Back to Journals » Cancer Management and Research » Volume 12
Expression of Integrin β6 and HAX-1 Correlates with Aggressive Features and Poor Prognosis in Esophageal Squamous Cell Carcinoma
Authors Li F, Shang Y, Shi F , Zhang L, Yan J, Sun Q, She J
Received 3 August 2020
Accepted for publication 15 September 2020
Published 2 October 2020 Volume 2020:12 Pages 9599—9608
DOI https://doi.org/10.2147/CMAR.S274892
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Rudolph Navari
Fanni Li,1 Yukui Shang,2 Feiyu Shi,3 Lei Zhang,3 Jun Yan,3 Qi Sun,3 Junjun She3
1Department of Talent Highland, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an 710061, People’s Republic of China; 2Department of Basic Medical Sciences, School of Medicine, Tsinghua University, Beijing 100084, People’s Republic of China; 3Department of General Surgery, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an 710061, People’s Republic of China
Correspondence: Qi Sun; Junjun She
Department of General Surgery, The First Affiliated Hospital of Xi’an Jiaotong University, 277 Yanta West Road, Xi’an, Shaanxi 710061, People’s Republic of China
Tel/Fax +86 29 85433720
Email [email protected]; [email protected]
Purpose: The development of esophageal squamous cell carcinoma (ESCC) is a complicated process in which cell adhesion and motility, mediated by integrins, are involved through connecting the cytoskeleton to extracellular matrix. Different mechanisms via which integrin β 6 participates in cancer invasion and metastasis have been described by numerous studies; however, the expression and clinical significance of integrin β 6 in ESCC remain unknown.
Methods: To investigate the differential expression of integrin β 6 in ESCC, qPCR and immunohistochemistry assays were performed in 10 paired human samples. A total of 137 ESCC samples were further enrolled to evaluate the expression levels of integrin β 6 and its endocytic trafficking regulator HS1-associated protein X-1 (HAX-1), followed by the evaluation of their correlation with clinicopathological parameters. The overall survival was analyzed using the Kaplan–Meier method, with significant variables further evaluated by multivariate Cox regression analyses.
Results: The expression of integrin β 6 was markedly increased in ESCC compared with matched adjacent normal tissues. Among the ESCC samples, positive expression of integrin β 6 was observed in 41.6% tumors, which was associated with histological differentiation, lymph node metastasis and TNM stage. High expression of HAX-1 was detected in 47.4% tumors, and there was a positive relationship between the expression levels of integrin β 6 and HAX-1. Furthermore, the expression of integrin β 6 and HAX-1 were independent unfavorable indicators for prognosis. Patients with positive integrin β 6 and high HAX-1 expression demonstrated worst outcomes.
Conclusion: The present findings suggested the predictive value of integrin β 6 and HAX-1 as independent indicators of poor prognosis for patients with ESCC, both of which may contribute to the tumor proliferation and metastasis, leading to ESCC progression. Therefore, combined targeting of integrin β 6 and HAX-1 may provide a potential novel approach for the treatment of ESCC.
Keywords: integrin, HAX-1, esophageal squamous cell carcinoma, prognosis
Introduction
As a common aggressive malignancy, esophageal cancer (EC) is characterized by a high incidence rate and lethality, exacerbated by distant metastasis and therapeutic resistance. Esophageal squamous cell carcinoma (ESCC) is the predominant EC subtype in East Asia in terms of histological classification, accounting for up to 90% of EC cases.1 While ongoing progress has been achieved with regards to surgical techniques and adjuvant therapies, the outcomes of ESCC remain unsatisfactory, with a 5-year overall survival rate ≤20%, and numerous patients are at a late stage at initial diagnosis.2 Therefore, it is important to further clarify the underlying mechanisms of ESCC progression, as well as identify novel biomarkers and potential therapeutic targets, which could improve the prognosis of patients with ESCC.
Cell motility and invasion are important steps in tumor progression and metastasis, in which the adhesion of the cellular cytoskeleton to the extracellular matrix (ECM) occurs. As a large family of transmembrane receptors on the cell surface, integrins act as anchoring and bidirectional signaling molecules that serve as a physical and functional bridge between cells and ECM.3 Currently, 24 distinct types of integrins have been discovered, composed of an α and a β subunit. Integrin β6 only partners with αv, giving rise to a single heterodimer αvβ6 that is unique, with an exclusive and specific expression in epithelial cells. Integrin β6 is barely detected in normal epithelia but becomes upregulated during carcinogenesis.4 By binding to corresponding ECM ligands, such as fibronectin and vitronectin, integrin β6 can activate downstream pathways so as to regulate a vast array of cellular behaviors including, but not limited to, epithelial-mesenchymal transition (EMT), transforming growth factor-β (TGF-β) activation and anoikis-resistance in a tumor context.5–7 Thus, integrin β6 can facilitate cell survival, proliferation, migration and tumorigenesis.
With regards to the mechanism of dysregulated integrin activity, endocytic trafficking is important for the regulation of integrin-mediated adhesion and migration in a dynamic manner by controlling the availability of integrin at the cell surface.8 Therefore, the assembly and disassembly of cellular adhesion contacts may be dynamically regulated via the trafficking of integrins.9,10 Moreover, the process of endocytosis simultaneously induces the ability of integrins to facilitate co-trafficking of growth factor receptors, referred to as intracytoplasmic “inside-in” signaling.11 The clathrin-mediated endocytosis of integrin β6, which could be regulated by HS1-associated protein X-1 (HAX-1), participates in cancer progression. The interaction of HAX-1 with integrin β6 could determine the net signaling output of integrin β6 on the cell surface, acting as a tight and elaborate means to regulate endocytosis of integrin β6 and thus consequent cell motility.12 Based on the role of endocytic trafficking in integrin β6-mediated migration,13 it is important to take endocytosis into consideration for the mechanistic study.
It has been reported that upregulation of integrin β6 occurs in a variety of carcinomas. However, the expression and clinical significance of integrin β6 in ESCC remain unknown. As an important regulator of endocytosis of integrin β6, HAX-1 has been revealed to be upregulated in ESCC, which is a risk factor of lymph node metastasis.14 However, it is yet to be elucidated whether HAX-1 is involved in integrin β6-mediated ESCC progression; thus, the present study aimed to investigate the expression of integrin β6 and HAX-1 in ESCC, followed by the evaluation of their correlation with various clinicopathological parameters and the prognosis of patients. The current study evaluated the prognostic value of integrin β6 and HAX-1, thus providing a theoretically potential basis for molecular studies and possible targets for the future therapeutic interventions of ESCC.
Patients and Methods
Patients and Tissue Samples
A total of 10 pair matched frozen ESCC and adjacent non-tumor tissues were collected from patients who received radical resection at The First Affiliated Hospital of Xi’an Jiaotong University. In addition, a series of 137 paraffin-embedded samples from patients with ESCC were selected between March 2011 and September 2014, with the enrollment criteria to exclude the influence of neoadjuvant therapy on the prognosis of patients as follows: a) R0 curative surgery, b) no neoadjuvant chemotherapy or radiotherapy, c) no perioperative death, and d) no death due to other diseases rather than ESCC. The median follow-up period was 52 months (range, 8–60 months). The pathologic TNM (pTNM) staging was based on the 8th edition of the American Joint Committee on Cancer.15 The protocol was approved (approval no. 2018-G-162) by the Institutional Medical Ethics Committee of The First Affiliated Hospital of Xi’an Jiaotong University, and was in agreement with the Declaration of Helsinki. Written informed consent was obtained from the patients prior to study commencement.
Quantitative Real-Time PCR (qPCR) and Immunohistochemistry (IHC)
RNA extraction, reverse transcription and qPCR assays were performed according to our previous study.16 The mRNA expression of integrin β6 was normalized to housekeeper β-actin for the calculation of fold change using the 2−ΔΔCt method. For IHC staining, the slide sections were blocked with normal goat serum after optimal epitope retrieval. Subsequently, the slides were incubated with primary monoclonal antibody against integrin β6 (1:200; Clone 442.5C4; Sigma-Aldrich) or HAX-1 (1:500; cat. no. 166845; Santa Cruz Biotechnology, Inc.) at 4°C overnight. After incubation with horseradish peroxidase (HRP)-conjugated secondary antibody, the staining was developed using a DAB peroxidase substrate (Beyotime Institute of Biotechnology). Non-specific IgG was utilized as the negative control for primary antibody.
Assessment of Integrin β6 and HAX-1 Immunostaining
The immunostaining of integrin β6 and HAX-1 was mainly expressed in the cytoplasm of tumor cells. The staining levels were based on the extent and intensity assessed by two blinded observers. The staining intensity was graded as 0 (no staining), 1 (weak staining), 2 (moderate staining) or 3 (strong staining), and the staining extent was scored as 0 (0%), 1 (1–25%), 2 (26–50%), 3 (51–75%) or 4 (76–100%). The IHC score for each ESCC sample was determined by the product of staining intensity and extent grade. Given the heterogeneity of IHC scores, the optimal cut-off value was finally determined by Log-rank tests with respect to the overall survival as previously reported.17 An IHC score of 8 was used as the cut-off value for positive or negative integrin β6 staining. As for HAX-1 immunostaining, the patients were classified into high or low expression group, with IHC score 9 as the cut-off value.
Statistics
Statistical analyses were performed using GraphPad Prism 5 software (La Jolla, CA, USA). A 2-tailed Wilcoxon matched pairs test was utilized to compare the expression levels of integrin β6 in paired ESCC and adjacent normal tissues. Chi-square tests were used to investigate the association of integrin β6 and HAX-1 expression levels with different clinicopathological factors. Spearman correlation analysis was used to evaluate the relationship between integrin β6 and HAX-1 expression levels. The overall survival was analyzed via the Kaplan–Meier method with Log rank tests, with significant variables further evaluated using multivariate Cox regression analyses. P<0.05 was considered to indicate a statistically significant difference.
Results
Upregulation of Integrin β6 in ESCC
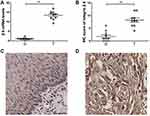
To examine whether there was a differential expression of integrin β6 in ESCC, qPCR analysis was performed in 10 paired human samples. The mRNA expression of integrin β6 was markedly increased in ESCC compared with matched adjacent normal tissues (Figure 1A). Subsequent IHC analysis of the same samples also demonstrated stronger immunostaining of integrin β6 in ESCC (Figure 1B–D).
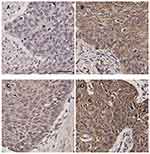
A total of 137 ESCC samples were hence enrolled to further investigate the staining of integrin β6 and HAX-1 via IHC, both of which were predominantly detected in the cytoplasm of tumor cells. Among the ESCC specimens, 57 cases (41.6%) demonstrated positive staining for integrin β6 (Figure 2A and B), and 65 cases (47.4%) showed high expression of HAX-1 (Figure 2C and D).
Relationship Between Integrin β6 and Clinicopathological Factors in ESCC
The relationship between integrin β6 and various clinicopathological factors is summarized in Table 1. There was a positive correlation between integrin β6 and lymph node metastasis, TNM stage and histological differentiation. The clinical cases with positive integrin β6 expression demonstrated a higher probability of lymph node metastasis compared with those with negative expression (P<0.001). In addition, there was an increased number of advanced-TNM-staged ESCC samples in integrin β6-positive cases compared with integrin β6 negative cases (P<0.001). Specimens with positive integrin β6 expression were more likely to exhibit poor differentiation (P=0.006). More importantly, patients with positive integrin β6 expression had worse outcomes compared with those with negative integrin β6 expression (Figure 3A; P<0.001).
 |
Table 1 Correlation of the Expression of Integrin β6 and HAX-1 with Clinicopathological Features in Human ESCC |
Relationship Between HAX-1 and Clinicopathological Factors in ESCC
There was a positive correlation between HAX-1 expression and tumor size, pT stage, lymph node metastasis and TNM stage (Table 2). ESCC specimens with high HAX-1 expression were more likely to demonstrate late pT stage (P<0.001) and lymph node metastasis (P=0.024) compared with those with low HAX-1 expression. Furthermore, there was an increased number of advanced-TNM-staged ESCC samples in high HAX-1 cases compared with low HAX-1 cases (P=0.016). It was also found that specimens with high HAX-1 staining were positively associated with tumor size (P=0.001). Additionally, patients with high HAX-1 expression had worse outcomes compared with those with low HAX-1 expression (Figure 3B; P=0.004).
 |
Table 2 Relationship Between Integrin β6 and HAX-1 in Human ESCC |
Correlation of Integrin β6 with HAX-1 in ESCC
The positive expression of integrin β6 was observed in 64.6% of samples highly expressing HAX-1, while the ratio of samples with low expression of HAX-1 was 20.8%. Spearman analysis further demonstrated that the expression of integrin β6 was positively correlated with HAX-1 expression (Table 2; r=0.444; P<0.001). The 137 ESCC cases enrolled in the present study were classified into four different groups based on integrin β6 and HAX-1 expression levels for Kaplan–Meier analysis, and the results indicated that patients with positive integrin β6 and high HAX-1 expression had worst outcomes compared with the other groups (Figure 4; P=0.003).
Univariate and Multivariate Analyses for Prognosis of Patients with ESCC
To investigate the potential value of integrin β6 and HAX-1 expression levels as predictors of poor prognosis for patient with ESCC, univariate and multivariate Cox regression analyses were performed. Univariate analysis suggested the ability of integrin β6 (P<0.001) and HAX-1 (P=0.004) expression levels to indicate poor outcomes, as well as clinicopathological parameters such as tumor size (P=0.032), histological differentiation (P=0.014), pT stage (P=0.025), lymph node metastasis (P=0.003) and TNM stage (P=0.017; Table 3). Subsequent multivariate analysis demonstrated that positive integrin β6 (P=0.004) and high HAX-1 expression (P=0.011) were independent unfavorable indicators for prognosis. In addition, pT stage (P=0.009) and lymph node metastasis (P=0.001) remained independent unfavorable prognostic indicators (Table 3).
 |
Table 3 Univariate and Multivariate Analyses of Clinicopathological Features for Overall Survival of 137 Patients with ESCC |
Discussion
Metastasis may occur at the early stage and initial diagnosis of ESCC, and along with the high incidence of post-operational recurrence, there are poor outcomes for patients with ESCC. Although pTNM stage classification is mostly utilized for survival reference, there is a great interest to discover alternative molecular biomarkers to supplement the pTNM staging system for early diagnosis and prognostic evaluation,18 which will facilitate the development of target therapy to treat highly lethal malignancy, such as ESCC. The development and progression of ESCC is a complicated process in which cancer cell adhesion, proliferation and motility mediated by integrins are involved by connecting the cytoskeleton to ECM. Different mechanisms via which integrin β6 participates in cancer invasion and metastasis have been described in previous studies,4 but a preliminary understanding of the role of integrin β6 in ESCC is lacking.
The present study for the first time characterized the expression of integrin β6 in ESCC, and demonstrated the mRNA expression of integrin β6 was significantly elevated in ESCC compared with corresponding normal tissues, which was also found at the protein level using IHC. In line with its oncogenic function, integrin β6 expression is elevated in a variety of malignancies, including human oral squamous cell carcinoma, suggesting its diagnostic value in colorectal cancer and cholangiocarcinoma.19–21 Furthermore, epithelial-specific integrin β6 has been reported to be a promising target for molecular imaging of cutaneous squamous cell carcinoma, head and neck squamous cell carcinoma, lung cancer and pancreatic ductal adenocarcinoma.22–24 Besides epigenetic regulation,25 the marked increase of integrin β6 expression during carcinogenesis could be induced by lysophosphatidic acid (LPA) via LPA receptor-1 coupling to Gαi, with subsequent activation of transcription factor Smad3 and Ets-1.19 Moreover, STAT3 is involved in the transcription of integrin β6.26 Cancer-associated fibroblasts (CAFs) in the tumor microenvironment (TME) may upregulate the expression of integrin β6 in colon cancer via the secretion of stromal cell-derived factor-1 (SDF-1) via the SDF-1/CXCR4 axis.27 Our previous study reported that interleukin-8 could increase integrin β6 expression via the ERK/Ets-1 pathway.28 In addition to the crosstalk between cancer cells and TME, integrin β6 could also be translationally regulated by eukaryotic initiation factor 4E (eIF4E), which contributes to tumor malignancy.29
The present study demonstrated that there were significant differences in histological differentiation, lymph node metastasis and TNM stage between positive and negative integrin β6 expression groups. It was identified that integrin β6 was an independent unfavorable indicator of prognosis for patients with ESCC. Integrin β6 serves as a poor prognostic indicator in multiple tumors, such as hilar cholangiocarcinoma16 and breast cancer.30 Integrin β6 can also initiate a cascade of downstream signaling pathways, as oppose to a single mechanism, to promote metastasis, which may explain why it sets the tumor stage and correlates with shortened survival.31 In particular, integrin β6 can induce the expression of MMP-3/9 in colon cancer cells via the ERK/Ets-1 pathway,32 contributing to invasion33 and chemo-resistance.34 Furthermore, integrin β6 promotes EMT via the TGF-β1/Smad2/3 signaling pathway,35 and the canonical Rho-Rac pathway may be downstream of integrin β6 via increased expression of MMP-9/15 in HER2 amplified breast cancer.36 In terms of TME, integrin β6 on cancer cells can activate fibroblasts via the TGF-β pathway, thus forming a positive feedback and mutually promotive crosstalk with CAF.27 In addition to intracellular pathway, integrin β6 can be transferred intercellularly via prostate cancer cell-derived small extracellular vesicles to microvascular endothelial cells, which enhances angiogenesis and progression.37
Disassembly of focal adhesions mediated by integrin via endocytosis is a key process for the facilitation of migration. Given the participation of abnormal integrin endocytosis in tumor progression, insights into the endocytic mechanisms by which integrin exerts its functions to promote invasiveness are vital to gain functional understanding of carcinogenesis.38 Ramsay et al provided the first evidence that the interaction with HAX-1 was crucial to the endocytosis of integrin β6 in the context of cancer.12 As such, the present study also investigated the expression of HAX-1 in ESCC samples. Indeed, high expression of HAX-1 was observed in ESCC cells, which was associated with tumor size, lymph node metastasis, advanced pT and TNM stage, consistent with a previous report.14 HAX-1 can modulate actomyosin contractility through RhoA/septin signaling, thus promoting cell adhesion and collective migration associated with breast cancer progression.39 Moreover, HAX-1 regulates proliferation, metastasis and angiogenesis of hepatocellular carcinoma cells by affecting Akt phosphorylation and FOXO3A expression.40 Endocytic trafficking is vital for the regulation of integrin-mediated malignant behaviors of tumor cells. Endocytosis participates in the dynamic process to redistribute the pools of integrin β6 from one part of the cell to another, allowing cancer cells to react more rapidly to adverse environmental stress.13 In the present study, it was found that there was a positive relationship between the expression levels of integrin β6 and HAX-1. Furthermore, patients with positive integrin β6 and high HAX-1 expression demonstrated worst outcomes, which is in accordance with the involvement of integrin β6 and HAX-1 in tumor progression. It should be noted that although 7 patients enrolled in the present study received chemotherapy or radiotherapy following radical surgery, adjuvant therapies were reported to fail to improve survival when compared with surgery alone and appeared less effective compared with neoadjuvant therapies.41 Hence, it is likely that adjuvant therapies would not impact the final analyses and results to a large degree. Still, additional multi-center studies with larger sample size are needed to validate the results obtained from the present study.
The discovery that integrin β6 promotes pathological processes marks it as a potential target. Indeed, integrin β6 has been investigated in therapeutic therapies, based on its role in cancer progression. The combination of integrin β6-targeted photodynamic therapy with immune checkpoint inhibition demonstrates enhanced anti-tumor efficacy with improved anti-tumor immunity and suppressed tumor growth.42 Additionally, integrin β6-targeted immunoliposomes show tumor-specific 5-fluorouracil delivery in colon cancer, offering a potential anticancer strategy to improve therapeutic efficacy.43 Integrin β6-targeted adenovirus vectors also provide a promising platform for local intraperitoneal treatment of ovarian cancer metastasis.44 Moreover, integrin β6-specific therapy developed from foot-and-mouth-disease virus could selectively eliminate β6-positive pancreatic cancer.45 Whilding et al reported the clinical evaluation of integrin β6 re-targeted chimeric antigen receptor T cell immunotherapy for tumors that express this integrin, providing a promising new molecular-specific therapy.46 Therefore, in the future mechanistic studies would be performed to further examine the role of HAX-1 in integrin β6-mediated ESCC progression, and evaluate the potential of HAX-1 and/or integrin β6-targeted therapy in the translation into clinical benefits.
In conclusion, the present study identified the upregulation of integrin β6 and its relevance to HAX-1, as well as unfavorable clinicopathological factors in ESCC. The current results further defined the predictive value of integrin β6 and HAX-1 as independent indicators of poor prognosis for patients with ESCC, both of which may contribute to tumor proliferation and metastasis, thus promoting ESCC progression. Therefore, combined targeting of integrin β6 and HAX-1 are required for future investigation, which may provide a potential novel approach for the treatment of ESCC.
Funding
This work was supported by the National Natural Science Foundation of China (81702362, 81803026, 81870380), Key Research and Development Program of Shaanxi (No. 2019KW-067), China Postdoctoral Science Foundation (2019T120922, 2018M631176), and Postdoctoral Science Foundation of Shaanxi Province (2018BSHYDZZ49).
Disclosure
The authors declare no conflict of interest in this work.
References
1. Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med. 2014;371(26):2499–2509.
2. Malhotra GK, Yanala U, Ravipati A, Follet M, Vijayakumar M, Are C. Global trends in esophageal cancer. J Surg Oncol. 2017;115(5):564–579. doi:10.1002/jso.24592
3. Te Molder L, Juksar J, Harkes R, Wang W, Kreft M, Sonnenberg A. Tetraspanin CD151 and integrin alpha3beta1 contribute to the stabilization of integrin alpha6beta4-containing cell-matrix adhesions. J Cell Sci. 2019;132(19):jcs235366. doi:10.1242/jcs.235366
4. Niu J, Li Z. The roles of integrin alphavbeta6 in cancer. Cancer Lett. 2017;403:128–137. doi:10.1016/j.canlet.2017.06.012
5. Lee C, Lee C, Lee S, Siu A, Ramos DM. The cytoplasmic extension of the integrin beta6 subunit regulates epithelial-to-mesenchymal transition. Anticancer Res. 2014;34(2):659–664.
6. Tod J, Hanley CJ, Morgan MR, et al. Pro-migratory and TGF-beta-activating functions of alphavbeta6 integrin in pancreatic cancer are differentially regulated via an Eps8-dependent GTPase switch. J Pathol. 2017;243(1):37–50. doi:10.1002/path.4923
7. Janes SM, Watt FM. Switch from alphavbeta5 to alphavbeta6 integrin expression protects squamous cell carcinomas from anoikis. J Cell Biol. 2004;166(3):419–431. doi:10.1083/jcb.200312074
8. De Franceschi N, Hamidi H, Alanko J, Sahgal P, Ivaska J. Integrin traffic - the update. J Cell Sci. 2015;128(5):839–852. doi:10.1242/jcs.161653
9. Nader GP, Ezratty EJ, Gundersen GG. FAK, talin and PIPKIgamma regulate endocytosed integrin activation to polarize focal adhesion assembly. Nat Cell Biol. 2016;18(5):491–503. doi:10.1038/ncb3333
10. Cao F, Zhou Y, Liu X, Yu CH. Podosome formation promotes plasma membrane invagination and integrin-beta3 endocytosis on a viscous RGD-membrane. Commun Biol. 2020;3(1):117. doi:10.1038/s42003-020-0843-2
11. Barrow-McGee R, Kishi N, Joffre C, et al. Beta 1-integrin-c-Met cooperation reveals an inside-in survival signalling on autophagy-related endomembranes. Nat Commun. 2016;7:11942.
12. Ramsay AG, Keppler MD, Jazayeri M, et al. HS1-associated protein X-1 regulates carcinoma cell migration and invasion via clathrin-mediated endocytosis of integrin alphavbeta6. Cancer Res. 2007;67(11):5275–5284. doi:10.1158/0008-5472.CAN-07-0318
13. Wang J, Wu J, Hong J, et al. PKC promotes the migration of colon cancer cells by regulating the internalization and recycling of integrin alphavbeta6. Cancer Lett. 2011;311(1):38–47. doi:10.1016/j.canlet.2011.06.025
14. Li M, Tang Y, Zang W, et al. Analysis of HAX-1 gene expression in esophageal squamous cell carcinoma. Diagn Pathol. 2013;8:47.
15. Rice TW, Patil DT, Blackstone EH. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: application to clinical practice. Ann Cardiothorac Surg. 2017;6(2):119–130. doi:10.21037/acs.2017.03.14
16. Sun Q, Dong X, Shang Y, Sun F, Niu J, Li F. Integrin alphavbeta6 predicts poor prognosis and promotes resistance to cisplatin in hilar cholangiocarcinoma. Pathol Res Pract. 2020;216(7):153022. doi:10.1016/j.prp.2020.153022
17. Wolf D, Wolf AM, Rumpold H, et al. The expression of the regulatory T cell-specific forkhead box transcription factor FoxP3 is associated with poor prognosis in ovarian cancer. Clin Cancer Res. 2005;11(23):8326–8331. doi:10.1158/1078-0432.CCR-05-1244
18. Shen Q, Shang B, Jiang B, Wang Y, Wang Z, Chen G. Overexpression of JAB1 promotes malignant behavior and predicts poor prognosis in esophageal squamous cell carcinoma. Thorac Cancer. 2020;11(4):973–982. doi:10.1111/1759-7714.13350
19. Xu M, Yin H, Cai Y, et al. Lysophosphatidic acid induces integrin beta6 expression in human oral squamous cell carcinomas cells via LPAR1 coupling to Galphai and downstream SMAD3 and ETS-1 activation. Cell Signal. 2019;60:81–90. doi:10.1016/j.cellsig.2019.04.008
20. Bengs S, Becker E, Busenhart P, et al. Beta6 -integrin serves as a novel serum tumor marker for colorectal carcinoma. Int J Cancer. 2019;145(3):678–685.
21. Li Z, Biswas S, Liang B, et al. Integrin beta6 serves as an immunohistochemical marker for lymph node metastasis and promotes cell invasiveness in cholangiocarcinoma. Sci Rep. 2016;6:30081. doi:10.1038/srep30081
22. Baart VM, van Duijn C, van Egmond SL, et al. EGFR and alphavbeta6 as promising targets for molecular imaging of cutaneous and mucosal squamous cell carcinoma of the head and neck region. Cancers. 2020;12(6):1474. doi:10.3390/cancers12061474
23. Saleem A, Helo Y, Win Z, et al. Integrin alphavbeta6 positron emission tomography imaging in lung cancer patients treated with pulmonary radiation therapy. Int J Radiat Oncol Biol Phys. 2020;107(2):370–376. doi:10.1016/j.ijrobp.2020.02.014
24. Ui T, Ueda M, Higaki Y, et al. Development and characterization of a (68)Ga-labeled A20FMDV2 peptide probe for the PET imaging of alphavbeta6 integrin-positive pancreatic ductal adenocarcinoma. Bioorg Med Chem. 2020;28(1):115189. doi:10.1016/j.bmc.2019.115189
25. Xu M, Yin L, Cai Y, et al. Epigenetic regulation of integrin beta6 transcription induced by TGF-beta1 in human oral squamous cell carcinoma cells. J Cell Biochem. 2018;119(5):4193–4204.
26. Niu W, Bo QY, Niu J, et al. Identification of integrin beta6 gene promoter and analysis of its transcription regulation in colon cancer cells. World J Gastrointest Oncol. 2020;12(5):526–534. doi:10.4251/wjgo.v12.i5.526
27. Peng C, Zou X, Xia W, et al. Integrin alphavbeta6 plays a bi-directional regulation role between colon cancer cells and cancer-associated fibroblasts. Biosci Rep. 2018;38(6).
28. Sun Q, Sun F, Wang B, et al. Interleukin-8 promotes cell migration through integrin alphavbeta6 upregulation in colorectal cancer. Cancer Lett. 2014;354(2):245–253. doi:10.1016/j.canlet.2014.08.021
29. Enyu L, Zhengchuan N, Jiayong W, et al. Integrin beta6 can be translationally regulated by eukaryotic initiation factor 4E: contributing to colonic tumor malignancy. Tumour Biol. 2015;36(8):6541–6550. doi:10.1007/s13277-015-3348-8
30. Moore KM, Thomas GJ, Duffy SW, et al. Therapeutic targeting of integrin alphavbeta6 in breast cancer. J Natl Cancer Inst. 2014;106(8). doi:10.1093/jnci/dju169.
31. Cantor DI, Cheruku HR, Nice EC, Baker MS. Integrin alphavbeta6 sets the stage for colorectal cancer metastasis. Cancer Metastasis Rev. 2015;34(4):715–734. doi:10.1007/s10555-015-9591-z
32. Gao H, Peng C, Liang B, et al. Beta6 integrin induces the expression of metalloproteinase-3 and metalloproteinase-9 in colon cancer cells via ERK-ETS1 pathway. Cancer Lett. 2014;354(2):427–437. doi:10.1016/j.canlet.2014.08.017
33. Yang GY, Guo S, Dong CY, et al. Integrin alphavbeta6 sustains and promotes tumor invasive growth in colon cancer progression. World J Gastroenterol. 2015;21(24):7457–7467.
34. Liu S, Wang J, Niu W, et al. The beta6-integrin-ERK/MAP kinase pathway contributes to chemo resistance in colon cancer. Cancer Lett. 2013;328(2):325–334. doi:10.1016/j.canlet.2012.10.004
35. Liu W, Sun T, Wang Y. Integrin alphavbeta6 mediates epithelial-mesenchymal transition in human bronchial epithelial cells induced by lipopolysaccharides of Pseudomonas aeruginosa via TGF-beta1-Smad2/3 signaling pathway. Folia Microbiol (Praha). 2020;65(2):329–338. doi:10.1007/s12223-019-00728-w
36. Desai K, Nair MG, Prabhu JS, et al. High expression of integrin beta6 in association with the Rho-Rac pathway identifies a poor prognostic subgroup within HER2 amplified breast cancers. Cancer Med. 2016;5(8):2000–2011.
37. Krishn SR, Salem I, Quaglia F, et al. The alphavbeta6 integrin in cancer cell-derived small extracellular vesicles enhances angiogenesis. J Extracell Vesicles. 2020;9(1):1763594. doi:10.1080/20013078.2020.1763594
38. Moreno-Layseca P, Icha J, Hamidi H, Ivaska J. Integrin trafficking in cells and tissues. Nat Cell Biol. 2019;21(2):122–132. doi:10.1038/s41556-018-0223-z
39. Balcerak A, Trebinska-Stryjewska A, Wakula M, et al. HAX1 impact on collective cell migration, cell adhesion, and cell shape is linked to the regulation of actomyosin contractility. Mol Biol Cell. 2019;30(25):3024–3036. doi:10.1091/mbc.E19-05-0304
40. Wu Z, Ai X, Hu H, et al. Hematopoietic-substrate-1 associated protein X-1 (HAX-1) regulates liver cancer cells growth, metastasis, and angiogenesis through Akt. Cancer Biol Ther. 2019;20(9):1223–1233. doi:10.1080/15384047.2019.1617562
41. Pasquali S, Yim G, Vohra RS, et al. Survival after neoadjuvant and adjuvant treatments compared to surgery alone for resectable esophageal carcinoma: a network meta-analysis. Ann Surg. 2017;265(3):481–491. doi:10.1097/SLA.0000000000001905
42. Gao L, Zhang C, Gao D, et al. Enhanced anti-tumor efficacy through a combination of integrin alphavbeta6-targeted photodynamic therapy and immune checkpoint inhibition. Theranostics. 2016;6(5):627–637. doi:10.7150/thno.14792
43. Liang B, Shahbaz M, Wang Y, et al. Integrinbeta6-targeted immunoliposomes mediate tumor-specific drug delivery and enhance therapeutic efficacy in colon carcinoma. Clin Cancer Res. 2015;21(5):1183–1195. doi:10.1158/1078-0432.CCR-14-1194
44. Uusi-Kerttula H, Davies J, Coughlan L, et al. Pseudotyped alphavbeta6 integrin-targeted adenovirus vectors for ovarian cancer therapies. Oncotarget. 2016;7(19):27926–27937. doi:10.18632/oncotarget.8545
45. Moore KM, Desai A, Delgado BL, et al. Integrin alphavbeta6-specific therapy for pancreatic cancer developed from foot-and-mouth-disease virus. Theranostics. 2020;10(7):2930–2942. doi:10.7150/thno.38702
46. Whilding LM, Parente-Pereira AC, Zabinski T, et al. Targeting of aberrant alphavbeta6 integrin expression in solid tumors using chimeric antigen receptor-engineered T cells. Mol Ther. 2017;25(1):259–273. doi:10.1016/j.ymthe.2016.10.012
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.