Back to Journals » Medical Devices: Evidence and Research » Volume 16
Extracorporeal Artificial Lungs: Co-Creating Future Technology – A Qualitative Analysis
Authors Dormann J , Wendt S, Dreher M, Ansems K, Rolland C, Spillner J, Szafran A, Breuer T, Pison C, Verbelen T, Benstoem C
Received 31 March 2023
Accepted for publication 10 July 2023
Published 28 July 2023 Volume 2023:16 Pages 201—210
DOI https://doi.org/10.2147/MDER.S415258
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Julia Dormann,1 Sebastian Wendt,1 Michael Dreher,2 Kelly Ansems,1 Carole Rolland,3 Jan Spillner,4 Agnieszka Szafran,1 Thomas Breuer,1 Christophe Pison,5– 7,* Tom Verbelen,8,* Carina Benstoem1,*
1Department of Intensive Care Medicine and Intermediate Care, Medical Faculty, RWTH Aachen University, Aachen, Germany; 2Department of Pneumology and Intensive Care Medicine, RWTH Aachen University, Aachen, Germany; 3Techniques de l’Ingénierie Médicale et de la Complexité (TIMC), Centre National de la Recherche Scientifique (CNRS), University Grenoble Alpes, Grenoble, France; 4Department of Thoracic Surgery, Medical Faculty, RWTH Aachen University, Aachen, Germany; 5Department of Pneumology and Physiology, CHU Grenoble Alpes; University Grenoble Alpes, Grenoble, France; 6Laboratory of Fundamental and Applied Bioenergetics, LBFA, Inserm1055, Grenoble, France; 7Département Universitaire des Patients Grenoble Alpes, University Grenoble Alpes, Grenoble, France; 8Department of Cardiovascular Sciences, KU Leuven and Department of Cardiac Surgery, University Hospitals Leuven, Leuven, Belgium
*These authors contributed equally to this work
Correspondence: Carina Benstoem, Department of Intensive Care Medicine and Intermediate Care, Medical Faculty, RWTH Aachen University, Pauwelsstr. 30, Aachen, D-52074, Tel +49 241 80 38038, Fax +49 241 80 33 82182, Email [email protected]
Background: Terminal lung diseases such as chronic obstructive pulmonary disease (COPD) and pulmonary hypertension (PH) in progression cause a large reduction in quality of life and may lead to bilateral lung transplantation (bLTx). An artificial portable lung could provide a bridge to lung transplantation, allowing patients to remain at home and mobile for longer. To advance the development of such an artificial lung, patient feedback is essential. The aim of this study is to analyze patient acceptance about an extracorporeal artificial lung and to implement these findings into the development.
Methods: In collaboration with a medical device developer, we presented a portable dummy oxygenator to patients with advanced lung disease, as potential end users. Data collection in Germany and France was based on two different methods: an online questionnaire and face-to-face interviews (F2F).
Results: A total of 604 participants answered the online questionnaire and 17 participants were included in the F2F interviews. The majority of participants (COPD n=140, PH n=17) were able to walk more than 1 km with a mean suffering pressure of 2.87 and 3, respectively. Six of the 17 F2F participants who could walk < 1 km were interested in an assistive device. The statistical value of Fisher’s exact test for suffering pressure and desire for a portable oxygenator was 0.45.
Conclusion: In patients with advanced lung disease, there is no statistically significant association between subjectively increased suffering pressure and desire for a portable oxygenator, so market introduction may be difficult. Potential end users should be implemented early in device development. Data collection via an online questionnaire combined with personal interviews has proven to be a successful approach here.
Keywords: chronic obstructive pulmonary disease, pulmonary hypertension, artificial lung, patient feedback
Introduction
Chronic Obstructive Pulmonary Disease (COPD) is a progressive, life-threatening lung disease characterised by breathlessness, coughing and susceptible to exacerbations and serious diseases.1,2 The prevalence of COPD has continuously increased in the last years and mortality continues to rise despite improved treatment options. COPD is the third leading cause of death worldwide with 3.000.000 deaths annually in 2019, with a prevalence of 251.000.000 patients in 2016.1 End-stage COPD patients are greatly affected in health-related quality of life.3 Available medication can only attenuate and prolong the disease and is very costly at 10.000 EUR per month.4
In severe COPD, hypoxia-related pulmonary hypertension is a prognosis-determining factor. Pulmonary hypertension (PH) results from restricted flow through the pulmonary circulation due to a pathological increase in pulmonary vascular resistance (PVR).5 The right ventricle (RV) generates higher pressures in the pulmonary circulation to overcome the elevated PVR, but cannot sustain this and will ultimately fail.5 Many lung diseases such as COPD, interstitial lung disease and alveolar hypoventilation syndromes lead to PH, compromising group 3 in the recently updated clinical classification of PH.5 Main symptoms are shortness of breath and exertional dyspnea. Around 1% of the global population is affected.6 Despite further advances in medical treatment, bilateral lung transplantation (bLTx) in terminal end-stages is the only curative option.7
COPD and PH patients face significant challenges with lung transplantation, primarily due to the limited availability of suitable organs and long waiting lists.8,9 Demand for lungs exceeds supply, resulting in long waiting times that exacerbate patients’ deteriorating respiratory conditions. This demonstrates the urgent need for solutions to address the organ shortage and speed up the transplantation process or to develop alternative treatment options. Research in the field of artificial lungs, although still in its infancy compared to ventricular assist devices, shows promise.10–12
Noninvasive ventilation has already been shown to recompensate ventilatory failure in severe hypercapnia and then lead to improvement in quality of life and exercise capacity. To advance the development and acceptance of artificial lungs, patient feedback is a fundamental component. Therefore, the aim of this study is to analyze patient acceptance and their insights towards a dummy of an extracorporeal artificial lung. A particular focus was on the health status and perceived suffering pressure of COPD and PH patients in general, making the results relevant for other manufacturers as well.
Methods
Study Design
In cooperation with a medical device developer, we presented a dummy of a specific portable oxygenator, which is still under development, to potential end-users. The intention was to get initial patient insights before clinical testing starts to see if there is an overall uptake from potential end-users. The device is an extracorporeal assist device which is intended to take over the function of the lungs and is supposed to function without a blood pump. For this purpose, the device is sutured to large blood vessels during open chest surgery and cannot simply be removed or replaced. So far, such products do not exist.
The data collection was based on two different methods, an online questionnaire and face-to-face (F2F) interviews. The online questionnaire was conducted to get insight in the patients’ suffering and mobility. F2F interviews were conducted in Germany and France to determine the extent to which patients suffering from COPD or PH would accept a portable oxygenator and their wishes for changes. The study protocol was approved by the Ethics Committee at the RWTH Aachen Faculty of Medicine, Germany (Red. No. EK126/19) and the Ethics Committee in Strasbourg, France (CPP Est IV Ref SI. 19.06.06.57116 IDRCB 2019-A01478-49). This sub-study was part of project “20664 RAS-Q: Bringing care home for COPD patients” that has received funding from European Institute of Innovation and Technology (EIT) Health. EIT Health is supported by the European Institute of Innovation and Technology, a body of the European Union receives support from the European Union´s Horizon 2020 Research and innovation programme. EIT Health had no role in study design, data collection, data analysis, data interpretation or writing of the study report. The study was conducted in accordance with the Declaration of Helsinki.
Setting
The online questionnaire was distributed in Germany and France and was available from 10.05.2019 to 01.11.2019. In Germany, it was distributed by snowball sampling. An e-mail containing a short summary of the objective, the link to reach the survey, a request to forward the information about the online questionnaire, with an information flyer for printing was sent. Additionally, we advertised the online questionnaire in German social media. An internet search was conducted to locate the e-mail addresses of the target group. In France, patients of an ambulatory care service named “AGIR á dom” based in Grenoble were asked to participate in the online questionnaire.
The recruitment for the F2F interviews occurred, in Germany, in the Clinic for Pneumology and Internal Intensive Medicine (Medical Clinic V) at the University Hospital RWTH Aachen. In France patients had been informed about the content of the interviews by the care service AGIR á dom.
Participants
Inclusion criteria for the online questionnaire were either COPD patients, PH patients or family members of both patient populations. Participants had to be at least 18 years old and able to speak either English, French, German or Dutch, as the questionnaire was available in these languages. This patient population was chosen to support the results of the F2F interviews and to get an overview of the suffering and desires of the patients and family members.
Inclusion criteria for the F2F interviews were: participant suffered from COPD or PH, possible end-users of a portable oxygenator, at least 18 years old and French or German native speaker, as the interviews were conducted in French or German. One participant suffers from both COPD and PH, since the PH disease was caused by the COPD disease, he is counted as a PH patient in the results section.
Data Sources/Measurements
The online questionnaire was created with the software LimeSurvey. First, the age of the participants was asked, because they had to be at least 18 years old. Then, the questionnaire was divided into three categories in order to create patient group categories: COPD patients, PH patients and family members each patient group. Further questions were: demographic data, mobility, smoke status, suffering pressure. Most of the questions were designed as single choice questions. The question regarding their average level of suffering should be rated with a scale from one to six, with one being the lowest and six being the highest level of suffering. The suffering pressure is intended to represent the respondent’s individual level of suffering, from low to high, in relation to their current condition. Before evaluation, a plausibility check was performed to exclude out-of-norm records. By submitting the questionnaire, consent was given to anonymous data processing.
In order to conduct semi-structured F2F interviews in which participants are encouraged to speak freely, pulmonologists and researchers developed an interview guide to increase the interviewer’s flexibility.13 Participants were asked about the average walking distance, feedback on the dummy of the portable oxygenator and their average level of suffering, which they were asked to indicate on a scale of one to five, with one being the lowest and five being the highest. In Germany, as soon as patients fulfilling the inclusion criteria were admitted as hospitalized, the treating physician informed the patient about the study and waited for consent. In France patients were informed about the content of the interviews by an outpatient care service. After given informed verbal consent and approval that the data would be processed anonymously, the interview started. Informed consent for the study was obtained from the treating physician as part of routine clinical practice. Considering that the interview was conducted in a completely anonymous manner, preventing any inferences about the patient’s identity, the ethics committee recommended the implementation of an informed verbal consent procedure. The consent provided by the participant was duly recorded in their medical records. During the interviews, a dummy of a potential portable oxygenator was shown, and the potential functioning was explained. The dummy corresponds to the original size, weight and carrying system of the oxygenator which is currently being developed. During the interviews, it was possible to touch, lift and wear the dummy, to improve the evaluability of the device. The interviewer explained how to wear the device as well as its exact functioning. The system requires a direct, permanent link to the blood system. The connection to the body is made via cannulas or vascular prostheses, which are sutured to the blood vessels. Consequently, the blood circulation is expanded outside the body. The device should be worn in front of the abdomen using the carrying system (see Figure 1). The system weighs about 2 kg.
 |
Figure 1 Person (1.54m height) wearing the dummy. |
Statistics
The data of the online questionnaire were evaluated descriptively with the SPSS software (V26, 2019). P-values below 0.05 were considered statistically significant. Continuous variables were reported as means ± standard deviation. Comparison between suffering pressure and acceptance of the device was performed with one-sided Fisher´s exact test. Therefore, values of suffering pressures between one and three were categorized as low and values between four and five as high.
The data of the F2F interviews were evaluated following the Framework analysis by Green and Thorogood.14 After familiarization with the data, identifying themes, data coding, organizing codes and themes the data were merged and mapped and interpreted using Microsoft Excel (Microsoft 2010).
Results
Participants
Online Questionnaire
The link to the online questionnaire was sent by email to 99 self-help groups, 306 pulmologists, to all German professional associations of pulmologists and to 606 lung sports groups in Germany.
During the collection period, the online questionnaire was accessed n=609 times. After a plausibility check of the given answers five records were removed, resulting in a total sample of 604 participants. Participant characteristics are provided in Table 1. Family members were not asked about their weight or height, thus reducing the total for the height and weight data by n=71 participants. Most of the questionnaire participants were female (55.79%), living in Germany (54.47%) and diagnosed with COPD (65,23%).
 |
Table 1 Characteristics Table |
F2F Questionnaire
The F2F interviews involved 17 participants in total, five COPD and two PH patients in Germany and four COPD, six PH patients in France. All participants answered the height, weight, and age questions, the mean values of which are shown in the characteristics table (see Table 1).
Walking Distance and Suffering Pressure
In total 93 COPD patients and 13 PH patients were able to walk <200 m, with a mean suffering pressure of respectively 4.27 and 4.62.
In total 74 COPD patients and 14 PH patients were able to walk between 200 m and 500 m, with a mean suffering pressure of respectively 3.72 and 3.71.
In total 69 COPD patients and 9 PH patients were able to walk between 500 m and 1 km, with a mean suffering pressure of respectively 3.52 and 2.22.
In total 140 COPD patients and 17 PH patients were able to walk >1 kilometer, with a mean suffering pressure of respectively 2.86 and 3.
Online Questionnaire
A total of 101 participants (90 COPD patients, 11 PH patients) were not able to walk 200 m, with an average suffering pressure of around 4.26 (SD=0.85) for COPD patients and 4.73 (SD=0.45) for PH patients (see Figure 2). Participants (n=84, 72 COPD patients, 12 PH patients) who could cover a walking distance between 200 m and 500 m had an average suffering pressure of 3.72 (SD=0.89) for COPD patients and 3.92 (SD=0.86) for PH patients. Participants (n=75, 67 COPD patients, 8 PH patients) who could cover a walking distance between 500 m and 1 km had an average suffering pressure of around 3.55 (SD=0.90) for COPD patients and 2.38 (SD=1.12) for PH patients. Most respondents, around 25.33% (n=153, 139 COPD patients, 14 PH patients), could overcome a walking distance of more than one kilometer and have an average suffering pressure of around 2.87 (SD=1.10) for COPD patients and 2.86 (SD=1.12) for PH patients. This question was answered by 413 participants.
 |
Figure 2 Walking distance in relation to the mean suffering pressure. |
F2F Questionnaire
A total of five participants (3 COPD patients, 2 PH patients) were able to walk less than 200 m, with an average suffering pressure of 4.67 (SD=0.47) for COPD patients and 4 (SD=1) for PH patients (see Figure 2). Participants (n=4, 2 COPD patients,2 PH patients) who could cover a walking distance between 200 m and 500 m had an average suffering pressure of 3.5 (SD=0.5) for COPD patients and 2.5 (SD=1.5) for PH patients. Participants (n=3, 2 COPD patients, 1 PH patient) who could cover a walking distance between 500 m and 1 km had an average suffering pressure of 2.5 (SD=0.5) for COPD patients and 1 (SD=0) for PH patients. A total of four respondents (1 COPD patients, 3 PH patients) could overcome more than one kilometer and have an average suffering pressure of 2 (SD=0) for COPD patients and 3.67 (SD=0.94) for PH patients. One participant did not answer the question about the walking distance but indicated an average suffering pressure of 3.
Suffering Pressure and Average Need of Help from Relatives Perspective
Relatives who participated in the online questionnaire were asked to rate the suffering pressure of their COPD or PH relative on a scale of 1 to 6. They were also asked how often the relative needs help in everyday life on average per week, with 1 being the lowest and 6 being the highest. Of the relatives, 25.35% participants (n=18) indicated that their relative did not need regular help and the level of suffering pressure was estimated to be around 2.39 on average (SD=1.11) (see Figure 3). There were 12.68% (n=9) of the participants who indicated that their relative needed help 1 to 2 days per week and the average level of suffering pressure was estimated to be around 4.11 (SD=1.07). There were 7.05% (n=5) of the participants who indicated that their relative needs help 3–4 days per week and has an average suffering pressure of 4.00 (SD=0.63). Another 26.76% of the relatives (n=19) indicated that their relative needs assistance every day with an average level of suffering pressure around 3.68 (SD=1.34). A total of 9.86% participants (n=7) indicated that they cannot leave their relative alone and the average level of suffering pressure was reported to be around 4.71 (SD=0.88). 18.31% of the participants (n=13) did not answer the question about the frequency of help. Of these, 10 participants gave an indication of the average suffering pressure, which is on average 4.20 (SD=1.66).
 |
Figure 3 Average need of help peer week in relation to the mean suffering pressure of relatives. |
Acceptance of the Device
The overall participants mean level of suffering in the F2F Interviews is around 3.29. The study participants were asked whether they could imagine having the described medical device inserted and undergoing the corresponding surgery. Participants who were interested in an assisting device had a mean suffering pressure of around 3 (SD=1.15) (see Figure 4). The suffering pressure of people who are not interested in an assisting device is on average around 3.33 (SD=1.33). Out of four patients who have reported a suffering pressure of five, three are not interested in an assistance system, one participant answered maybe. The Fishers exact test regarding the correlation between suffering pressure and the desire of an oxygenator is statistically not significant, p=0.45.
 |
Figure 4 Mean suffering pressure in relation to the answer if they would undergo the surgery for the implantation of the oxygenator. |
Of four patients able to walk more than 1 km, three of them were interested in the concept of the portable oxygenator and one is not, but at “a last resort” the patient would accept such a system (see Figure 5).
 |
Figure 5 Walking distance in relation to the answer if they would undergo the surgery for the implantation of the oxygenator. |
Of three patients able to walk between 500 m and 1 km, two of them were interested in the concept of the portable oxygenator and one is not. One patient answered: “it is making think about the illness a lot”.
Out of 17 participants interviewed, nine rejected the device, because they perceived it as too heavy (n=2), have not commented their answer (n=2), because of the anaesthesia (n=2), the level of suffering was not yet high enough (n=2) or felt they were too old (n=1).
During the interview, 11 patients described the device as too heavy. One participant commented: “It’s very heavy, cumbersome, and this weight I would carry it if I had to, for 1h. Without doing extraordinary things”. Another participant answered that he “would put it nearby as soon as possible”.
In total 14 participants want more flexibility in how the device is carried: that the device can be carried in another way (n=10), that it can be removed for sleeping (n=1), that it would be hidden inside the body (n=2), that it can be turned down (n=1). The back was often mentioned as an example of other forms of carrying, comparable to a backpack that you can take off for sitting or attach to a trolly or wheelchair.
Feedback for the Manufacturer
As a final question, participants in the F2F interviews were asked for any feedback for the manufacturer of the product with requests for changes; for better clarity, the responses have been summarized in Table 2. In total, six participants did not have a recommendation.
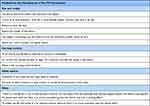 |
Table 2 Feedback for the Manufacturer of the F2F Participants (Headings and Categories in Bold) |
Discussion and Conclusion
The aim of this study is to analyze the acceptance of patients and their attitude towards an extracorporeal artificial lung using a tool suitable for this purpose. In general, the current state of health and the classification of the individual suffering pressure is very individual. But in average the suffering pressure is higher, the more the ability to move, especially their walking distance is limited. This is true for the participants of the online questionnaire as well as for the F2F questionnaire. The relatives also support the assumption: the worse the general condition and the more help is needed, the suffering pressure is estimated to be the highest. However, it could not be shown that increased suffering pressure and limited mobility automatically lead to a willingness of the participants of the F2F questionnaire to undergo surgery for a portable oxygenator. The invasive surgical procedure tended to have a deterrent effect on participants. Participants engaged very actively with the questionnaire and indicated many requests for changes that would lead to greater acceptance of the oxygenator. Despite statements like “if it improves everyday life, the design is secondary”, the design seems to play a major role. The most important result of the questionnaire and the F2F interviews is that it would be difficult to bring the presented device to the market. The manufacturer should redesign the device, into one that is lighter and removable.
A limitation of the study is that the online questionnaire was only sent to those e-mail addresses that were available on the Internet and in some cases were no longer active. Furthermore, we do not know to what extent the questionnaire was distributed by the snowball effect as planned. In France, only one ambulatory care service was commissioned to distribute the questionnaire.
Further, the size of the F2F survey was limited and the questionnaire was conducted in two different languages. Also, the participants did not confirm the correctness of their information after transcription and mapping.
When comparing the two questionnaires, there is the limitation that the online questionnaire asked about distress on a scale of one to six and the F2F questionnaire asked on a scale of one to five.
A lot of research has already been done to develop the dummy, but patients should have been involved in the development of the device at an earlier stage.
In planning the F2F interviews and online questionnaire, we unsystematically searched for comparable studies. In planning these interviews and the survey, we did not find any comparable examples reporting that potential end users had been involved this early in the medical device development process in general, but we were able to show that this would be necessary. If this were the case, the very expensive and time-consuming development process could be carried out more efficiently and in a more targeted manner, and, if necessary, could be adapted to the needs of potential end users at an earlier stage. There is still a need for research in this area.
Disclosure
Dr Sebastian Wendt reports he is a full time employee at Abiomed Europe GmbH since July 2020 which was for him after the relevant time for this project. None of the authors have any relevant conflict of interest to declare.
References
1. WHO. Chronic obstructive pulmonary disease (COPD). Available from: https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd).
2. Vestbo J. COPD: definition and phenotypes. Clin Chest Med. 2014;35(1):1–6. doi:10.1016/j.ccm.2013.10.010
3. Siafakas NM. Management of Chronic Obstructive Pulmonary Disease. Sheffield, UK: ERJ Open Research; 2006.
4. Byng D, Lutter JI, Wacker ME, et al. Determinants of healthcare utilization and costs in COPD patients: first longitudinal results from the German COPD cohort COSYCONET. Int J Chron Obstruct Pulmon Dis. 2019;14:1423–1439. doi:10.2147/COPD.S201899
5. Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1):1801913. doi:10.1183/13993003.01913-2018
6. Hoeper MM, Ghofrani H-A, Grünig E, et al. Pulmonary Hypertension. Dtsch Arztebl Int. 2017;114(5):73–84. doi:10.3238/arztebl.2017.0073
7. George MP, Champion HC, Pilewski JM. Lung transplantation for pulmonary hypertension. Pulm Circ. 2011;1(2):182–191. doi:10.4103/2045-8932.83455
8. Hull TD, Leya GA, Axtell AL, et al. Lung transplantation for chronic obstructive pulmonary disease: a call to modify the lung allocation score to decrease waitlist mortality. J Thorac Cardiovasc Surg. 2022;164(4):1222–1233.e1211. doi:10.1016/j.jtcvs.2021.11.086
9. Verleden GM, Gottlieb J. Lung transplantation for COPD/pulmonary emphysema. Eur Respir Rev. 2023;32(167):220116. doi:10.1183/16000617.0116-2022
10. Naito N, Cook K, Toyoda Y, et al. Artificial Lungs for Lung Failure: JACC Technology Corner. J Am Coll Cardiol. 2018;72(14):1640–1652. doi:10.1016/j.jacc.2018.07.049
11. Orizondo RA, Cardounel AJ, Kormos R, et al. Artificial Lungs: current Status and Future Directions. Curr Trans Rep. 2019;6(4):307–315. doi:10.1007/s40472-019-00255-0
12. Camboni D, Philipp A, Arlt M, et al. First Experience With a Paracorporeal Artificial Lung In Humans. ASAIO J. 2009;55(3):304–306. doi:10.1097/MAT.0b013e31819740a0
13. Pope C, Mays N. Qualitative research in health care. BMJ. 2006;320:114.
14. Green JTN, Thorogood, N. Qualitative Methods for Health Research.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
