Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Expression Patterns of Macrophage Migration Inhibitory Factor and Its Gene Variants (MIF-173 G˃C) in Verruca Vulgaris
Authors Hassan MH , Abuhamdah S , Elsadek BE , Abdelwahab A, Abd-Elhamid TH , Fayed HM , Abbass A, Abdallah AAM , Mohamed M , Abd-Elmagid WM
Received 1 March 2022
Accepted for publication 26 May 2022
Published 10 June 2022 Volume 2022:15 Pages 1073—1085
DOI https://doi.org/10.2147/CCID.S363916
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 6
Editor who approved publication: Dr Jeffrey Weinberg
Mohammed H Hassan,1 Sawsan Abuhamdah,2,3 Bakheet EM Elsadek,4 Ashraf Abdelwahab,5 Tarek Hamdy Abd-Elhamid,6 Hanan M Fayed,7 Amany Abbass,8 Ahmed Alamir Mahmoud Abdallah,9 Marwa Mohamed,5 Wafaa Mohamed Abd-Elmagid5
1Department of Medical Biochemistry, Faculty of Medicine, South Valley University, Qena, 83523, Egypt; 2College of Pharmacy, Al Ain University, Abu Dhabi, United Arab Emirates; 3Department of Biopharmaceutics and Clinical Pharmacy, Faculty of Pharmacy, The University of Jordan, Amman, Jordan; 4Department of Biochemistry and Molecular Biology, Faculty of Pharmacy, Al-Azhar University, Assiut Branch, Assiut, 71524, Egypt; 5Department of Dermatology, Venereology and Andrology, Faculty of Medicine, Sohag University, Sohag, Egypt; 6Department of Histology and Cell Biology, Faculty of Medicine, Assiut University, Assiut, Egypt; 7Department of Chemical and Clinical Pathology, Faculty of Medicine, South Valley University, Qena, 83523, Egypt; 8Department of Chemical and Clinical Pathology, Faculty of Medicine, Sohag University, Sohag, Egypt; 9Medical Biochemistry Department, Faculty of Medicine, Sohag University, Sohag, Egypt
Correspondence: Mohammed H Hassan, Department of Medical Biochemistry, Faculty of Medicine, South Valley University, Qena, 83523, Egypt, Tel +20 109 8473605, Email [email protected]; [email protected]
Introduction: Verruca vulgaris is a benign hyperkeratotic proliferation of the epidermis. Few studies look at the differences in serum and tissue macrophage migration inhibitory factor (MIF) levels in verruca vulgaris, as well as its gene polymorphisms that have yet to be explored. The current study provided in-depth evaluation of MIF in serum and tissues of patients with verruca vulgaris, and establishes for the first time the possible association of MIF gene polymorphisms with common warts.
Methods: This case-control study included 50 patients who were diagnosed clinically as common warts in comparison with 50 age and sex-matched controls. Clinical examination was done on all included cases. Serum MIF was measured using enzyme-linked immunosorbent assay (ELISA), while its tissue expression was analyzed using Western blotting and immunohistochemical techniques for the included participants. Analysis of MIF-173 G˃C single nucleotide polymorphism was performed by polymerase chain reaction (PCR) using restriction fragment length polymorphism (RFLP) technique.
Results: The overall results revealed significantly lower MIF tissue expression in lesional and perilesional skin biopsies from cases compared to the controls using Western blot and immunohistochemical analysis. Yet, the difference in the serum MIF levels between cases and controls was not significant (p ˃ 0.05). GC genotype of the studied MIF rs755622 G>C SNP could be considered as a protective genetic factor against the occurrence of verruca vulgaris among Egyptians with OR (95% CI) equal 0.444 (0.199– 0.989).
Conclusion: MIF and its genetic variants are thought to play a pathogenic role in verruca vulgaris development and recurrence.
Keywords: macrophage migration inhibitory factor, MIF rs755622 G>C SNP, common warts, RFLP-PCR, western blot, immunohistochemistry
Introduction
The human papillomavirus (HPV) causes verruca vulgaris (common wart), a benign hyperkeratotic proliferation of the epidermis. Warts are very widespread all over the world, and the infection is frequently contracted as a child. Warts can cure on their own, but adult warts are more tenacious and difficult to treat. They are most typically found on the hands and fingers, and they can cause physical and psychological distress in those who are affected.1,2 The virus’s eradication is linked to viral antigens being exposed to the host’s circulating immunity and the emergence of a delayed immune response. The virus primarily stimulates a T-cell humoral response, and prolonged infection can be detected in patients with a low T-cell population (HIV infection, post-chemotherapy).3
Many studies have explored the significant role of cytokines in the pathogenesis, progression, and development of numerous viral-induced dermatological diseases (via influencing both innate and cellular immune responses).4–8 Because viruses can hijack host machinery to accelerate viral replication, cutaneous viral infection poses a particular challenge to the skin’s immune system.9 The macrophage migration inhibitory factor (MIF) is an example of a cytokine that is important in the host immune response but has been little investigated in the pathophysiology of verruca vulgaris. During the earliest antiviral reactions against HPV in cutaneous tissues, the host’s innate immunity plays a significant role. To evade immune responses, HPV modifies multiple host cellular pathways, resulting in virus-mediated immunosuppression.9
Migration inhibitory factor is found in a range of cell types, including B cells, T cells, and macrophages, and plays a role in innate immunity mediation by promoting host inflammatory reactions through the start of pro-inflammatory, hormonal, and enzymatic activities.10 MIF also regulates host inflammatory responses by modifying cellular mechanisms such as T-cell dissemination, p53-dependent apoptosis reduction, and glucocorticoid immunosuppression counterbalancing.11
The promoter region of the MIF gene (OMIM: 153620) contains a single nucleotide polymorphism (SNP) 173 G>C (rs755622) that closely corresponds with the susceptibility and severity to numerous autoimmune and inflammatory diseases.12,13
Previous two recent studies were done to assess the role of MIF levels in patients with common warts, based on ELISA only,14,15 and both of them had opposing opinion regard this issue. Here, we hypothesized that “MIF and its gene polymorphism may play a role in either the development and/or recurrence of verruca vulgaris”. To fill the existing gap in the literature and to test our hypothesis, we conducted our study using ELISA, Western blot method, and immunohistochemistry to analyse MIF expression in blood and skin tissues to investigate the potential role of MIF in the pathogenesis of verruca vulgaris. We also look for the first time into the significance of the MIF 173 G>C (rs755622) SNP in the development and recurrence of verruca vulgaris.
Materials and Methods
Study Design and Participants
The study involved 50 verruca vulgaris patients who were chosen at random from the Dermatology Outpatient Clinic at Sohag University Hospital, Faculty of Medicine, Sohag University, Sohag, Egypt. The included patients were compared to a control group of 50 unrelated, healthy, age- and sex-matched participants. After receiving approval from the Research Committee at Sohag University’s Faculty of Medicine, the current study was done between June 2019 and May 2021. Before the study began, each subject was told of the study’s purpose and given written informed agreement to participate. The research was carried out following the Helsinki Declaration. We adjusted the sample size to attain 80% power and a 5% confidence level of significance (type I error).
Inclusion and Exclusion Criteria
The study included both males and females who were 18 years or older who had verruca vulgaris but had not received systemic (immunotherapy) or topical treatment for at least a month before the skin samples were taken. Patients who were pregnant, breastfeeding, or had a history of systemic illnesses such as autoimmune disorders, diabetes mellitus, malignancies, or hypertension were excluded from the study.15
Dermatological Assessment of the Included Patients
Each patient was given a detailed medical history and a complete clinical examination, which covered age, sex, medical and family history, and disease characteristics (including duration, number, and distribution of common warts, primary or recurrent lesions).
Laboratory Workup
Blood and Tissue Samples
Five mL of blood was taken from each participant and separated into two parts: the first (2 mL) was evacuated into EDTA-containing tubes and immediately frozen at −80°C until MIF genetic analysis. While the remaining amount (3 mL) was evacuated into a separator gel tube, centrifuged at 822 g for 15 minutes, and the separated sera were divided into aliquots using 1-mL cryotubes and stored at −20°C for biochemical MIF assays later.
After being locally anaesthetized with 2% lidocaine, skin punch biopsies (4 mm) were taken from a) the lesion and b) the skin around warts (perilesional skin) of the included cases and c) the back of the controls at the Dermatology Department of Sohag University Hospital, and each skin biopsy was divided into two parts: The first part was homogenised in ice-cold Tris-HCl lysis buffer, pH 7.4, containing 1% protease inhibitor cocktail (Cell Signaling Technology, Inc., MA, USA) using a Potter-Elvehjem rotor-stator homogenizer (glass/teflon homogenizer), fitted with a teflon pestle, and stored in a frozen matter at −80°C for later assessment of tissue MIF expression by Western blotting technique.16 For immunohistochemical examination of MIF expression, the second part was fixed in 10% formalin.
Biochemical Assays of Serum MIF Levels
Serum MIF levels were measured by ELISA multiskan EX micro-plate-photometer (STAT FAX-2100; Thermo Scientific, Palm City, FL, USA), using a commercially available ELISA assay kit supplied by Chongqing Biospes Co., Ltd, Chongqing, China (Catalog No. BYEK3015).
Western Blotting Assessments of MIF Expression Levels
Skin tissue specimens were homogenized in ice-cold RIPA lysis buffer (Sigma-Aldrich, Milan, Italy), containing 1% protease inhibitor cocktail (Cell Signaling Technology, Inc., MA, USA, Cat#5871) using Potter-Elvehjem rotor-stator homogenizer (glass/teflon homogenizer), fitted with a Teflon pestle and stored frozen at −70°C for subsequent assessment of tissue MIFexpressions by Western blotting technique. Proteins in each corresponding liver tissue sample were denatured at 95°C for 5 minutes in 2× Laemmli buffer followed by the addition of 5% 2-mercaptoethanol. SDS–PAGE electrophoresis was achieved by loading 30 µg protein per lane at 75 volts through resolving gel 10% followed by 125 volts for approximately 2 hours and transferred to a PVDF membrane using T-77 ECL semidry transfer unit (Amersham BioSciences UK Ltd) for 2 hours. Immunoblotting was performed by incubating the PVDF membrane in TBS buffer containing 0.1% Tween and 5% non-fat milk for one hour at 4°C, followed by overnight incubation separately at 4°C with a rabbit polyclonal anti-MIF antibody (Chongqing Biospes Co. ltd, China, Cat# YPA2198) at a dilution of 1:1000. After being washed three times with TBST buffer, each membrane was incubated for 1 hour at room temperature with an alkaline phosphatase-conjugated goat anti-rabbit secondary antibody (Novus Biologicals, LLC, Littleton, CO, USA, Cat# NB7157) at a dilution of 1:5000. After being washed four times in TBST, the membrane-bound antibody was detected with a commercially available BCIP/NBT substrate detection Kit (Genemed Biotechnologies, Inc., CA, USA, Cat# 10=0007). Equivalent protein loading for each lane was confirmed by stripping and re-blotting each membrane at 4°C against a mouse monoclonal anti-β-actin antibody (Novus Biologicals, LLC, Littleton, CO, USA, NBP1-47423) at a dilution of 1:5000. The analysis was repeated 3 times to assure the reproducibility of results (see Supplementary Data). Quantification was performed using ImageJ software and expressed as the band density relative to that of β-actin.17,18
Immunohistochemical Analysis of MIF
Skin samples were fixed in 10% formalin for immunohistochemistry. Fixated samples were rinsed in tap water, dehydrated with ethanol at increasing concentrations, cleaned with xylene, and embedded in paraffin. In descending grades of ethanol, paraffin sections were deparaffinized and rehydrated. Epitopes were extracted by boiling sections in citrate buffer (pH 6.0) in the microwave. Endogenous peroxidase was inhibited by soaking slides in ethanol containing 3% hydrogen peroxide. For 60 minutes at room temperature, sections were incubated with rabbit anti-human MIF (Chongqing Biospes Co., Ltd, Chongqing, China, dilution 1:50). After washing in TBS-T, sections were incubated for 2 hours at room temperature with HRP-conjugated goat anti-rabbit secondary antibodies (Thermofisher Scientific, Rockford, IL, USA) at a dilution of 1:250. After washing in TBS-T, sections were incubated for 3 minutes with 0.05% diaminobenzidine (DAB) and 0.01% H2O2 to visualise the reaction product. We skipped the primary antibodies during the staining of several of our slides to avoid non-specific binding.
MIF-173 G˃C Single Nucleotide Polymorphism Detection Assay
Extraction of Genomic DNA
G-spinTM total DNA extraction kit protocol (iNtRON Biotechnology, Inc, Korea) was used to extract genomic DNA from whole blood according to the manufacturer’s instructions. For subsequent genetic analysis, the isolated DNA was stored at −80°C.
MIF Genotyping
All participants were genotyped using polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP), for one restriction site in the MIF gene, MIF-173 G˃C (rs755622) single nucleotide polymorphism. Two specific oligonucleotide primers were used that are complementary to the 3’ ends of each of the sense and antisense strands of the DNA target (supplied by Beijing SBS Genetech, China): The forward primer 5′-ACTAAGAAAGACCCGAGGC-3′. The reverse primer 5′-GGGGCACGTTGGTGTTTAC-3′, in concordance with previous protocols.19,20
After mixing 12.5μl from the PCR master mix solution (Catalog no. 25028, iNtRON Biotechnology, Korea) with 1 μL forward primer, 1 μL reverse primer (both primers with a concentration of 10 nmol), 8.5 μL nuclease-free water, and 2μl of extracted DNA with a total volume 25 μL, the following PCR conditions were used: the following temperature profile was used for 35 cycles after 10 min of initial denaturation at 94°C: 94°C for 30 seconds, 60°C for 30 seconds, 72°C for 60 seconds, and a final extension of 10 min, using Biometra thermal cycler (Serial no.2603204, Biometra, Germany). The PCR products, (Figure 1A), were 366-bp in size, using a 50-bp DNA ladder (Catalog No. 24072, iNtRON Biotechnology, Korea) and were digested at 37◦C for 3 hours using (FastDigest Alu1 FD0014, Lot: 00147479; Thermo Fisher Scientific), where 10 μL of the PCR reaction mixture added to 2 μL of 10X buffer, 2 μL of the restriction enzyme and mixed with 18 μL nuclease-free water, then loaded in gel electrophoresis (serial no. 283BR11101, Bio-Rad-pac 300, Italy), using 2% agarose gel stained with 5μL ethidium bromide.
A 50-bp DNA ladder was used to visualize DNA fragments under ultraviolet light (using U.V. Transilluminator 2000, serial no. 642-1045, Bio-Rad, Italy). The MIF restriction fragment lengths were two fragments for Wild genotype (GG) variant of 98 and 268 bp, 3 fragments of 205, 98, and 63 bp for homozygous (CC) mutant genotype, and 4 fragments of 268, 205, 98, and 63 bp for heterozygous (GC) mutant genotype (Figure 1B). A small percentage of the participants’ DNA samples (10%−15%) were picked at random and re-genotyped to ensure that the genotyping results were reproducible.
Statistical Analysis
SPSS version 19 was used for data entry and analysis (Statistical Package for Social Science). For parametric data, we used numbers, percentages, and mean standard deviation (SD), while for non-parametric data, we used median and inter-quartile range (IQR). To compare qualitative variables, the Chi-square test and Fisher exact test were used. The odds ratio (OR) was estimated using 95% confidence intervals (CI). When P ˂ 0.05, the P-value is considered statistically significant. The studied SNP followed the Hardy Weinberg (HW) equation.21,22 Hardy–Weinberg equilibrium was performed by the chi-square G test “Goodness of Fit” with 1 degree of freedom using website SNPs-Institut für Humangenetik, SNPs, Association studies, De Finetti, Hardy–Weinberg browser; https://ihg.gsf.de/ihg/snps.html.
Results
Demographic and Clinical Data of the Study Groups
The current study included 100 participants categorized into 50 patients with verruca vulgaris (16 males and 34 females) with their mean age of 26.96 years ±10.46 SD and 50 controls (24 males and 26 females) with their mean age 26.54 years ±10.07 SD. There were no significant differences between cases and controls regarding age and sex, indicating matching of the study groups (p˃0.05).
Regarding the distribution of verruca vulgaris lesions in the included cases, the majority were in the extremities (96%). Fifty-six percent of patients have a single lesion and the remaining cases (44%) have multiple warts. The mean duration of the disease was 8.52 months±8.0 “SD”. Eighty-six percent of the included patients have primary lesions while the remaining 14% have recurrent lesions. Positive family history for the disease was present in 16% of the included cases.
Serum MIF Levels Among the Study Groups and Their Relation with Demographic and Clinical Data
Although there were lower median serum levels of MIF among patients with verruca vulgaris (20.97 μg/L) compared to the controls (22.3 μg/L), not reach a significant difference (p˃0.05) (Table 1 and Figure 2). There was a lack of significant difference in the median MIF serum levels among the included cases in terms of gender, number of lesions, or recurrence, p˃0.05 for all (Table 2).
 |
Table 1 The Median Serum MIF Levels in the Study Groups |
 |
Table 2 Median Serum Levels Among Patients with Verruca Vulgaris in Terms of Demographic and Clinical Data of the Included Cases |
 |
Figure 2 Serum MIF levels among patients with verruca vulgaris and the controls. |
Western Blot and Immunohistochemical Analysis of MIF Expression in the Skin Tissues of the Studied Groups
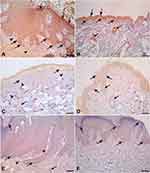
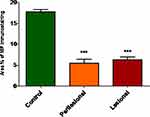
Local expression of tissue MIF using Western blot revealed significantly lower MIF expression in both lesional and perilesional skin biopsies compared to the controls with a lack of significant difference in MIF expression between lesional and perilesional biopsies (Figure 3). Using the immunohistochemical technique, analysis of MIF expression in biopsies of control subjects revealed that intense MIF immunostaining was observed in cells of the basal and granular layers of the epidermis (Figure 4A and B, arrows), capillary endothelial cells of papillary dermis (Figure 4A, arrowheads) and skin appendages (Figure 4B, double headed arrows). In contrast, biopsies from perilesional regions showed marked decrease in MIF immunostaining in the epidermis (Figure 4C and D, arrows), capillaries endothelial cells (Figure 4C, arrowheads) and skin appendages (Figure 4C, double headed arrow). Similarly, biopsies from lesional regions exhibit weak MIF immunostaining in the epidermis (Figure 4E and F, arrows), capillaries endothelial cells (Figure 4E, arrowheads) and skin appendages (Figure 4E, double headed arrow). To confirm our observations, we assessed the area percent of MIF immunostaining using ImageJ software. We found that the area percent of MIF immunostaining was 17.80±1.729% in control group and 5.524±3.022% and 6.295±2.230% in perilesional and lesional groups, respectively. The area percent of MIF immunostaining was significantly lower in skin biopsies from perilesional and lesional regions compared to biopsies taken from control subjects (P < 0.05) (Figure 5).
Genotypes and Allele Frequencies of MIF-173 G˃C (rs755622) SNP in the Study Groups and Its Relation with Serum MIF Levels
There were significantly higher GG (wild homozygous) genotypes among patients with verruca vulgaris (62%) compared to the controls (42%) and significantly lower frequency of GC (heterozygous mutant) genotype among cases (38%) compared to the controls (58%) indicating that GC genotype of the studied MIF rs755622 G>C SNP is a protective factor against the occurrence of verruca vulgaris among Egyptians with OR (95% CI) equal 0.444 (0.199–0.989). CC genotype (mutant homozygous) could not be detected in our study groups. Although G-allele was more frequent among cases (81%) than controls (71%) and C-allele was more frequent among controls (29%) than cases (19%) but they did not reach significant levels (p = 0.098 for both) (Table 3). There was a lack of significant difference in the median MIF serum levels among the included cases in terms of genotypes and alleles of the studied MIF rs755622 G>C SNP (Table 4).
 |
Table 3 MIF rs755622 G>C Genotypes and Allele Frequencies Among the Studied Groups |
 |
Table 4 Median Serum MIF Levels Among Patients with Verruca Vulgaris in Terms of MIF rs755622 G>C Genotypes and Alleles |
Genotypes and Allele Frequencies of MIF-173 G˃C (rs755622) SNP in Terms of Verruca Vulgaris Characteristics
There was a lack of significant differences in the genotypes and allele frequencies of MIF rs755622 G>C among cases with single verruca vulgaris lesion compared to those who have multiple lesions (p˃0.05), with significantly frequent (GC) genotype among cases with the recurrent disease while GG genotype and G-allele were significantly frequent among cases with primary disease (p˂0.05 for all) (Table 5).
 |
Table 5 MIF rs755622 G>C Genotypes and Alleles Frequencies in Terms of the Veruuca Vulgaris Lesions’ Characteristics |
Discussion
In the eradication of warts, cell-mediated immunity (CMI) plays an important role. Patients with human immunodeficiency virus (HIV) infection have shown a link between cellular immunological deficiencies and HPV infection and related morbidities.23 MIF is widely expressed in different tissues, including the skin, and is thought to play a significant role in cell-mediated immunity. It is recognised to play a key function in the skin when it comes to inflammation, immunological response, wound healing, and skin disease.24,25 MIF has been named as “an incriminating agent in dermatological disorders”.26 Limited research, however, has looked into its function in the pathophysiology of dermatological disorders.27 MIF has been thought to play a key function as a proinflammatory cytokine since its discovery; however, MIF is now thought to modulate the inflammatory “set point” by controlling the release of other pro-inflammatory mediators.28 Glucocorticoids enhanced rather than suppressed MIF production, and this mechanism regulates inflammatory and immunological responses.29,30
Our findings revealed significantly lower MIF expression levels in lesional and perilesional skin biopsies among patients with verruca vulgaris compared to healthy controls (using Western blot and immunohistochemistry techniques), with no significant differences in expression levels between lesional and perilesional skin biopsies within the patients’ group. Our immunohistochemical analysis revealed high MIF expression in the basal and granular layers of the epidermis of normal subjects. This observation was in line with Brocks et al.31 In contrast, perilesional and lesional skin biopsies showed marked reduction of MIF expression in the epidermis. Studies have linked MIF expression with epidermal Langerhans cell migration and T-lymphocytes proliferative responses during pathological skin conditions.32 Interestingly, Coleman et al have associated wart regression with T lymphocytes and macrophage infiltration.33 On the other hand, defective cell-mediate immunity has been linked with non-regressing wart.33 Taken together, suppression of MIF expression in verruca vulgaris, we observed here, could be through a paracrine signal from the wart virus and/or virus-infected cells.
Furthermore, when healthy controls were compared to patients with common warts, we found greater median values of circulating MIF, though not to a significant level. In line with our findings, El-Hamd et al14 reported significantly lower serum MIF levels among cases with common warts, using ELISA assays. On the contrary, Sorour et al15 reported significantly higher MIF levels in lesional and perilesional skin biopsies from patients with verruca vulgaris compared to the controls using ELISA assays. Although, because tissue homogenate samples vary in their protein content, ELISA assays of any tissue protein marker should be divided by the total protein content of every sample to obtain correct marker assays, this was not done in the cited study. A recent study by Nassar et al34 supported our findings and reported low serum MIF levels using ELISA method, at base line with significant rise in the MIF levels two weeks after the last session of intralesional Candida antigen injection of common warts among responders to this type of therapy. They supposed the upregulation of MIF after immunotherapy confirms its essential role in antigen presentation, stimulation of delayed type hypersensitivity (which represent the major part in immunity against intracellular pathogen) and release of Th1 cytokines.
Macrophage migration inhibitory factor modulates lymphocyte trafficking and inhibits regulatory effects on cytotoxic CD8+ T cells.35 MIF was found to play a role in the recovery of hepatic cells infected with HSV-2 in a mice model.36 MIF levels are high in viral infections such as the influenza virus and the human immunodeficiency virus, in addition to bacterial infection.37,38 An efficient immune response eliminates the majority of HPV infections. HPV is very unique and full of mystery. Failure to remove HPVs encourages the growth of warts, which is mostly due to HPV’s ability to evade the body’s immune defence system. This process also includes a number of inflammatory reactions that have only recently been discovered. Pro-inflammatory cytokines and tumour necrosis factor are significant mediators of skin and mucosal inflammation development. Epidermal cells have been shown to release cytokines in response to a variety of stimuli, including viral infection.39–41 T cells and macrophages activated by MIF release proinflammatory cytokines such as tumour necrosis factor (TNF-α), interleukins (IL-1β, IL-2, IL-6, IL-8) and interferon (IFN-γ), which result in inflammatory responses. As a result, MIF influences both the innate and adaptive immune responses.30
In our study, we found no significant variations in MIF serum levels in terms of sex, lesion distribution, or disease recurrence. This was in agreement with Sorour et al15 who reported similar findings.
Analyzing polymorphisms in the MIF gene’s promoter region [MIF 173 G˃C (rs755622)] is a significant determinant of disease risk factors, and it could lead to better prevention and treatment options. On chromosome 22q11.2, the human MIF gene is located. A functional variation in MIF’s 5’ promoter region, which replaces G with C at position −173, appears to impact promoter activity in a cell-type dependent manner.42,43 As far as we know, this is the first study that assesses the possible association of MIF-173 G˃C single nucleotide gene polymorphism with the risk of development and recurrence of verruca vulgaris. Our results showed significantly high frequency of wild homozygous (GG) genotype with significantly lower frequency of heterozygous mutant (GC) genotype among Egyptian patients with common warts, thus GC could be considered as a protective genetic factor against the development of verruca vulgaris with OR (95% CI) equal 0.444 (0.199–0.989). Additionally, there was a significantly frequent (GC) genotype among cases with recurrent common warts, which means that (GG) genotype is frequently occurring among patients with verruca vulgaris, but patients having (GC) are more liable to recurrent affection. Of course, these findings are required to be confirmed using larger-scale studies.
Conclusion
The current research supports the possible role of MIF in the pathogenesis of verruca vulgaris as evidenced by the significant reduction of its expression in those patients. Moreover, the genetic variants of MIF (rs755622) were significantly associated with the disease occurrence and recurrence.
Study’s Limitations
The small sample size was the main study limitation; therefore, the current findings, particularly the MIF-173 G˃C single nucleotide polymorphism, which is the first study to identify its likely role in verruca vulgaris, would need to be validated in larger scale studies.
Data Sharing Statement
The datasets used and analyzed in this study are available upon reasonable request.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was partially funded by Faculty of Medicine, South Valley University, Qena 83523, Egypt.
Disclosure
The authors declare no conflicts of interest.
References
1. Gibbs S. Local treatments for cutaneous warts: systematic review. BMJ. 2002;325:461. doi:10.1136/bmj.325.7362.461
2. Ralph J, O’Grady C, Boggs J, Barry R. Remission of verruca vulgaris following incisional punch biopsy. Clin Exp Dermatol. 2021;46:1163–1165. doi:10.1111/ced.14613
3. Deligeoroglou E, Giannouli A, Athanasopoulos N, et al. HPV infection: immunological aspects and their utility in future therapy. Infect Dis Obstet Gynecol. 2013;2013:540850. doi:10.1155/2013/540850
4. Ma JE, Brewer JD. Merkel cell carcinoma in immunosuppressed patients. Cancers. 2014;6(3):1328–1350. doi:10.3390/cancers6031328
5. Drago F, Ciccarese G, Broccolo F, et al. The role of cytokines, chemokines, and growth factors in the pathogenesis of pityriasis rosea. Mediators Inflamm. 2015;2015:438963. doi:10.1155/2015/438963
6. Cush SS, Reynoso GV, Kamenyeva O, et al. Locally produced IL-10 limits cutaneous vaccinia virus spread. PLoS Pathog. 2016;12(3):e1005493. doi:10.1371/journal.ppat.1005493
7. Garcia M, Alout H, Diop F, et al. Innate immune response of primary human keratinocytes to west nile virus infection and its modulation by mosquito saliva. Front Cell Infect Microbiol. 2018;8:387. doi:10.3389/fcimb.2018.00387
8. Sahu U, Biswas D, Prajapati VK, Singh AK, Samant M, Khare P. Interleukin-17-A multifaceted cytokine in viral infections. J Cell Physiol. 2021;236(12):8000–8019. doi:10.1002/jcp.30471
9. Lei V, Petty AJ, Atwater AR, Wolfe SA, MacLeod AS. Skin viral infections: host antiviral innate immunity and viral immune evasion. Front. Immunol. 2020;11:593901. doi:10.3389/fimmu.2020.593901
10. Donn RP, Ray DW. Macrophage migration inhibitory factor: molecular, cellular and genetic aspects of a key neuroendocrine molecule. J Endocrinol. 2004;182:1–9. doi:10.1677/joe.0.1820001
11. Leech M, Lacey D, Xue JR, et al. Regulation of p53 by macrophage migration inhibitory factor in inflammatory arthritis. Arthritis Rheum. 2003;48:1881–1889. doi:10.1002/art.11165
12. Castañeda-Moreno VA, De la Cruz-Mosso U, Torres-Carrillo N, et al. MIF functional polymorphisms (−794 CATT 5–8 and −173 G>C) are associated with MIF serum levels, severity and progression in male multiple sclerosis from western Mexican population. J Neuroimmunol. 2018;320:117–124. doi:10.1016/j.jneuroim.2018.04.006
13. De la Cruz-Mosso U, Bucala R, Palafox-Sánchez CA, et al. Macrophage migration inhibitory factor: association of −794 CATT5–8 and −173 G>C polymorphisms with TNF-α in systemic lupus erythematosus. Hum Immunol. 2014;75(5):433–439. doi:10.1016/j.humimm.2014.02.014
14. El-Hamd MA, Assaf HA, Nada EA. Possible role of interleukin-17 and macrophage migration inhibitory factor in cutaneous warts. J Cosmet Dermatol. 2018;17:1250–1253. doi:10.1111/jocd.12472
15. Sorour NE, Hamed AM, Tabl HAM, Ahmed AAA. Assessment of macrophage migration inhibitory factor in patients with verruca vulgaris. Clin Cosmet Investig Dermatol. 2019;12:591–595. doi:10.2147/CCID.S209269
16. Assaf HA, AbdelMaged WM, Elsadek BE, Hassan MH, Adly MA, Ali SA. Survivin as a novel biomarker in the pathogenesis of acne vulgaris and its correlation to insulin-like growth factor-I. Dis Markers. 2016;2016:7040312. doi:10.1155/2016/7040312
17. Elsadek B, Mansour A, Saleem T, Warnecke A, Kratz F. The antitumor activity of a lactosaminated albumin conjugate of doxorubicin in a chemically induced hepatocellular carcinoma rat model compared to sorafenib. Dig Liver Dis. 2017;49:213–222. doi:10.1016/j.dld.2016.10.003
18. Hassan MH, Abuhamdah S, Abdel-Bary M, et al. Circulating and local nuclear expression of survivin and fibulin-3 genes in discriminating benign from malignant respiratory diseases: correlation analysis. Biosci Rep. 2021;41(1):BSR20203097. doi:10.1042/BSR20203097
19. Col-Araz N, Oguzkan-Balci S, Baspinar O, Sever T, Balat A, Pehlivan S. Mannose binding lectin and macrophage migration inhibitory factor gene polymorphisms in Turkish children with cardiomyopathy: no association with MBL2 Codon 54 A/B genotype, but an association between MIF −173 CC genotype. Int J Med Sci. 2012;9(6):506–512. doi:10.7150/ijms.4787
20. El-Mahdy RI, Saleem TH, Essam OM, Algowhary M. Functional variants in the promoter region of macrophage migration inhibitory factor rs755622 gene (MIF G173C) among patients with heart failure: association with echocardiographic indices and disease severity. Heart Lung. 2021;50:92–100.
21. Hardy GH. Mendelian proportions in a mixed population. Science. 1908;28:49–50. doi:10.1126/science.28.706.49
22. Stark AE. A clarification of the Hardy-Weinberg law. Genetics. 2006;174:1695–1697. doi:10.1534/genetics.106.057042
23. Palefsky J, Holly E, Ralston M, Jay N. Prevalence and risk factors for human papillomavirus infection of the anal canal in human immunodeficiency virus (HIV)-positive and HIV-negative homosexual men. J Infect Dis. 1998;177:361–367. doi:10.1086/514194
24. Zhao Y, Shimizu T, Nishihira J, et al. Tissue regeneration using macrophage migration inhibitory factor (MIF)-impregnated gelatin microbeads in cutaneous wounds. Am J Pathol. 2005;167:1519–1529. doi:10.1016/S0002-9440(10)61238-2
25. Shimizu T. The role of macrophage migration inhibitory factor (MIF) in ultraviolet radiation-induced carcinogenesis. Cancers. 2010;2:1555–1564. doi:10.3390/cancers2031555
26. Feily A, Yaghoobi R, Pazyar N. Macrophage migration inhibitory factor as an incriminating agent in dermatological disorders. Indian J Dermatol. 2013;58(2):157. doi:10.4103/0019-5154.108068
27. Garcia-Orozco A, Martinez-Magaña IA, Riera-Leal A, et al. Macrophage inhibitory factor (MIF) gene polymorphisms are associated with disease susceptibility and with circulating MIF levels in active non-segmental vitiligo in patients from western Mexico. Mol Genet Genomic Med. 2020;8(10):e1416. doi:10.1002/mgg3.1416
28. Kuai SG, Ou QF, You DH, et al. Functional polymorphisms in the gene encoding macrophage migration inhibitory factor (MIF) are associated with active pulmonary tuberculosis. Infect Dis. 2016;48(3):222–228. doi:10.3109/23744235.2015.1107188
29. Calandra T, Bernhagen J, Metz CN, et al. MIF as a glucocorticoid‐induced modulator of cytokine production. Nature. 1995;377(6544):68–71. doi:10.1038/377068a0
30. Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol. 2003;3(10):791–800. doi:10.1038/nri1200
31. Brocks T, Fedorchenko O, Schliermann N, et al. Macrophage migration inhibitory factor protects from nonmelanoma epidermal tumors by regulating the number of antigen-presenting cells in skin. FASEB J. 2016;31:526–543. doi:10.1096/fj.201600860R
32. Shimizu T, Abe R, Nishihira J, et al. Impaired contact hypersensitivity in macrophage migration inhibitory factor-deficient mice. Eur J Immunol. 2003;33(6):1478–1487. doi:10.1002/eji.200323751
33. Coleman N, Birley HD, Renton AM, et al. Immunological events in regressing genital warts. Am J Clin Pathol. 1994;102:768–774. doi:10.1093/ajcp/102.6.768
34. Nassar A, Nofal A, Bakr NM, Essam R, Alakad R. Correlation of serum interleukin 17 and macrophage migration inhibitory factor levels with clinical response to intralesional candida antigen and their potential use as predictors of clinical outcome in patients with multiple common warts. J Cosmet Dermatol. 2021;1–9. doi:10.1111/jocd.14688
35. Abe R, Peng T, Sailors J, Bucala R, Metz CN. Regulation of the CTL response by macrophage migration inhibitory factor. J Immunol. 2001;166:747–753. doi:10.4049/jimmunol.166.2.747
36. Mogensen SC. Macrophage migration inhibition as a correlate of cell-mediated immunity to herpes simplex virus type 2 in mice. Immunobiology. 1982;162(1):28–38. doi:10.1016/S0171-2985(11)80014-8
37. Hou XQ, Gao YW, Yang ST, Wang CY, Ma ZY, Xia XZ. Role of macrophage migration factor inhibitor in influenza H5N1 virus pneumonia. ActaVirologica. 2009;53(4):225–231.
38. Delaloye J, De Bruin IJA, Darling EA, et al. Increased macrophage migration factor inhibitor (MIF) plasma levels in acute HIV-1 infection. Cytokine. 2012;60(2):338–340. doi:10.1016/j.cyto.2012.07.027
39. Scott M, Nakagawa M, Moscicki AB. Cell-mediated immune response to human papillomavirus infection. Clin Diagn Lab Immunol. 2001;8:209–220. doi:10.1128/CDLI.8.2.209-220.2001
40. Stanley MA, Pett MR, Coleman N. HPV: from infection to cancer. BiochemSoc Trans. 2007;35:1456–1460. doi:10.1042/BST0351456
41. Frazer IH. Interaction of human papillomaviruses with the host immune system: a well evolved relationship. Virology. 2009;384:410–414. doi:10.1016/j.virol.2008.10.004
42. Donn R, Alourfi Z, De Benedetti F, et al. Mutation screening of the macrophage migration inhibitory factor gene: positive association of a functional polymorphism of macrophage migration inhibitory factor with juvenile idiopathic arthritis. Arthritis Rheum. 2002;46:2402–2409. doi:10.1002/art.10492
43. Renner P, Roger T, Calandra T. Macrophage migration inhibitory factor: gene polymorphisms and susceptibility to inflammatory diseases. Clin Infect Dis. 2005;41(Suppl 7):S513–S519. doi:10.1086/432009
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.