Back to Journals » Infection and Drug Resistance » Volume 11
Extensively drug-resistant Acinetobacter baumannii and Proteeae association in a Romanian intensive care unit: risk factors for acquisition
Authors Muntean D , Licker M , Horhat F , Dumitrașcu V, Săndesc D, Bedreag O, Dugăeșescu D, Coșniță DA, Krasta A , Bădițoiu L
Received 27 April 2018
Accepted for publication 29 August 2018
Published 8 November 2018 Volume 2018:11 Pages 2187—2197
DOI https://doi.org/10.2147/IDR.S171288
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Joachim Wink
Delia Muntean,1,2 Monica Licker,1,2 Florin Horhat,1 Victor Dumitrașcu,1 Dorel Săndesc,1,2 Ovidiu Bedreag,1,2 Dorina Dugăeșescu,1 Dan A Coșniță,1 Anca Krasta,2 Luminița Bădițoiu1
1Victor Babeș University of Medicine and Pharmacy, Timisoara, Romania; 2Pius Brînzeu Emergency Clinical County Hospital, Timisoara, Romania
Purpose: The purpose of this study was to identify risk factors for extensively drug-resistant (XDR) Acinetobacter baumannii (AB) and XDR Proteeae association in the largest intensive care unit (ICU) in Western Romania.
Materials and methods: This retrospective case-controlled study was conducted between January 2016 and December 2016 in the ICU of the “Pius Brînzeu” County Emergency Clinical Hospital of Timișoara. Data were collected, in strict confidentiality, from the electronic database of the Microbiology Laboratory and the hospital’s electronic medical records. Risk factors were investigated by logistic regression. Independent variables with P≤0.05 and OR >1 (95% CI >1) in the univariate analysis were entered into multivariate sequenced analysis.
Findings: The incidence density of coinfection with XDR AB and XDR Proteeae was 5.31 cases per 1,000 patient-days. Independent risk factors for the association of XDR AB and XDR Proteeae were represented by the presence of tracheostomy and naso-/orogastric nutrition ≥ 8 days. In addition, pressure ulcers were independent predictive factors for infections with all three infection types. Previous antibiotic therapy was an independent risk factor for the acquisition of XDR-AB strains, alone or in association, while the prolonged hospitalization in the ICU, blood transfusion, and hemodialysis appear as independent risk factors for single infections.
Conclusion: This association of XDR AB and XDR Proteeae may well not be limited to our hospital or our geographical area.
Keywords: extensive drug resistance, ICU, infections, risk factors
Introduction
Multidrug-resistant organisms (MDROs) responsible for health care-associated infections (HCAIs) have become prevalent and their association has become increasingly common with devastating outcomes. On the other hand, the increased use of broad-spectrum antimicrobial drugs selects MDROs. In the intensive care unit (ICU), acquisition of MDROs depends not only on antimicrobial use but also upon the severity of the illness, invasive procedures, infected or colonized patients with MDROs, and ICU contact pressure.1–9
Infections due to Acinetobacter baumannii (AB) have been detected mainly in critically ill patients and are associated with an increased risk of mortality.7 AB has become the prototype of extensively drug-resistant (XDR) pathogens, being sensitive to only a few antimicrobial agents, and of late, worrying trends have started to occur.10,11 The average percentage of invasive strains with combined resistance (to fluoroquinolones, aminoglycosides, and carbapenems) in European Union (EU)/European Economic Area (EEA) was 31.7% in 2016, while 4% were resistant to colistin, mostly in Southern Europe, Southeastern Europe, and the Baltic countries.12 According to EARS-Net data, in 2015, 12 of 27 EU/EEA countries recorded a level of 50% or higher for carbapenem-resistant AB strains.13 Most of these strains are involved in the etiology of HCAIs such as ventilator-associated pneumonia, postoperative wound infections, urinary tract infections associated with permanent catheterization, blood stream infections, or meningitis associated with ventricular shunt.
Colistin and tigecycline remain the only effective drugs for the management of carbapenem-resistant AB strains.14–16 However, besides the emergence of colistin resistance and nephrotoxicity/neurotoxicity issues, treatment with colistin raises the issue of superinfection with other pathogens that are naturally resistant to colistin, such as Proteeae, Serratia marcescens, Pseudomonas mallei, and Burkholderia cepacia.17,18 In addition, when deciding upon empirical treatment for the association of XDR AB and enterobacteria with natural resistance to colistin and tigecycline, the clinician needs to take this resistance into consideration.
Recent studies have shown that certain Romanian ICUs are experiencing a high incidence of infection with multidrug-resistant (MDR) AB and Proteus mirabilis.19 According to the CARMIN-ROM study, performed in 2015, carbapenem resistance to AB-invasive isolates was 82.1%, which places Romania on the third place among EARS-Net countries.20
The aim of this study was to identify the risk factors for XDR AB and XDR Proteeae association in the largest ICU from Western Romania.
Materials and methods
Setting and study design
This retrospective case–control study was conducted over a period of 1 year, between January 2016 and December 2016, in the “Pius Brînzeu” County Emergency Clinical Hospital of Timișoara with 1,100 beds and an ICU with 27 beds dedicated to both medical and surgical pathologies.
All patients who were admitted to the ICU during the study period were evaluated, except for those with a length of stay of less than 1 day in the ICU or those who had positive cultures before/upon admission to the ICU. Four subsamples of patients were considered according to the pathogens involved:
- S I – all patients identified with HCAIs caused by the association of XDR AB and XDR Proteeae;
- S II – all patients identified with HCAIs caused only by XDR AB;
- S III – all patients identified with HCAIs caused only by XDR Proteeae; and
- S IV – patients matched by age group, ward, and hospitalization date with cases from S I, but who did not develop infections.
Demographics and risk factors
Data were collected in strict confidentiality from the electronic database of the Microbiology Laboratory and the hospital’s electronic medical records. Owing to the retrospective design of the study, informed consent was not required, but the study was approved by the ethics committee of the “Pius Branzeu” Timisoara Emergency Clinical County Hospital (ref. no. 130/13, September 2017).
The following data were collected: gender, age, the clinical ward from which patients were transferred, length of stay in the ICU, discharge status, infection type, risk factors (mechanical ventilation, central venous catheterization, urinary catheterization, tracheostomy, gastrostomy, use of dialysis, blood transfusion, vasopressor therapy, presence of wounds or pressure ulcers, duration of antibiotic use prior to isolation of XDR AB and XDR Proteeae), immune status (immunosuppressive pathology, radiotherapy/chemotherapy in the last 3 months), comorbidities, and physical status at the time of admission.
HCAIs were defined according to 2012/506/EU European Parliament Decision, implemented at the national level.21
Inclusion in The American Society of Anesthesiologists Physical Status Classification System has complied with the definitions of the latest approved version of October 15, 2014.22
Comorbidities were quantified using the age-adjusted Charlson Comorbidity Index, with the inclusion of the following: diabetes; mild/moderate to severe liver pathology; malignancy; chronic kidney diseases; cardiac, pulmonary, or peripheral vascular, cerebrovascular, hematological diseases; dementia; gastroduodenal ulcer; and HIV infections.23
Improved evolution was defined as removing the patient from mechanical ventilation and balancing hemodynamic, acid–base, and electrolytic statuses, while stationary evolution was used when the patient’s evolution did not change after the time of admission to the ICU.
Case fatality rate was defined as the number of deaths due to a specific disease among patients with this pathology. The fatality attributable to health care-associated pathology was calculated as the difference between the fatality recorded in the sample of cases and the fatality recorded in the control sample.
The incidence density was defined as the number of new cases that occurred in the ICU during a defined period per total number of patient-days during a defined period multiplied by 1,000.
Laboratory methods
Microbiological identification was done according to morphological, cultural, and biochemical characteristics. The antimicrobial sensitivity tests were performed by automated Vitek 2 system (bio-Mérieux, Marcy l’Etoile, France) according to the Clinical and Laboratory Standards Institute (CLSI).24
According to a study by Magiorakos et al,10 XDR pathogens were defined as being non-susceptible to at least one agent in all but two or fewer antimicrobial categories (colistin and minocycline for AB and carbapenems or amikacin or fluoroquinolones for Proteeae). The phenotypic confirmation of extended spectrum beta-lactamases (ESBL) production was done using the synergy test between extended-spectrum cephalosporins and clavulanic acid. Carbapenemase production was demonstrated by combined disc methods (KPC, MBL and OXA-48 Confirm kit; Rosco Diagnostica, Taastrup, Denmark).24–27
Statistical analyses
The database was analyzed using the IBM SPSS Statistics 20. Continuous numeric variables were characterized by mean values and 95% CI, and the category type was characterized by value and percentage. Testing data distribution was performed using the Shapiro–Wilk test. Comparison of category variables was performed by the chi-squared test with Fischer’s exact test, and the Mann–Whitney U test was used for continuous variables. Risk factors for infections were investigated by logistic regression. Independent variables with P≤0.05 and OR>1 (95% CI>1) in the univariate analysis were entered into multivariate sequenced analysis. Only variables that clearly met the risk factor criteria were included to reduce the intervention of hazard as much as possible. To avoid collinearity, only independent variables were included. Model selection was performed based on the Nagelkerke R2 coefficient and the deviation from the theoretical model, estimated by the Hosmer and Lemeshow goodness of fit test. Statistical significance was calculated by two-tailed tests, and the significance threshold was set at P-values ≤0.05.
Results
Descriptive data
Of the 998 patients admitted to the ICU during the study period, 50 met the inclusion criteria in S I, 53 in S II, and 52 in S III, and the control group consisted of 112 uninfected patients. At a total of 9,416 patient-days, co-infection with XDR AB and XDR Proteeae recorded an incidence density of 5.31 cases per 1,000 patient-days. Infections with single XDR-AB and XDR Proteeae strains were identified with a similar incidence density of 5.63 and 5.52 cases per 1,000 patient-days, respectively. The sample characteristics and exposure to possible predictive factors are listed in Table 1.
Case fatality rate was 88% for patients with co-infection, 54.72% for those included in the S II, 57.69% for those included in the S III, and 31.25% for those included in the S IV. Thus, the fatality attributable to health care-associated pathology in S I (56.75%) was twice as high as the one recorded in S II (23.47%) or S III (26.44%).
Hospital-acquired (HA) pneumonia was present in 82% of cases in S I, as well as 66.04% in S II, and 51.92% in S III (P=0.001), considering that preexisting lung disease such as COPD was identified in only 2.00% of the cases in S I, 5.66% of the cases in S II, 5.77% of the cases in S III, and 3.57% of the cases in S IV, and bronchiectasis was not found in any of the 267 patients included in the four samples. HA urinary tract infections were more common in S III (P=0.001), and surgical site infections were significantly more prevalent among patients in S II (P=0.033).
In S I, 36.00% (n=18) of the patients received colistin, despite the fact that Proteeae strains are naturally resistant to this drug.
Univariate analysis
To highlight the risk factors, we compared not only S I, S II, and S III with S IV but also S I with S II and S III. Univariate analysis identifies predictive factors for S I, presented in Table 2.
OR corroborated to 95% CI, and statistical significance resulted in the following categories of predictive factors:
- Risk factors for all three infection types: tracheostomy, pressure ulcers, blood transfusion, central catheterization ≥8 days, hospitalization in ICU ≥8 days, urinary catheterization ≥8 days, naso-/orogastric nutrition ≥8 days, mechanical ventilation >2 days, and broad-spectrum cephalosporin or carbapenem administration;
- Single infection risk factors: general surgery for XDR-AB infection, hemodialysis for XDR-AB infection, and immunosuppressive pathology both for S II and S III;
- Co-infection-associated risk factors: gastrostomy, nasogastric nutrition, mechanical ventilation, and tigecycline administration. Comparative analysis of S I vs S II additionally highlights neurosurgical interventions as a risk factor for the association studied (OR=2.70 [1.21–6.01], P=0.018);
- Single- and co-infection-associated risk factors: previous empirical antibiotherapy, vasopressor therapy, and colistin administration for XDR-AB strains; ASA >5 and piperacillin/tazobactam (PIP/TAZ) administration for XDR-Proteeae strains.
Multivariate analysis
Multivariate analysis through logistic regression identified the presence of tracheostomy and naso-/orogastric nutrition ≥8 days as significant, independent of co-infection risk factors (Table 3). Pressure ulcers (the consequence of prolonged bed rest) are independent predictive factors for all three studied types of infections produced by MDROs. Previous antibiotic therapy was an independent risk factor for the acquisition of XDR-AB strains, alone or in association, while the prolonged hospitalization in the ICU, blood transfusion, and hemodialysis appeared as independent risk factors for single infections.
  | Table 3 Multivariate analysis of risk factors Abbreviation: ICU, intensive care unit. |
Discussion
This study, performed to highlight the risk factors involved in the association of XDR AB and XDR Proteeae, was conducted in a Romanian ICU, which faces a high incidence of MDROs, both AB and Proteeae species. In another study conducted in the same unit between 2012 and 2013, the incidence density rate for MDR AB was 4.68 per 1,000 patient-days and for the ESBL-producing P. mirabilis, it was 4.17 per 1,000 patient-days.19 This epidemiologic status persists despite the fact that cases reported by hospital departments (surgical departments included) are sporadic (both regarding HCAI and bacterial multiresistance) in a combined active and passive surveillance system. We mention that the hospital is included in the national sentinel system for the identification of HCAI in high-risk departments and the antimicrobial resistance patterns of strains causing invasive infections. This endemic situation of MDROs is not punctiform, especially in ICUs of Southeast Europe.12,13
In this context, identifying risk factors for the association of XDR AB and XDR Proteeae could influence the therapeutic course for patients hospitalized in the ICU, where any deterioration of the general condition due to infectious pathology, attacking a profoundly affected condition, may result in the loss of the patient. Surveillance data of MDROs could help the clinician to establish the appropriate empiric therapy. The intensive use of colistin or tigecycline in the context of increased incidence of carbapenem-resistant strains favors the emergence of natural resistant microorganisms, such as Proteeae.
Descriptive analysis shows that, from a demographic perspective, patients co-infected with XDR AB and XDR Proteeae were the youngest, with statistically significant differences, vs those infected with only XDR AB (P=0.041).
The association of pathogens resulted in triple than average ICU hospitalization time when compared to single infections with AB strains (45.26 vs 13.47 days), more than two times longer hospitalization when compared to that of single infection with Proteeae strains (45.26 vs 20.58 days) and more than 10 times longer hospitalization when compared to that in the control sample (which was 4.43 days).
Analyzing the origin of patients included in S I, it was noticed that ~50% of patients were transferred from neurosurgery (38%) and neurology (10%), which suggests impairment of consciousness, the need for assisted ventilation, tracheostomy, naso-/orogastric nutrition, and a prolonged length of hospital stay. Emergency ICU hospitalization, directly from the emergency room, also involves a severe pathology with a high probability of multiple invasive diagnostic or therapeutic maneuvers.
Comorbidity analysis correlated with physical status showed that patients infected with association of pathogens (S I) had the lowest level of chronic pathology (correlated with the lowest age). The mean value of the Charlson Comorbidity Index adjusted for age was 1.96. In contrast, the average ASA Physical Status Classification System was 4.88 and placed them at the limit between severe systemic pathology with vital risk and death, with little chance of survival in the absence of surgery. The uninfected control sample consisted of patients with severe systemic disease (lowest ASA: 3.89), but with the most important comorbidities (Charlson Comorbidity Index adjusted at age was 3.62).
The association between XDR AB and XDR Proteeae increased the average number of antibiotherapy days by more than six times compared to the antibioprophylaxis in S IV, by more than two times compared to the antibiotherapy period revealed in S III, and 2.5 times compared to the antibiotherapy period revealed in S II. According to internal guidelines, cefuroxime was predominantly used in perisurgical antibiotherapy protocols, while in the ICU, the empirical therapy of patients with clinical and paraclinical signs of infection accompanied by deterioration of the clinical condition, third-generation cephalosporins, PIP/TAZ, or carbapenems were administered. Colistin or tigecycline was administered to patients in whom carbapenem-resistant genes were identified by direct examination of the bronchial aspirate with the GeneXpert System (Cepheid, Sunnyvale, CA, USA). The final therapy of infections with these XDR pathogens included the association of colistin or tigecycline with carbapenems, aminoglycosides, or fluoroquinolones, depending on the sensitivity of pathogens, the infection site, and the renal function of the patient.
In terms of invasive procedures at risk of HCAI pathology, the average number of days of mechanical ventilation in S I increased by three times compared to that in S II and S III (31.94 days vs 10.34/9.69 days). All patients in the four samples were subjected to invasive mechanical ventilation, but for different time periods, and some of them later benefited from noninvasive intermittent ventilation. The average number of days with central catheterization increased by more than three times (43.14 days vs 13.47/13.02 days). Urinary catheterization duration increased by 3.46 times compared with that of S II and 2.28 times compared with that of SIII (46.42 days vs 13.40/20.29 days). Naso-/orogastric nutrition duration increased by more than five times (39.18 days vs 6.75/7.75 days).
In this study, the presence of tracheostomy and naso-/orogastric nutrition ≥8 days was not identified as co-infection risk factors. Previous antibiotic therapy was an independent risk factor for the acquisition of XDR-AB strains, associated or not. Only in univariate analysis, the administration of large-spectrum cephalosporins and carbapenems has been identified as a predictive factor for all three types of infection. Previous administration of colistin was a predictive factor for the acquisition of XDR-AB strains, associated or not, and the PIP/TAZ prescription was a predictive factor for the XDR-Proteeae infection. Tigecycline was the only antimicrobial agent close to the co-infection prediction threshold.
Numerous studies have assessed antibiotic-associated risk factors for infection with MDR AB. Three classes of them have been most frequently implicated: third-generation cephalosporins, carbapenems, and fluoroquinolones.28
In a Lebanese ICU, Moghnieh et al29 identified four parameters as independent risk factors for acquisition of XDR AB: urinary catheter placement for >6 days, presence of gastrostomy tube, use of carbapenems or PIP/TAZ, and ICU contact pressure for >4 days.
A case–control study published in 2014 identified in univariate analysis the following risk factors for XDR AB-associated HCAIs: bed rest over 30 days, hemodialysis with catheter placement, tracheotomy and prior use of glycopeptides, carbapenems, PIP/TAZ, and fourth-generation cephalosporins. In the multivariate analysis, independent factors have been observed: bed rest for 30 days and prior use of imipenem, meropenem, PIP/TAZ, and fourth-generation cephalosporins.30
In another study performed in 2015, the main risk factors for XDR AB were previous carbapenem use and high Sequential Organ Failure Assessment score and for pan drug-resistant AB, previous use of colistin, carbapenems, and high Simplified Acute Physiology Score.31
Similarly, the use of broad-spectrum antibiotics such as carbapenems and PIP/TAZ and also central venous catheters were identified as risk factors independently associated with XDR-AB bacteremia.32
In another study, prior exposure to carbapenems, use of mechanical ventilation, and chronic kidney disease were independent factors associated with imipenem-resistant Gram-negative bacilli septicemia.33
Since 2003, Tumbarello et al34 identified infections caused by MDR Providencia stuartii as an emerging problem, with the risk factors for these infections being advanced age, previous hospitalization, neoplastic disease, and previous antibiotic therapy with cephalosporins, quinolones, or aminoglycosides.
The study limitations are based on the particular epidemiological situation found in a single ICU with a specific program of infection control measures. In addition, monitoring only during hospitalization in the ICU has created problems in identifying exposure to certain risk factors, in particular, previous antibiotic use or immunosuppressive therapy.
Conclusion
This association of XDR AB and XDR Proteeae may well not be limited to our hospital or our geographical area. This problem could be found throughout the world.
Independent risk factors for the association of XDR-AB and XDR-Proteeae were represented by the presence of tracheostomy and naso-/orogastric nutrition ≥8 days. In addition, pressure ulcers were independent predictive factors for all the three studied MDROs infections. Previous antibiotic therapy was an independent risk factor for the acquisition of XDR-AB strains, alone or in association, while the prolonged hospitalization in the ICU, blood transfusion, and hemodialysis appeared to be independent risk factors for single infections.
Disclosure
The authors report no conflicts of interest in this work.
References
Nseir S, Grailles G, Soury-Lavergne A, Minacori F, Alves I, Durocher A. Accuracy of American Thoracic Society/Infectious Diseases Society of America criteria in predicting infection or colonization with multidrug-resistant bacteria at intensive-care unit admission. Clin Microbiol Infect. 2010;16(7):902–908. | ||
Cisneros JM, Rodriguez-Bano J, Fernandez-Cuenca F, et al; Spanish Group for Nosocomial Infection (GEIH) for the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC). Risk-factors for the acquisition of imipenem-resistant Acinetobacter baumannii in Spain: a nationwide study. Clin Microbiol Infect. 2005;11(11):874–879. | ||
Playford EG, Craig JC, Iredell JR. Carbapenem-resistant Acinetobacter baumannii in intensive care unit patients: risk factors for acquisition, infection and their consequences. J Hosp Infect. 2007;65(3):204–211. | ||
Seligman R, Ramos-Lima LF, Oliveira V do A, Sanvicente C, Sartori J, Pacheco EF. Risk factors for infection with multidrug-resistant bacteria in non-ventilated patients with hospital-acquired pneumonia. J Bras Pneumol. 2013;39(3):339–348. | ||
Zhou HY, Yuan Z, Du Y. Prior use of four invasive procedures increases the risk of Acinetobacter baumannii nosocomial bacteremia among patients in intensive care units: a systematic review and meta-analysis. Int J Infect Dis. 2014;22:25–30. | ||
Falagas ME, Karveli EA, Siempos II, Vardakas KZ. Acinetobacter infections: a growing threat for critically ill patients. Epidemiol Infect. 2008;136(8):1009–1019. | ||
Falagas ME, Rafailidis PI. Attributable mortality of Acinetobacter baumannii: no longer a controversial issue. Crit Care. 2007;11(3):134. | ||
Villalón P, Valdezate S, Cabezas T, et al. Endemic and epidemic Acinetobacter baumannii clones: a twelve-year study in a tertiary care hospital. BMC Microbiol. 2015;15:47. | ||
Perez F, Hujer AM, Hujer KM, Decker BK, Rather PN, Bonomo RA. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2007;51(10):3471–3484. | ||
Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. | ||
Spellberg B, Bonomo RA. Combination Therapy for Extreme Drug-Resistant Acinetobacter baumannii: Ready for Prime Time? Crit Care Med. 2015;43(6):1332–1334. | ||
ECDC [webpage on the Internet]. Surveillance of antimicrobial resistance in Europe. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net); 2016; Stockholm. Available from: https://ecdc.europa.eu/en/publications-data/antimicrobial-resistance-surveillance-europe-2016. Accessed July 27, 2017. | ||
European Centre for Disease Prevention and Control. Carbapenem-Resistant Acinetobacter baumannii in Healthcare Settings-8 December 2016. Stockholm: ECDC; 2016. | ||
Kwon SH, Ahn HL, Han OY, La HO. Efficacy and safety profile comparison of colistin and tigecycline on the extensively drug resistant Acinetobacter baumannii. Biol Pharm Bull. 2014;37(3):340–346. | ||
Katip W, Uitrakul S, Oberdorfer P. Clinical outcomes and nephrotoxicity of colistin loading dose for treatment of extensively drug-resistant Acinetobacter baumannii in cancer patients. Infect Drug Resist. 2017;10:293–298. | ||
Khawcharoenporn T, Pruetpongpun N, Tiamsak P, Rutchanawech S, Mundy LM, Apisarnthanarak A. Colistin-based treatment for extensively drug-resistant Acinetobacter baumannii pneumonia. Int J Antimicrob Agents. 2014;43(4):378–382. | ||
Falagas ME, Kasiakou SK. Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin Infect Dis. 2005;40(9):1333–1341. | ||
Bartlett JG, Auwaerter PG, Pham PA. Johns Hopkins ABX Guide: Diagnosis & Treatment of Infectious Diseases. 2nd ed. Jones & Bartlett Publishers; Burlington, MA, United States. 2010, Vol. 526. | ||
Axente C, Licker M, Moldovan R, et al. Antimicrobial consumption, costs and resistance patterns: a two year prospective study in a Romanian intensive care unit. BMC Infect Dis. 2017;17(1):358. | ||
Popescu GA, Șerban R, Niculcea A. CARMIN-ROM 2015 (Consumul de antibiotice, Rezistența microbiană și Infecții Nosocomiale în România-2015); 2015. Available from: https://cnscbt.ro/index.php/analiza-date-supraveghere/infectii-nosocomiale-1/684-consumul-de-antibiotice-rezistenta-microbiana-si-infectii-nosocomiale-in-romania-2015/file. Accessed July 27, 2017. | ||
EUR-Lex Access to European Union law. 2012/506/EU: Commission implementing decision of 8 August 2012 amending decision 2002/253/EC laying down case definitions for reporting communicable diseases to the community network under decision No 2119/98/EC of the European Parliament and of the council (notified under document C(2012) 5538) text with EEA relevance. Off J Eur Union 262, 27.9.2012, p. 1–57. | ||
American Society of Anesthesiologists [webpage on the Internet]. ASA Physical Status Classification System. ASA House of Delegates; 2014. Available from: https://www.asahq.org/resources/clinical-information/asa-physical-status-classification-system. Accessed July 27, 2017. | ||
MD+ CALC [webpage on the Internet]. Charlson Comorbidity Index (CCI). Available from: https://www.mdcalc.com/charlson-comorbidity- index-cci. Accessed July 27, 2017. | ||
Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twenty Third Informational Supplement, CLSI Document M100-S23. Wayne, PA: CLSI; 2013. | ||
Hrabák J, Chudáčková E, Papagiannitsis CC. Detection of carbapenemases in Enterobacteriaceae: a challenge for diagnostic microbiological laboratories. Clin Microbiol Infect. 2014;20(9):839–853. | ||
Giske CG, Martinez-Martinez L, Cantón R, et al. EUCAST Guidelines for Detection of Resistance Mechanisms and Specific Resistances of Clinical and/or Epidemiological Importance; 2013. Version 1.0:4–10. | ||
Miriagou V, Tzelepi E, Kotsakis SD, Daikos GL, Bou Casals J, Tzouvelekis LS. Combined disc methods for the detection of KPC- and/or VIM-positive Klebsiella pneumoniae: improving reliability for the double carbapenemase producers. Clin Microbiol Infect. 2013;19(9):E412–E415. | ||
Paterson DL. The epidemiological profile of infections with multidrug-resistant Pseudomonas aeruginosa and Acinetobacter species. Clin Infect Dis. 2006;43 (Suppl 2):S43–S48. | ||
Moghnieh R, Siblani L, Ghadban D, et al. Extensively drug-resistant Acinetobacter baumannii in a Lebanese intensive care unit: risk factors for acquisition and determination of a colonization score. J Hosp Infect. 2016;92(1):47–53. | ||
Chan MC, Chiu SK, Hsueh PR, Wang NC, Wang CC, Fang CT. Risk factors for healthcare-associated extensively drug-resistant Acinetobacter baumannii infections: a case-control study. PLoS One. 2014;9(1):e85973. | ||
Inchai J, Liwsrisakun C, Theerakittikul T, Chaiwarith R, Khositsakulchai W, Pothirat C. Risk factors of multidrug-resistant, extensively drug-resistant and pandrug-resistant Acinetobacter baumannii ventilator-associated pneumonia in a Medical Intensive Care Unit of University Hospital in Thailand. J Infect Chemother. 2015;21(8):570–574. | ||
Ng TM, Teng CB, Lye DC, Apisarnthanarak A. A multicenter case-case control study for risk factors and outcomes of extensively drug-resistant Acinetobacter baumannii bacteremia. Infect Control Hosp Epidemiol. 2014;35(1):49–55. | ||
Chen IL, Lee CH, Ting SW, Wang LY. Prediction of imipenem-resistant microorganisms among the nosocomial critically ill patients with Gram-negative bacilli septicemia: a simple risk score. Infect Drug Resist. 2018;11:283–293. | ||
Tumbarello M, Citton R, Spanu T, et al. ESBL-producing multidrug-resistant Providencia stuartii infections in a university hospital. J Antimicrob Chemother. 2004;53(2):277–282. |
Supplementary material
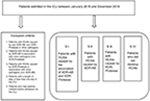  | Figure S1 Study design. Abbreviations: AB, Acinetobacter baumannii; HCAIs, health care-associated infections; ICU, intensive care unit; XDR, extensively drug-resistant. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.