(5):515–529. | ||
Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5177(177):ra138. | ||
Brudno JN, Somerville RP, Shi V, et al. Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of B-cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease. J Clin Oncol. 2016;34(10):1112–1121. | ||
Zhu YM, Wu Z, Tan YP, et al. Anti-CD19 chimeric antigen receptor T-cell therapy for adult Philadelphia chromosome-positive acute lymphoblastic leukemia: Two case reports. Medicine. 2016;95(51):e5676. | ||
Chen Y, Cheng Y, Suo P, et al. Donor-derived CD19-targeted T cell infusion induces minimal residual disease-negative remission in relapsed B-cell acute lymphoblastic leukaemia with no response to donor lymphocyte infusions after haploidentical haematopoietic stem cell transplantation. Br J Haematol. 2017;179(4):598–605. | ||
Békássy AN, Hermans J, Gorin NC, Gratwohl A. Granulocytic sarcoma after allogeneic bone marrow transplantation: a retrospective European multicenter survey. Acute and Chronic Leukemia Working Parties of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 1996;17(5):801–808. | ||
Poon LM, Hamdi A, Saliba R, et al. Outcomes of adults with acute lymphoblastic leukemia relapsing after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19(7):1059–1064. | ||
Oliff A, Ramu NP, Poplack D. Leukemic relapse 5½ years after allogeneic bone marrow transplantation. Blood. 1978;52(2):281–284. | ||
Lawson SE, Darbyshire PJ. Use of donor lymphocytes in extramedullary relapse of childhood acute lymphoblastic leukaemia following bone marrow transplantation. Bone Marrow Transplant. 1998;22(8):829–830. | ||
de La Cámara R, Figuera A, Steegmann JL, et al. Allogeneic bone marrow transplantation for high risk acute lymphoblastic leukemia. Results from a single institution. Bone Marrow Transplant. 1992;9(6):433–438. | ||
Cormier MG, Armin AR, Daneshgari F, Castelli M. Unusual extramedullary relapse of acute lymphoblastic leukemia in a bone marrow transplant patient. J Surg Oncol. 1987;36(4):290–294. | ||
Robillard DT, Kutny MA, Chewning JH, Arbuckle JL. Extramedullary relapse of acute lymphoblastic leukemia presenting as abnormal uterine bleeding: a case report. J Pediatr Adolesc Gynecol. 2017;30(3):431–434. | ||
Tsuruchi N, Okamura J. Childhood acute lymphoblastic leukemia relapse in the uterine cervix. J Pediatr Hematol Oncol. 1996;18(3):311–313. | ||
Dai H, Zhang W, Li X, et al. Tolerance and efficacy of autologous or donor-derived T cells expressing CD19 chimeric antigen receptors in adult B-ALL with extramedullary leukemia. Oncoimmunology. 2015;4(11):e1027469. | ||
Weng J, Lai P, Qin L, et al. A novel generation 1928zT2 CAR T cells induce remission in extramedullary relapse of acute lymphoblastic leukemia. J Hematol Oncol. 2018;11(1):25. | ||
Park JH, Rivière I, Gonen M, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378(5):449–459. | ||
Li S, Zhang J, Wang M, et al. Treatment of acute lymphoblastic leukaemia with the second generation of CD19 CAR-T containing either CD28 or 4-1BB. Br J Haematol. 2018;181(3):360–371. | ||
Zhang Y, Zhang W, Dai H, et al. An analytical biomarker for treatment of patients with recurrent B-ALL after remission induced by infusion of anti-CD19 chimeric antigen receptor T (CAR-T) cells. Sci China Life Sci. 2016;59(4):379–385. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

