Back to Journals » Patient Preference and Adherence » Volume 17
Extracorporeal Left Ventricular Assist Device as a Bridge to Surgery for Ventricular Septal Rupture After Acute Myocardial Infarction
Received 16 September 2023
Accepted for publication 1 November 2023
Published 8 November 2023 Volume 2023:17 Pages 2871—2876
DOI https://doi.org/10.2147/PPA.S436512
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jongwha Chang
Lu Tong, Long Wu, Nianguo Dong
Department of Cardiovascular Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, People’s Republic of China
Correspondence: Nianguo Dong, Email [email protected]
Abstract: Ventricular septal rupture (VSR) after acute myocardial infarction (AMI) is a rare but often fatal complication. Surgery is considered the preferred treatment, although the optimal timing is discussed. The immediate preoperative hemodynamic status significantly impacts postoperative outcomes, making mechanical circulatory support (MCS) devices crucial for perioperative hemodynamic stability. We present the case of a 61-year-old woman with no remarkable cardiological history admitted to our hospital with a diagnosis of AMI and VSR. Due to hemodynamic instability and cardiogenic shock, we utilized an intra-aortic balloon pump (IABP) and an extracorporeal left ventricular assist device (extra-VAD) as a bridge to surgery. After 17 days of IABP support, the patient experienced hemodynamic instability, elevated lactate levels, pulmonary edema, and eventually bedside endotracheal infiltration inventor-assisted breathing. Subsequently, the IABP was removed, and the patient underwent 6 days of extra-VAD therapy, resulting in hemodynamic stability, a decline in lactate levels, and a reduction in pulmonary edema on X-ray. Surgical coronary artery bypass grafting and VSR repair were successfully performed without periprocedural complications, and the patient was subsequently discharged. Extra-VAD is useful for serious cardiogenic shock in patients with VSR after AMI and may be considered a reasonable approach as a bridge to surgery.
Keywords: ventricular septal rupture, acute myocardial infarction, extracorporeal left ventricular assist device
Introduction
Ventricular septal rupture (VSR) after acute myocardial infarction (AMI) is a complication that frequently results in serious cardiogenic shock and poor prognosis and can be fatal. Surgical repair is the leading treatment of choice, and its timing is the most important factor affecting survival rates.1 Early surgery has been associated with high mortality rates and risk of recurrent ventricular rupture, and although delayed intervention allows for the repair of scar tissue, it includes the risk of rupture extension, cardiogenic shock, and multiple organ dysfunction syndrome (MODS); moreover, the patient may die while awaiting surgery.2,3 The immediate preoperative hemodynamic status significantly influences postoperative outcomes.4 Different mechanical circulatory support (MCS) devices, such as intra-aortic balloon pump (IABP), Impella, Tandem Heart, and extracorporeal membrane oxygenation (ECMO), have distinct hemodynamic effects, insertion methods, monitoring requirements, and clinical applicability. Multiple factors must be considered when selecting the appropriate MCS device.5–8 Advancements in MCS technology significantly improved the success rate in treating cardiogenic shock, and delayed and elective surgical procedures are strategies acknowledged by several researchers.2,3
Although the concept of perioperative mechanical circulatory support in high-risk patients with VSR and cardiogenic shock has been reported,9 the use of an extracorporeal left ventricular assist device (extra-VAD) as a bridge to surgical VSR repair and coronary artery bypass grafting has not been described, particularly in China. In this report, we present a case of successful application of an extra-VAD as a bridge to surgical repair in a patient with cardiogenic shock due to VSR after AMI.
Case Description
A 61-year-old woman with no previous cardiological history was admitted to our hospital due to anteroseptal myocardial infarction (MI) after 4 days of experiencing chest pain, palpitations, and dyspnea at a regional hospital. Upon admission, the patient had difficulty breathing, a little chest tightness, rest in a 30° sitting position, and cold limbs. The physical examination showed invasive blood pressure of 80/45 mmHg with norepinephrine at 0.06 µg/kg/min, no urine output within two hours, a heart rate of 114 beats per minute, regular heart sounds with a mild systolic murmur, a respiratory rate of 25 breaths per minute, arterial oxygen saturation of 95%, and wheezing sounds in the lungs.
An electrocardiogram revealed ST elevation in leads V1 to V6 of 2 mm; high-sensitivity troponin I was markedly increased, and the lactate level was 4.6 mmol/L. Transthoracic echocardiogram showed regional wall motion abnormalities consistent with left anterior descending territory ischemia, including akinesis of the mid and apical segments of the anteroseptum, along with a ventricular aneurysm appearance at the apex. The estimated size of the VSR at the ventricular apex was 17 mm, accompanied by moderate dilation of the left ventricle (LV) cavity. The biplane LV ejection fraction of Simpson was estimated at 49%. Tricuspid annular plane systolic excursion and right ventricular fractional area changes were within normal limits.
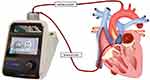
A diagnosis of cardiogenic shock after AMI and VSR was established, requiring mechanical circulatory support (MCS). The right femoral artery was punctured, and IABP was implanted. Cardiogenic shock was reduced, followed by improved hemodynamic stability, urine output recovery, and normalized lactate levels. After 7 days of IABP assistance, coronary angiography revealed occlusion of the left anterior descending artery (thrombolysis in myocardial infarction [TIMI 0]), stenosis of 90% in the diagonal branches (TIMI 3), and stenosis of 80% in the right coronary artery (TIMI 3). Due to poor cardiac function and the use of contrast agents during coronary angiography. Then, the heart rate raised to 124 beats per minute and premature ventricular beats were observed, the patient’s heart function deteriorated with less urine. The patient presented hemodynamic instability, pulmonary edema on radiography and tracheal intubation connected to a ventilator, an increase in the VSR size (estimated at 18 mm), a decrease in the biplane LV ejection fraction of Simpson (estimated at 48%), elevated lactate levels (5.6 mmol/L), and worsened cardiogenic shock after 17 days of IABP support. Considering that right heart function is acceptable, the potential interference with LV recovery and pulmonary circulation overload by veno-arterial ECMO, a domestically developed investigational maglev extra-VAD, MoyoAssist® (magAssist Inc., Suzhou, China), was chosen for humanitarian use with the informed consent of the family of the patient. The IABP was removed, and we used minimally invasive methods to establish extra-VADs without open heart surgery. The method of drainage was to puncture the femoral vein with a 21 Fr venous cannula through the atrial septum to the left atrium. Perfusion was performed by separating and incising the axillary artery with an 8 mm Intel Vasco artificial blood vessel. This 8 mm artificial vessel was used to connect the axillary artery to the tubing to reduce the risk of thrombosis at the contact site. The left atrial canal is generally circled three times in the left atrium, avoiding catheter displacement and left atrial and pulmonary vein injury. The extra-VAD was implanted to establish the left atrium, left ventricular assist pump, and axillary artery circulation. The details are shown in Figure 1. The pump flow rate was maintained at 2–3 L/min by adjusting the pump speed to provide adequate cardiac support without suction. The pump speed is usually between 1500 and 2000 rpm. In general, we used heparin sodium for anticoagulation. The activated clotting time was maintained within 180–200 seconds, and the activated partial thromboplastin time was maintained within 55–70 seconds. No complications were observed during the entire usage period. Extra-VAD therapy was administered for 6 days, resulting in decreased lactate levels and a reduction in pulmonary edema. Eventually, the patient was assessed to have stable circulation, improved organ function, and normal lactate, and 27 days after acute myocardial infarction, the risk of surgical intervention was relatively low. Successful surgical coronary artery bypass grafting and VSR repair were performed, and the patient was discharged. All clinical events are presented in Figure 2.
Discussion
VSR is a rare and fatal complication of AMI, with an incidence of approximately 1.3–2%.10 For patients with VSR, determining the surgical timing is challenging, and the treatment of cardiac shock is more complicated. In the acute stage, these patients are prone to cardiac shock and myocardial edema, leading to complex operations and high mortality rates.2,11 Recently, with the advancement of MCS, the hemodynamic stability of some patients with VSR can be achieved through mechanical assistance, which enables many patients to safely go through the acute phase and undergo delayed and selective surgery, thus avoiding unnecessary risks.12,13 Therefore, delayed and elective surgical procedures are strategies acknowledged by several researchers.
Extra-AVD as a form of MCS can help patients stabilize cardiac function and further bridge subsequent treatment. In foreign countries, the left ventricular assist device is implanted surgically.14 However, in our center, most extra-VAD implantation is minimally invasive. Minimally invasive extra-VAD implantation can improve patient outcomes.15
Following recent literature and guidelines16,17 and clinical practice experience, our center proposes a personalized deferred surgical treatment strategy for VSR, the details are shown in Figure 3. Mechanical circulatory support is preferred for patients with cardiogenic shock, and those with a good response can undergo surgical treatment approximately 30 days after the onset of MI; patients who respond poorly to mechanical circulatory support or experience circulatory instability during treatment require active emergency surgical intervention; and a few patients can have the symptoms of ventricular septal perforation relieved through drug treatment, maintaining a stable condition over a longer period. For these patients, selective surgical treatment can be considered approximately 30 days after MI, according to their conditions.
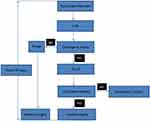 |
Figure 3 Delay and elective surgical treatment of VSR after AMI in Wuhan Union Hospital. Abbreviations: ECLS, extracorporeal life support (including IABP, Impella, Tandem Heart, ECMO, etc). |
To our knowledge, this is the first reported case in which an extra-VAD was used as a bridge to surgery for VSR after AMI with cardiogenic shock. Here, cardiogenic shock worsened, and pulmonary edema increased while the patient received IABP assistance. Subsequently, the IABP was removed, and an extra-VAD was chosen to assist left ventricular function and stabilize hemodynamics. The use of extra-VAD can ensure organ perfusion and provide opportunities for surgery. After 6 days of extra-VAD support, cardiogenic shock was alleviated. The patient’s general state and organ function were carefully evaluated. Finally, the patient underwent successful surgery and could be discharged from the hospital.
Accurate assessment of the hemodynamic condition, precise management during mechanical circulatory support-assisted treatment, and careful selection of the optimal timing for surgical strategies were key factors in achieving successful treatment in this case. Not all similar cases will have favorable results using this method. Further studies encompassing multiple centers, larger sample sizes, and prospective designs are needed to validate the findings of this study.
Conclusion
The extra-VAD provides a novel treatment approach for achieving hemodynamic stability and maintaining cardiac function in patients with VSR awaiting surgery. Further practical experience is necessary to enhance the application of this method.
Date Availability Statement
This is a case report without statistical analysis of the raw medical record data. All medical data involving the patient are documented in the patient’s medical record. If necessary, more detailed imaging data or laboratory data can be provided to the corresponding authors or the first authors.
Ethics Statement
As the dissemination and promotion of medical experience, the patient was very willing to submit his case data to a professional journal. Therefore, she signed an informed consent form to publish this case report. The present study involved human participants, and it was conducted considering ethical responsibilities according to the World Medical Association and the Declaration of Helsinki. Publication of the case details was approved by the Ethics Committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (ChiCTR2200055529).
Informed Consent for Publication
The patient agreed to publish her medical data, including imaging data and laboratory data, and signed informed consent.
Author Contributions
All authors made a significant contribution to the work reported, whether in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was not supported by any external funds.
Disclosure
All the authors declare that they have no conflicts of interest in this medical case report and have not received any financial support.
References
1. Papalexopoulou N, Young CP, Attia RQ. What is the best timing of surgery in patients with post-infarct ventricular septal rupture? Interact Cardiovasc Thorac Surg. 2013;16(2):193–196. doi:10.1093/icvts/ivs444
2. Xi EP, Zhu J, Zhu SB, et al. Percutaneous closure of a post-traumatic ventricular septal defect with a patent ductus arteriosus occluder. Clinics. 2012;67(11):1281–1283. doi:10.6061/clinics/2012(11)10
3. Zhao K, Li B, Sun B, Tao D, Jiang H, Wang H. Survival and risk factors associated with surgical repair of ventricular septal rupture after acute myocardial infarction: a single-center experience. Front Cardiovasc Med. 2022;9:933103. doi:10.3389/fcvm.2022.933103
4. Birnbaum Y, Fishbein MC, Blanche C, Siegel RJ. Ventricular septal rupture after acute myocardial infarction. N Engl J Med. 2002;347(18):1426–1432. doi:10.1056/NEJMra020228
5. Rihal CS, Naidu SS, Givertz MM, et al.; Society for Cardiovascular Angiography and Interventions (SCAI); Heart Failure Society of America (HFSA); Society of Thoracic Surgeons (STS); American Heart Association (AHA), and American College of Cardiology (ACC). 2015 SCAI/ACC/HFSA/STS clinical expert consensus statement on the use of percutaneous mechanical circulatory support devices in cardiovascular care: endorsed by the American Heart Association, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; Affirmation of Value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention. J Am Coll Cardiol. 2015;65(19):e7–e26. doi:10.1016/j.jacc.2015.03.036
6. Thiele H, Jobs A, Ouweneel DM, et al. Percutaneous short-term active mechanical support devices in cardiogenic shock: a systematic review and collaborative meta-analysis of randomized trials. Eur Heart J. 2017;38(47):3523–3531. doi:10.1093/eurheartj/ehx363
7. Thiele H, Ohman EM, De waha-thiele S, Zeymer U, Desch S. Management of cardiogenic shock complicating myocardial infarction: an update 2019. Eur Heart J. 2019;40(32):2671–2683. doi:10.1093/eurheartj/ehz363
8. Cheng R, Hachamovitch R, Kittleson M, et al. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: a meta-analysis of 1866 adult patients. Ann Thorac Surg. 2014;97(2):610–616. doi:10.1016/j.athoracsur.2013.09.008
9. Burkhoff D, Cohen H, Brunckhorst C, O’Neill WW; TandemHeart Investigators Group. A randomized multicenter clinical study to evaluate the safety and efficacy of the TandemHeart percutaneous ventricular assist device versus conventional therapy with intraaortic balloon pumping for treatment of cardiogenic shock. Am Heart J. 2006;152(3):469.e1–8. doi:10.1016/j.ahj.2006.05.031
10. Crenshaw BS, Granger CB, Birnbaum Y, et al. Risk factors, angiographic patterns, and outcomes in patients with ventricular septal defect complicating acute myocardial infarction. GUSTO-I (Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries) Trial Investigators. Circulation. 2000;101(1):27–32. doi:10.1161/01.cir.101.1.27
11. Zhu J, Xi EP, Zhu SB. Percutaneous closure of traumatic ventricular septal defects: device selection and reasoning. Clinics. 2014;69(2):150–151. doi:10.6061/clinics/2014(02)12
12. Li P, Wu L, Dong N. First experience of magnetically levitated extracorporeal left ventricular assist device for cardiogenic shock in China. ESC Heart Fail. 2022;9(2):1471–1473. doi:10.1002/ehf2.13769
13. Burkhoff D, Sayer G, Doshi D, Uriel N. Hemodynamics of mechanical circulatory support. J Am Coll Cardiol. 2015;66(23):2663–2674. doi:10.1016/j.jacc.2015.10.017
14. Zubair MH, Brovman EY. Lateral thoracotomy versus sternotomy for left ventricular assist device implantation. Curr Opin Anesthesiol. 2023;36(1):25–29. doi:10.1097/ACO.0000000000001211
15. Wachter K, Franke UFW, Rustenbach CJ, Baumbach H. Minimally invasive versus conventional LVAD-implantation-an analysis of the literature. Thorac Cardiovasc Surg. 2019;67(3):156–163. doi:10.1055/s-0038-1627455
16. O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61(4):485–510. doi:10.1016/j.jacc.2012.11.018
17. Arslan F, Bongartz L, Ten Berg JM, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: comments from the Dutch ACS working group. Neth Heart J. 2018;26(9):417–421. doi:10.1007/s12471-018-1134-0
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.